Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6278
Revised: July 29, 2013
Accepted: August 20, 2013
Published online: October 7, 2013
AIM: To compare efficacy of combined lamivudine (LAM) and adefovir dipivoxil (ADV) therapy with that of entecavir (ETV) monotherapy for hepatitis B virus (HBV)-related decompensated liver cirrhosis.
METHODS: A total of 120 naïve patients with HBV-related decompensated cirrhosis participated in this study. Sixty patients were treated with combined LAM and ADV therapy (LAM + ADV group), while the other 60 were treated with ETV monotherapy (ETV group) for two years. Tests for liver and kidney function, alpha-fetoprotein, HBV serum markers, HBV DNA load, prothrombin time (PT), and ultrasonography or computed tomography scan of the liver were performed every 1 to 3 mo. Repeated measure ANOVA and the χ2 test were performed to compare the efficacy, side effects, and the cumulative survival rates at 48 and 96 wk.
RESULTS: Forty-five patients in each group were observed for 96 wk. No significant differences in HBV DNA negative rates and alanine aminotransferase (ALT) normalization rates at weeks 48 (χ2 = 2.12 and 2.88) and 96 (χ2 = 3.21 and 3.24) between the two groups were observed. Hepatitis B e antigen seroconversion rate in the LAM + ADV group at week 96 was significantly higher in the ETV group (43.5% vs 36.4%, χ2 = 4.09, P < 0.05). Viral breakthrough occurred in 2 cases (4.4%) by week 48 and in 3 cases (6.7%) by week 96 in the LAM + ADV group, and no viral mutation was detected. In the ETV group, viral breakthrough occurred in 1 case (2.2%) at the end of week 96. An increase in albumin (F = 18.9 and 17.3), decrease in total bilirubin and in ALT (F = 16.5, 17.1 and 23.7, 24.8), reduced PT (F = 22.7 and 24.5), and improved Child-Turcotte-Pugh and the model for end-stage liver disease scores (F = 18.5, 17.8, and 24.2, 23.8) were observed in both groups. The cumulative rates of mortality and liver transplantation were 16.7% (10/60) and 18.3% (11/60) in the LAM + ADV and ETV groups, respectively.
CONCLUSION: Both LAM + ADV combination therapy and ETV monotherapy can effectively inhibit HBV replication, improve liver function, and decrease mortality.
Core tip: This study compared the de novo efficacy of combined lamivudine (LAM) and adefovir dipivoxil (ADV) therapy with that of entecavir (ETV) monotherapy for patients with hepatitis B virus (HBV)-related decompensated liver cirrhosis. Both LAM + ADV combination therapy and ETV monotherapy can effectively inhibit HBV replication, improve liver function, and decrease mortality. The data obtained in this study demonstrate the efficacy and the safety of these treatment regimens for 96 wk in patients with HBV-related decompensated liver cirrhosis.
-
Citation: Lian JS, Zeng LY, Chen JY, Jia HY, Zhang YM, Xiang DR, Yu L, Hu JH, Lu YF, Zheng L, Li LJ, Yang YD.
De novo combined lamivudine and adefovir dipivoxil therapyvs entecavir monotherapy for hepatitis B virus-related decompensated cirrhosis. World J Gastroenterol 2013; 19(37): 6278-6283 - URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6278
Cirrhosis is the end stage of chronic liver damage and is characterized by fibrosis resulting in the distortion and destruction of normal liver architecture. Functional liver tissue is destroyed and replaced by regenerating nodules that do not fully restore lost liver function. Cirrhosis may be due to various causes, including hepatitis B virus (HBV) infection, hepatitis C (HCV) and alcohol consumption[1,2]. Chronic infection with HBV accounts for 30% of hepatic cirrhosis globally[3]. In China, about 93 million people are carriers of HBV, with 20 million people chronically infected. Within a five-year period, 10% to 20% of patients with chronic hepatitis B develop cirrhosis[4]. The five-year survival rates of patients with compensated cirrhosis and of those with decompensated cirrhosis (determined by the presence of ascites, hepatoencephalopathy, and/or history of variceal bleeding) were 84% and 14%, respectively[5]. Cirrhosis precedes most cases of hepatocellular carcinoma (HCC), with 70% to 90% of HCC developing from liver cirrhosis or inflammation[6]. Antiviral agents are assumed to reduce decompensated cirrhosis and HCC development[7], however, agents such as lamivudine (LAM) and telbivudin show high drug resistance. The latest chronic hepatitis B prevention and treatment guidelines suggest the selection of a higher genetic barrier to resistant antivirals, such as entecavir (ETV) and tenofovir, for patients with HBV-related liver cirrhosis[8,9]. However, based on the paradigm that drug combination therapy is more effective than monotherapy for the treatment of human immunodeficiency virus and HCV, the same approach may be appropriate for chronic hepatitis B. This study was designed to compare the two-year efficacy of de novo combination therapy of LAM and adefovir dipivoxil (ADV) with that of ETV monotherapy in patients with decompensated liver cirrhosis.
From January 2008 to March 2009, 120 patients diagnosed with HBV-related decompensated liver cirrhosis at the First Affiliated Hospital of the Zhejiang University School of Medicine (Hangzhou, China) were recruited into this study. The diagnosis was based on medical history, the results of physical examination, biochemical, endoscopic and ultrasound findings, and radiological signs of cirrhosis. All patients were 18 to 65 years old, with ≥ 103 copies/mL HBV DNA, 7 to 12 (inclusive) Child-Turcotte-Pugh (CTP) score, ≥ 50 mL calculated serum creatinine clearance, ≥ 75 g/L hemoglobin, ≥ 2.5 × 109/L total white blood cells, ≤ 20 ng/mL α-fetoprotein, and no evidence of HCC. None of the patients had been treated with antiviral drugs, including interferon-α or nucleos(t)ides. Patients with hepatitis delta virus, hepatitis C virus, or had human immunodeficiency virus (HIV) co-infection were excluded. Patients with HCC, autoimmune hepatitis, alcoholic liver cirrhosis, hepatorenal syndrome, grade 3 or 4 hepatic encephalopathy, spontaneous bacterial peritonitis, and severe heart, renal, and brain diseases were also excluded. All patients who participated in this study provided informed consent and were aware of the procedures to be conducted. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University.
The study was designed as a prospective case-control study. The patients were randomly assigned to the ETV monotherapy (60 patients) group and the de novo LAM and ADV combination therapy (60 patients) group. Baseline data of the two groups were compared to ensure comparability. Patients in the combination therapy group were prescribed 100 mg LAM and 10 mg ADV per day, while the monotherapy group received 0.5 mg ETV per day.
Serum hepatitis B viral markers, including hepatitis B surface antigen (HBsAg), antibody to HBsAg, hepatitis B e antigen (HBeAg), antibody to HBeAg and antibody to hepatitis B core antigen, were detected by commercially available enzyme immunoassays (Abbott Laboratories; Chicago, IL, United States). Serum HBV DNA was measured by polymerase chain reaction with a linear range between 1 × 103 and 5 × 108 copies/mL (Shanghai ZJ Bio-Tech Co., Ltd., China).
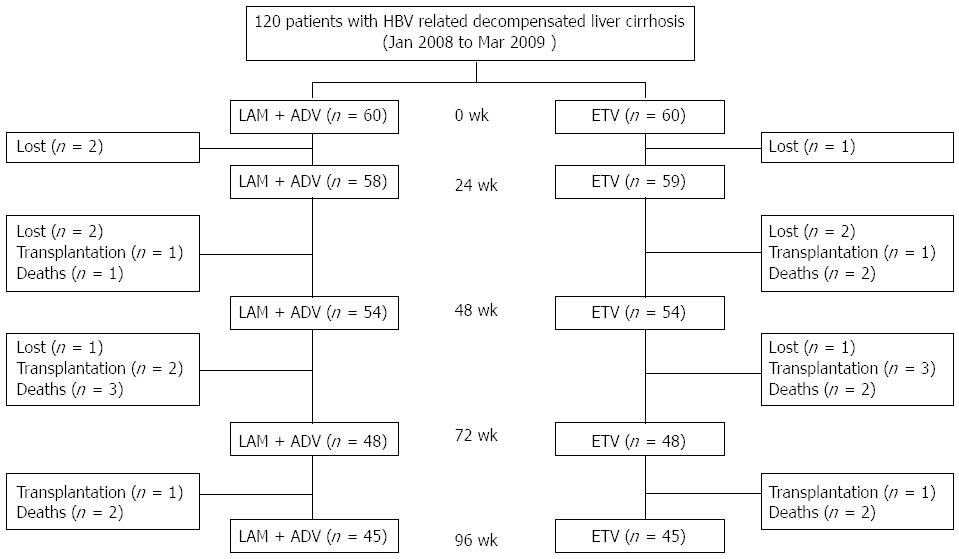
Follow-up observations in the two groups were performed at the start and during weeks 4, 12, 24, 36, 48, 60, 72, 84, and 96. Follow-up clinical assessments included physical examination, HBeAg and antibodies to the e antigen, quantitative HBV DNA, serum biochemistry, alpha-fetoprotein, renal function, prothrombin time (PT), and ultrasonography or computed tomography scan. The lower limit of detection of DNA used in this study was 1.0 × 103 copies/mL (Shanghai ZJ Bio-Tec Co., Ltd, China). The condition of the patients after 96 wk is shown in Figure 1.
SPSS 16.0 software was used for data analysis. Measurements were presented as mean ± SD and comparisons were conducted following analysis of the results using the Student’s t test. Proportions were presented as percentage (%). Rate comparisons were performed using the χ2 test. A P value < 0.05 was considered significant.
In the two years of follow-up observations, of the 60 patients who received LAM and ADV combination therapy, 5 cases were lost, 4 cases underwent liver transplantation, 6 cases died, and 45 cases survived until the end of the observation period. The 45 remaining cases comprised 16 females and 29 males. The mean patient age was 53.1 ± 8.8 years. Of the 60 patients who received ETV monotherapy, 4 cases were lost, 5 cases underwent liver transplantation, 6 cases died, and 45 cases survived until the end of the observation period. The 45 remaining cases comprised 17 females and 28 males. The mean patient age was 53.2 ± 7.4 years. The baseline characteristics of the patients were similar, and no statistically significant differences were observed (Table 1).
| Variables | LAM + ADV (n = 45) | ETV (n = 45) | t | χ2 | P value |
| Age (yr) | 53.1 ± 8.8 | 53.2 ± 7.4 | 0.23 | > 0.05 | |
| Male/female | 29/16 | 28/17 | 3.10 | > 0.05 | |
| HBV DNA (log10 copy/mL) | 6.56 ± 1.13 | 6.61 ± 1.15 | 0.33 | > 0.05 | |
| ALT (U/L) | 98.1 ± 21.6 | 99.8 ± 17.2 | 0.23 | > 0.05 | |
| TBil (μmol/L) | 51.6 ± 8.9 | 49.2 ± 6.8 | 0.31 | > 0.05 | |
| Alb (g/L) | 29.2 ± 0.7 | 29.5 ± 1.2 | 0.24 | > 0.05 | |
| PT (s) | 16.3 ± 2.3 | 16.5 ± 1.9 | 0.21 | > 0.05 | |
| CTP score | 8.4 ± 1.7 | 8.6 ± 2.1 | 0.16 | > 0.05 | |
| MELD score | 13.7 ± 3.5 | 12.9 ± 6.7 | 0.25 | > 0.05 | |
| HBeAg positive rate | 23 (51.1) | 22 (48.9) | 2.13 | > 0.05 | |
| Ascites | 22 (48.9) | 21 (46.7) | 2.46 | > 0.05 | |
| HE | 6 (13.3) | 7 (15.5) | 3.13 | > 0.05 | |
| UGB | 9 (22.2) | 8 (17.8) | 3.35 | > 0.05 |
Of the 45 patients in the LAM and ADV combination group, 51.1% (23/45) and 86.7% (39/45) achieved undetectable HBV DNA by weeks 48 and 96, respectively. Of the 45 patients in the ETV group, 60% (27/45) and 88.9% (40/45) achieved undetectable HBV DNA by weeks 48 and 96, respectively. No statistical differences were observed between the two groups by weeks 48 and 96 (P > 0.05).
In the LAM and ADV combination therapy group, 71.1% (32/45) and 88.9% (40/45) of patients achieved ALT normalization by week 48 and 96, respectively. In the ETV treatment group, 68.9% (31/45) and 91.1% (41/45) achieved ALT normalization by week 48 and 96, respectively. No statistical difference was observed between the two groups at week 48 and 96 (P > 0.05).
Of the 45 patients who received the LAM and ADV combination treatment, 30.4% (7/23) and 43.5% (10/23) achieved HBeAg seroconversion by weeks 48 and 96, respectively. Similarly, 27.3% (6/22) and 36.4% (8/22) of the patients who received ETV monotherapy achieved HBeAg seroconversion by weeks 48 and 96, respectively. No statistical difference was observed between the two groups at week 48, while the HBeAg seroconversion rate in the LAM and ADV combination group at week 96 was significantly higher than that in the ETV monotherapy group (43.5% vs 36.4%, χ2 = 4.09, P < 0.05).
Of the respondents, 2 and 3 patients in the LAM and ADV combination group and 1 and 2 patients in the ETV monotherapy group developed virological breakthrough by weeks 48 and 96, respectively. No genetic mutations were detected in either patient group. The obtained differences were not statistically different (P > 0.05).
After 96 wk of treatment, the albumin level in patients in the LAM and ADV combination group increased significantly compared with the baseline level (F = 18.9, P < 0.05), whereas ALT and TBil decreased significantly compared with the baseline levels (F = 16.5 and 23.7, respectively, P < 0.05). In addition, PT was significantly shortened (F = 22.7, P < 0.05), and both CTP and MELD scores decreased significantly compared with the baseline scores (F = 18.5 and 24.2, respectively, P < 0.05). A decrease of more than 2 points in the CTP score in 31 (68.9%) cases was observed and is shown in Table 2.
| Characteristics | LAM + ADV combination group | ETV monotherapy group | ||||
| 0 wk | 48 wk | 96 wk | 0 wk | 48 wk | 96 wk | |
| Alb (g/L) | 29.2 ± 0.7 | 32.2 ± 0.5 | 36.7 ± 0.2a | 28.9 ± 1.2 | 31.9 ± 0.4 | 36.4 ± 0.6a |
| TBil (μmol/L) | 51.6 ± 8.9 | 30.8 ± 7.5 | 19.1 ± 6.2a | 47.2 ± 6.8 | 31.6 ± 6.8 | 18.2 ± 3.9a |
| ALT (U/L) | 98.1 ± 21.6 | 56.1 ± 21.3 | 34.7 ± 12.8a | 99.8 ± 17.2 | 54.2 ± 15.7 | 32.5 ± 11.5a |
| PT (s) | 16.3 ± 2.3 | 14.3 ± 1.6 | 12.6 ± 2.1a | 16.5 ± 1.9 | 13.8 ± 2.0 | 12.9 ± 3.7a |
| CTP score | 8.4 ± 1.7 | 6.8 ± 1.9 | 5.5 ± 3.7a | 8.6 ± 2.1 | 6.7 ± 2.5 | 5.7 ± 1.3a |
| MELD score | 13.7 ± 3.5 | 9.8 ± 3.1 | 7.6 ± 1.8a | 12.9 ± 6.7 | 9.6 ± 4.3 | 7.9 ± 2.3a |
After 96 wk, patients who received ETV treatment exhibited a significant increase in albumin level compared with the baseline level (F = 17.3, P < 0.05). In contrast, ALT and TBil decreased significantly compared with baseline levels (F = 17.1 and 24.8, P < 0.05). PT was significantly shortened (F = 24.5, P < 0.05), and CTP and MELD scores decreased significantly compared with the baseline levels (F = 17.8 and 23.8, P < 0.05). A decrease in the CTP score by more than 2 points was evident in 30 (66.7%) cases.
The LAM and ADV combination group and the ETV monotherapy group showed no significant differences in albumin level or in ALT, TBil, PT, CTP, and MELD scores by weeks 48 and 96 (Table 2).
All patients in this study responded well to both LAM and ADV combination therapy and ETV monotherapy. Creatinine levels in four cases in the LAM and ADV combination therapy group and in one case in the ETV monotherapy group were more than twice the baseline values, but were still lower than upper limit of normal. No patient developed lactic acidosis in either group.
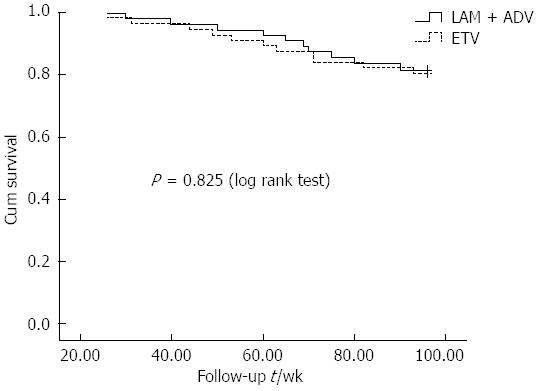
In the combination group, the cumulative mortality (including liver transplantation) was 16.7% (10/60) during the follow-up period, and included 2 cases of upper gastrointestinal bleeding, 2 cases of hepatic encephalopathy, 1 case of secondary bacterial infection, and 1 case of hepatorenal syndrome. Four patients had undergone liver transplantation in this group. In the ETV monotherapy group, the cumulative mortality (including liver transplantation) was 18.3% (11/60), and included 3 cases of upper gastrointestinal bleeding, 2 cases of secondary bacterial infection, and 2 cases of hepatorenal syndrome. Three patients had undergone liver transplantation. These findings are illustrated in Figure 2.
Increasing evidence shows that suppression of HBV replication results in the reduction of hepatic necroinflammation and consequently, improvement of liver function in patients with HBV-related decompensated liver cirrhosis. Antiviral therapy associated with improved outcomes in patients with HBV-related decompensated cirrhosis, including postponement or prevention of liver transplantation, reducing the incidence of HCC[10-12].
LAM was the first oral agent approved for the treatment of chronic hepatitis B (CHB) and currently has a well-established safety and efficacy profile. Liaw et al[13] reported that continuous treatment with LAM delays clinical progression of CHB infection in patients by significantly reducing the incidence of hepatic decompensation and HCC. ADV benefits pre- and post-transplant patients with LAM-resistant CHB, including decompensated cirrhotics, by suppressing HBV DNA and by improving the CTP score[14,15]. In China, ADV is relatively cheap and a large number of CHB patients, including cirrhotics, have received LAM and ADV combination therapy. According to the latest guidelines, the patients with liver cirrhosis and those who have received a liver graft for HBV-related cirrhosis should be considered for de novo combination therapy because of the risk of clinical deterioration if they develop drug resistance[16]. But the data to support a role of combination therapy in these patients were limited. On the other hand, ETV demonstrates very low rates of resistance in nucleoside-naïve patients and is recommended for patients with HBV-related decompensated cirrhosis[8,17]. Therefore, a comparison of the efficacy and safety between LAM and ADV combination therapy and ETV monotherapy for patients with HBV-related decompensated cirrhosis is urgent. Our study showed that 51.1% and 86.7% of patients in the LAM and ADV combination group achieved undetectable HBV DNA by weeks 48 and 96, respectively, while 60% and 88.9% of patients in the ETV treatment group achieved undetectable HBV DNA by weeks 48 and 96, respectively. In addition, both de novo combination of LAM and ADV therapy and ETV monotherapy significantly increased albumin level and decreased TBil, PT, CTP, and MELD scores compared with baseline. More importantly, 68.9% of patients in the combination group and 66.7% of patients in the monotherapy group had a decrease in their CTP score of more than 2 points after 96 wk of treatment. A total of 73.7% of patients in the combination group and 71.1% of patients in the monotherapy group exhibited an increase in the CTP score at the end of 96 wk. No genetic mutations in either treatment group were detected. In this study, no statistically significant difference was observed between the LAM and ADV combination therapy group and the ETV monotherapy group in terms of the serological conversion rate by 48 wk, while the HBeAg seroconversion rate in the LAM and ADV combination group at week 96 was significantly higher than that in the ETV group. This finding is similar to the results of previous studies[18].
HBV-related decompensated cirrhosis requires a longer duration of antiviral therapy and consideration of the effect and safety of these drugs are essential. LAM has been shown to be safe. ADV, in contrast, is mainly excreted by the kidney and has an impact on renal function during long-term antiviral therapy[19]. Our study confirms that ADV treatment of decompensated cirrhosis is safe and effective. However, in the subsequent stages of treatment, doctors should closely monitor kidney function and adjust the treatment plan as soon as renal function is found to be abnormal.
The best treatment method for late-stage HBV-related decompensated liver cirrhosis is liver transplantation. However, transplantation is very expensive and there is a worldwide donor shortage. Liver transplantation is considered in the treatment of decompensated cirrhosis only when the CTP score for grade C or the MELD score is more than 20 points. Upon detection of HBV DNA, patients with decompensated cirrhosis should be immediately treated with antiviral therapy to improve liver function and to reduce the need for liver transplantation.
Lange et al[20] reported on 16 patients with liver cirrhosis and chronic hepatitis B who were treated with ETV. Five of these patients developed lactic acidosis (all with MELD scores > 20) during ETV treatment. Lactic acidosis was lethal for one patient, while for other patients, the symptoms were resolved after termination/interruption of ETV treatment. In the present study, no cases of lactic acidosis were observed during the follow-up period of 96 wk.
In conclusion, both de novo LAM and ADV combination therapy and ETV monotherapy are effective in patients with HBV-related decompensated cirrhosis, with no differences in the level of HBV DNA suppression, liver function improvement, resistance rate, and on confirmed changes in renal parameters and in cumulative survival rate. The data obtained in this study demonstrate the efficacy and the safety of these treatment regimens for 96 wk in patients with HBV-related decompensated liver cirrhosis, as well as their evident therapeutic benefits in both groups.
The mortality rate of hepatitis B virus (HBV)-related decompensated cirrhosis is very high. Recommended treatment options are monotherapy with high genetic barrier nucleos(t)ide analogues or combination therapy with no cross resistance nucleos(t)ide analogues. There has been no report regarding the entecavir monotherapy or de novo lamivudine and adefovir dipivoxil combination therapy in these patients.
De novo combination therapy with lamivudine and adefovir dipivoxil is better than lamivudine monotherapy in patients with HBV-related decompensated live cirrhosis. But there is no head to head research to compare the entecavir, a high genetic barrier nucleoside analogue monotherapy with de novo lamivudine and adefovir dipivoxil combination therapy for those patients. In this study, the authors demonstrated that both entecavir monotherapy and de novo lamivudine and adefovir dipivoxil combination therapy were effective for patients with HBV-related decompensated liver cirrhosis.
Many clinical studies showed that the combined therapy is effective for patients with human immunodeficiency virus and hepatitis C virus infection. And entecavir is effective for patients with HBV-related decompensated liver cirrhosis. This is the first head to head study to report that both de novo lamivudine and adefovir dipivoxil combination therapy and entecavir monotherapy are effective for patients with HBV-related decompensated liver cirrhosis.
By understanding that both de novo lamivudine and adefovir dipivoxil combination therapy and entecavir monotherapy are effective for patients with HBV-related decompensated liver cirrhosis, this study may represent a future strategy for therapeutic intervention in patients with HBV-related decompensated liver cirrhosis.
De novo combination therapy means combination with two or more drugs from the beginning of the treatment. Monotherapy means use one drug form the beginning of the treatment. The diagnosis of decompensated liver cirrhosis was based on clinical, laboratory, previous histological, ultrasonographic and radiological signs of cirrhosis with Child-Turcotte-Pugh (CTP) score. The CTP score is a system to assess the disease stage for decompensated cirrhotic patients.
This is a good clinical study in which the authors compared the effects of de novo lamivdine and adefovir dipivoxil combination therapy with entecavir monotherapy for HBV-related decompensated liver cirrhosis patients. The authors concluded that both de novo lamivudine and adefovir dipivoxil combination therapy and entecavir monotherapy are effective for patients with HBV-related decompensated liver cirrhosis.
P- Reviewers Bock CT, Said ZNA S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Myers RP, Shaheen AA, Hubbard JN, Kaplan GG. Characteristics of patients with cirrhosis who are discharged from the hospital against medical advice. Clin Gastroenterol Hepatol. 2009;7:786-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787-799, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1764] [Cited by in F6Publishing: 1760] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 4. | Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630-1635. [PubMed] [Cited in This Article: ] |
| 6. | Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 8. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2323] [Cited by in F6Publishing: 2339] [Article Influence: 194.9] [Reference Citation Analysis (0)] |
| 9. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 724] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 10. | Guan R, Lui HF. Treatment of hepatitis B in decompensated liver cirrhosis. Int J Hepatol. 2011;2011:918017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Fontana RJ. Management of patients with decompensated HBV cirrhosis. Semin Liver Dis. 2003;23:89-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, Anschuetz G, Davis R, Gardner SD, Brown NA. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002;123:719-727. [PubMed] [Cited in This Article: ] |
| 13. | Liaw YF, Raptopoulou-Gigi M, Cheinquer H, Sarin SK, Tanwandee T, Leung N, Peng CY, Myers RP, Brown RS, Jeffers L. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology. 2011;54:91-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann H, Samuel D, Zeuzem S, Villeneuve JP. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Carey I, Harrison PM. Monotherapy versus combination therapy for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2009;18:1655-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1107] [Cited by in F6Publishing: 1032] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 19. | Viganò M, Lampertico P, Colombo M. Drug safety evaluation of adefovir in HBV infection. Expert Opin Drug Saf. 2011;10:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, Sarrazin C. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001-2006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |