Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6020
Revised: June 26, 2013
Accepted: July 30, 2013
Published online: September 28, 2013
AIM: To investigate the metabolic enzymatic capacity of the colon mucosa to detoxify noxious carcinogenic compounds.
METHODS: We investigated the activity of 2 conjugating enzymes-the microsomal uridine glucuronosyltransferase (UGT) and the cytosomal glutathione S-transferase (GST) in the uninvolved mucosa of the colon transversum and sigmoideum in patients with adenomatous polyps and colorectal cancer. Biopsies were taken from the mucosa during colonoscopies which were done for clinical (diagnostic) reasons. After storage, the biopsy material was homogenized and after differential centrifugation the enzyme assays were performed with 4-nitrophenol (UGT) and 1-chloro 2,4-dinitrobenzene (GST) as substrates.
RESULTS: About 48 patients were included of which 28 had adenomas and 20 had colorectal carcinomas confirmed by histopathology. Enzyme activities were expressed as nmol/mg per minute protein for the GST and as pmol/mg per minute protein for the UGT. Analysis of variance (F-test) indicated that both enzymes were more widely distributed in adenoma than in cancer patients. The means ± SD were smaller for cancer patients: GST for adenomas 268 ± 152 vs 241 ± 69 for carcinomas and UGT for adenomas 197 ± 200 vs 150 ± 86 for carcinomas.
CONCLUSION: Compared to patients with adenomatous colon polyps those with colorectal carcinoma exhibited a lower capacity of detoxifying enzyme metabolism and their activities clustered over a smaller range.
Core tip: Protective enzymes can conjugate carcinogenic chemicals. The functional capacity of these enzymes is diminished in patients with colorectal cancer and in some patients with colon adenomas.
- Citation: Hoensch HP, Roelofs HM, Edler L, Kirch W, Peters WH. Disparities of conjugating protective enzyme activities in the colon of patients with adenomas and carcinomas. World J Gastroenterol 2013; 19(36): 6020-6025
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6020
Colorectal cancer is an important cause of cancer death in Western countries. In Europe, colorectal cancer (CRC) is the second cause of death from malignant disease after lung cancer[1]. It is estimated that up to 10% of CRC cases can be attributed to hereditary factors leaving approximately 90% of so called sporadic colorectal cancer cases, which may be attributed to diet and lifestyle factors[2]. Epidemiological studies have shown the importance of dietary habits in the risk for CRC. Diets low in fruit and vegetables, and high in red meat and fat are associated with an increased risk of CRC[3,4]. Humans may be daily exposed to a large variety of toxic or even carcinogenic compounds, present in food[5] or as a result of lifestyle habits such as smoking or use of alcohol[6,7]. However, humans possess a highly efficient system of defense against such harmful compounds. Detoxification enzymes such as UDP-glucuronosyltransferases (UGTs)[8] and glutathione S-transferases (GSTs)[9] are responsible for the efficient modification and detoxification of harmful molecules. These enzymes are present in many tissues and also in the gastrointestinal tract in esophagus, stomach, small intestine, large intestine and in the liver[8-11]. UDP-glucuronosyltransferases catalyze the conjugation with glucuronic acid of a wide variety of exogenous compounds (e.g., drugs, pesticides, tobacco smoke components such as benzo(a)pyrene) as well as endogenous compounds (e.g., bilirubin, bile acids, steroid hormones)[8]. Glutathione S-transferases catalyze the reaction of glutathione with mainly exogenous electrophiles (e.g., polycyclic aromatic hydrocarbons, heterocyclic amines) and endogenous products of oxidative stress[9]. The conjugates formed by these enzyme reactions are generally less toxic than their precursors and are more water-soluble, which facilitates their biliary and renal excretion. The gastrointestinal tract is in direct contact with potentially toxic or (pre) carcinogenic agents, ingested by food, medication, drugs, etc. and the intestinal mucosa acts as a first-line barrier[12]. Tissue-specific expression of the different isoforms of GSTs and UGTs in colon and liver was demonstrated to result in the differences in enzyme activities as measured in these tissues[10,11,13,14].
Earlier, we demonstrated an inverse relationship between GST enzyme activity and cancer risk in several organs of the gastrointestinal tract[15], suggesting that the levels of phase II detoxification enzymes could be pivotal in cancer prevention. After comparison of detoxification levels in small intestine (a site of low cancer risk) and large intestine (high cancer risk), we even postulated that the levels of detoxification enzymes in the colon could be critically low[16].
We now investigated mucosal GST and UGT detoxification activities in normal mucosa of patients with colorectal adenomas, which are at risk to develop colorectal cancer, and in patients who already did develop colorectal cancer. Individual susceptibility to CRC could be partly due to low levels of detoxification enzymes.
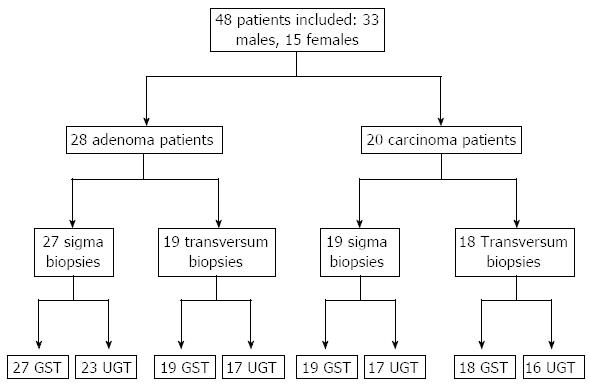
Fifty-one patients gave their written informed consent to use additional biopsy material for this study, the protocol of which was approved by the Ethics Committee of the university of Dresden/Germany. Colonoscopies were performed exclusively for clinical (diagnostic) reasons in all patients and colorectal cancer or adenomas were confirmed in n = 48 patients. Figure 1 shows the characteristics of the 48 patients included in the statistical analyses of the study. Endoscopic findings were confirmed by histopathology using a standard protocol. Information on the clinical variables was taken from the patients’ clinical files. Consecutive patients with adenomatous polyps or colorectal cancers were included regardless of localization, size, stage and histological grading. All patients with neoplasia of the colon were included if there was pathologically proven neoplasia. We excluded patients with insufficient clinical data and those without biopsies of the uninvolved mucosa.
Forceps biopsies of the colon mucosa were taken from the uninvolved mucosa of the transversum and the sigmoid colon. Biopsy material was shock-frozen and stored in liquid nitrogen until analysis. Biopsies were weighed and homogenized by 15 strokes in a plastic/plastic potter in 5 volumes of a buffer solution (pH = 7.4) containing 0.25 mol/L saccharose, 20 mmol/L Tris/HCl and 1 mmol/L dithiothreitol (all chemicals were from Sigma, Zwijndrecht, The Netherlands). Half of the total homogenate was frozen at -80 °C in small aliquots and used for the UGT assay. The other half of the homogenate was used for preparation of cytosol by centrifugation for 60 min at 150000 g and at 4 °C in a 42.2Ti rotor (Beckman Optima L-70K). The supernatant (cytosol) was frozen at -80 °C in small aliquots and used for the GST assay. Protein content was determined in cytosol (2 × 5 μL) and homogenate (2 × 5 μL) and GST enzyme activity in the cytosolic fractions (2 × 10 μL) was determined with 1-chloro-2,4-dinitrobenzene as substrate as described before[17]. UGT enzyme activity in the homogenates (1× 20 μL) was measured with 4-nitrophenol as substrate according to Strassburg et al[18] Usually, 2-3 biopsy particles per patient were taken from each site (uninvolved colon transversum and colon sigmoideum). For some patients not enough biopsy material was obtained to perform the complete set of enzyme assays (Figure 1). Combined activity was calculated as the mean of the activity of sigmoid and transversum when both locations were biopsied per patient, otherwise as the activity of one of the two locations only.
Patient characteristics were analyzed using descriptive statistical methods. Enzyme activities in the different groups analyzed were described using means and standard deviations and compared using the t-test. An F-test was used to compare the variances in the two groups. The correlation between GST and UGT was described numerically using the Spearman correlation coefficient and graphically using linear regression exhibiting both confidence intervals (for the means) and prediction intervals for the actual values. SAS/STAT software, Version 9.3 was used for all statistical analyses. P-values ≥ 0.05 were identified as not significant (NS) throughout this paper.
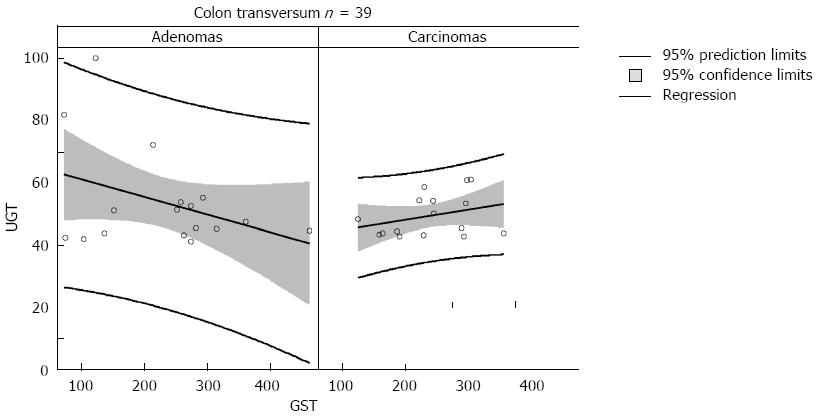
We investigated whether patients with adenomas or CRC differed in their colon capacity to metabolize noxious chemical compounds using the activities of detoxifying conjugating phase II enzymes as biomarkers. Table 1 shows the patients’ demographic characteristics: 20 patients had colorectal carcinomas with stage 2 and 3 and 28 patients had adenomatous polyps of various location, size and histological types. In 3 patients the diagnosis remained unclear as to the type of neoplasia. The means for GST enzyme activity were lower in the cancer patients than in adenoma patients (except for the transversum), but this difference did not reach statistical significance (Table 2). The UGT means were also lower in cancer patients even when distinguishing between the transversal and sigmoidal location. While the means of enzyme activities were not significantly different when using a t-test it was obvious that the ranges and the standard deviations were much wider for the adenoma patients. Adenoma patients had a wider distribution and cancer patients aggregated at a lower level and over a smaller range. This difference, indicated by the size of the standard deviations could be manifested by using the F-test which showed a statistically significant difference for UGT both in the transversum and sigmoid and for GST in the sigmoid. Figure 2 shows the individual distribution of the UGT and GST enzyme activity in the colon transversum of adenoma patients (left panel) and CRC patients (right panel) in the form of a regression analysis with the 95%CIs (for the means) and prediction intervals (for the actual values). The range was narrower for the cancer patients. No statistically significant differences of enzyme levels were found between the transversum and sigma, both, for UGT and GST. For this reason the means from the transversum and the sigma were also pooled for the analysis of the combined enzyme levels.
| Characteristics | Colorectal cancer (n = 20) | Adenomas (n = 28) | P-value |
| Sex | 0.0011 | ||
| Male | 9 (45) | 24 (86) | |
| Female | 11 (55) | 4 (14) | |
| Age, yr, mean (min-max) | 68.4 | 68.4 | NS2 |
| (39-86) | (51-88) | ||
| Alcohol | 0.0211 | ||
| Yes | 3 (16) | 15 (54) | |
| No | 17 (84) | 13 (46) | |
| Smoking | NS1 | ||
| Yes | 3 (16) | 2 (16) | |
| No | 17 (84) | 26 (84) | |
| Aspirin | NS1 | ||
| Yes | 2 (10) | 5 (18) | |
| No | 18 (90) | 23 (82) | |
| NSAIDs | 0 (0) | 1 (3) | NS1 |
| Yes | 20 (100) | 27 (97) | |
| No | |||
| BMI (kg/m2) | 25.1 | 25.6 | NS2 |
| Enzyme activities | Adenomas (n = 28) | Carcinomas (n = 20) | P-value (F-test) |
| GST (nmol/mg per minute) | |||
| Transversum | 225 ± 104 (19) | 237 ± 72 (18) | 0.1342 |
| Sigmoid | 265 ± 152 (27) | 232 ± 78 (19) | 0.0046 |
| Combined | 268 ± 152 (28) | 241 ± 69 (20) | 0.0007 |
| UGT (pmol mg per minute) | |||
| Transversum | 231 ± 269 (17) | 159 ± 122 (16) | 0.0015 |
| Sigmoid | 170 ± 158 (23) | 144 ± 96 (17) | 0.0456 |
| Combined | 197 ± 200 (27) | 150 ± 86 (19) | 0.0005 |
The enzyme activities could be influenced by a variety of clinical factors. Using the clinical charts we examined the major clinical variables to find out if these were affecting the enzyme levels. Both enzymes activities were not influenced by age, alcohol and nicotin consumption, use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) and body mass index (BMI). Female patients had lower enzyme levels and less variability than males (Table 3). There were more males than females among the polyp patients (24 vs 4) than among the cancer patients (9 vs 11). Also, alcohol consumption was more prevalent in the polyp patients (15 vs 13) than in the cancer patients (3 vs 17). No differences were detected between both groups for nicotin use, aspirin or other NSAID medications and body weight.
| Male | Female | t-test | F-test | |
| GST (combined) | 258 ± 139 (36) | 235 ± 68 ( 15) | 0.54 | 0.006 |
| Transversum | 218 ± 94 (26) | 251 ± 71 (13) | 0.28 | 0.310 |
| Sigmoid | 267 ± 137 (34) | 202 ± 75 (14) | 0.10 | 0.020 |
| UGT (combined) | 198 ± 180 (34) | 146 ± 99 (15) | 0.30 | 0.020 |
| Transversum | 231 ± 238 (23) | 153 ± 108 (12) | 0.29 | 0.009 |
| Sigmoid | 168 ± 142 (29) | 141 ± 110 (13) | 0.54 | 0.360 |
We measured the kinetic detoxifying enzyme activity in the uninvolved normal appearing mucosa of patients with benign and malign neoplasia of the colon. Usually enzyme values can be expressed using mRNA and protein levels, but these parameters do not reflect the functional activity within the epithelial cells of the mucosa. Using kinetic data we could obtain direct information on the metabolic capacity of the tissue. Both UGT and GST had a significantly different distribution between patients with benign neoplasia (adenomas) and those with cancerous neoplasia (CRC). The type of neoplasia was associated with the enzyme levels and an increased variance was found in the polyp patients. On the other hand the enzyme levels could inform on the degree of neoplasia and its evolution. Cancer patients had a smaller range at a lower level. This could mean that cancer patients had lost some of the mucosal detoxifying potential, predisposing them to develop cancer. The wide range of enzyme values could indicate that some patients with adenomas who were in the lower range might be at risk to develop cancer. It is possible that adenoma patients who are in the higher range are protected from cancer development since they possess a higher protective enzyme capacity. This hypothesis needs to be validated by longitudinal follow-up studies of enzyme values in polyp patients over long periods of time. Previously, we found in the rectum of healthy controls GST-levels of 321 ± 29 nmol/mg per minute protein (n = 10)[19] which were higher than those reported in this paper.
The UGT enzyme system is located in the endoplasmic reticulum of the mucosal intestinal cells and these enzymes can detoxify hazardous chemical compounds - mostly lipophilic - which penetrate deep into the interior of the cells. The GST enzymes are located within the cytoplasm of the cells and use intracellular glutathione to protect the cells from electrophilic chemicals. Both enzymes perform conjugation reactions and thereby render xenobiotics water-soluble (phase II metabolism). The conjugated metabolites can be readily excreted by the liver or the kidney. Phase II enzymes can be induced by their substrates and are influenced by environmental chemicals and clinical factors in the human body[20]. In contrast to our previous publication[21] there were no significant differences of enzyme levels relating to the location between the upper and the lower segments. However, this might be due to the different patient population (mainly normal and inflammatory findings) investigated previously. Female gender was associated with a higher prevalence of carcinomas and lower enzyme activities. This might have contributed to the observed enzyme disparities. One limitation of this study was the small number of patients in the 2 groups. Furthermore due to the small sample size enzyme values at the m-RNA level could not be studied. Further studies should include enzyme measurements at the protein and mRNA level. More patients and addition of a control group with nonneoplastic diseases of the colon should be included.
Our results suggest that protective enzymes could be diminished in the colon mucosa of cancer patients. Reduced activities of protective enzymes could lead to increased susceptibility to develop colorectal cancer.
We want to thank Sascha Mack for helping us to collect the patient data.
It is still controversial which factors determine the development of colorectal neoplasia. Exogenous dietary agents can be broken down by protective enzymes in the colon mucosa.
The cellular mutations of the tumor tissue in colorectal cancer (CRC) are thoroughly investigated but preneoplastic aberrations in the mucosa of the colon that lead to development of neoplasia are rarely investigated. The authors studied the properties of the uninvolved mucosa in patients with colon neoplasia by investigating the activities of protective enzymes.
The direct comparison of activities of conjugating protective enzymes in carcinoma and adenoma patients has not been reported so far. It is highly informative to find out whether 2 types of neoplasia with different degree of malignant potential exhibit variances in the distribution of enzyme activities. We found that lower enzyme activities were associated with CRC but adenoma patients still retained sufficient activities. Susceptibility to develop CRC could depend on the enzyme levels expressed by the preneoplastic mucosa. Low enzyme levels could be a risk factor for adenoma patients to develop CRC.
By inducing protective enzymes with dietary agents it might be feasible to prevent neoplasia in the colon.
Conjugating enzymes represent Phase 2 of drug metabolism. They mainly break down lipophilic carcinogenic compounds.
This paper makes good efforts in trying to understand that, protective enzymes could be diminished in the colon mucosa of cancer patient and reduced activities of these enzymes could lead to increased susceptibility to develop colon cancer.
P- Reviewer Alshehabi Z S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 507] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Houlston RS, Peto J. Genetics of common cancers. Inherited predisposition to cancer. London: Chapman Hall Medical 1996; 208-226. [Cited in This Article: ] |
| 3. | Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | World Cancer Research Fund. Food, nutrition and the prevention of cancer: a global perspective. Washington: American Institute for Cancer Research 1997; . [Cited in This Article: ] |
| 5. | Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2192] [Cited by in F6Publishing: 1885] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 6. | Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435-7451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 802] [Cited by in F6Publishing: 729] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 7. | Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1179] [Cited by in F6Publishing: 1085] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 9. | Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2409] [Cited by in F6Publishing: 2364] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 10. | Coles BF, Chen G, Kadlubar FF, Radominska-Pandya A. Interindividual variation and organ-specific patterns of glutathione S-transferase alpha, mu, and pi expression in gastrointestinal tract mucosa of normal individuals. Arch Biochem Biophys. 2002;403:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Basu NK, Ciotti M, Hwang MS, Kole L, Mitra PS, Cho JW, Owens IS. Differential and special properties of the major human UGT1-encoded gastrointestinal UDP-glucuronosyltransferases enhance potential to control chemical uptake. J Biol Chem. 2004;279:1429-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Hoensch HP, Hutt R, Hartmann F. Biotransformation of xenobiotics in human intestinal mucosa. Environ Health Perspect. 1979;33:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Tukey RH, Strassburg CP. Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol Pharmacol. 2001;59:405-414. [PubMed] [Cited in This Article: ] |
| 14. | Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181-182:453-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Peters WH, Roelofs HM, Hectors MP, Nagengast FM, Jansen JB. Glutathione and glutathione S-transferases in Barrett’s epithelium. Br J Cancer. 1993;67:1413-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Peters WH, Kock L, Nagengast FM, Kremers PG. Biotransformation enzymes in human intestine: critical low levels in the colon? Gut. 1991;32:408-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Hoensch H, Morgenstern I, Petereit G, Siepmann M, Peters WH, Roelofs HM, Kirch W. Influence of clinical factors, diet, and drugs on the human upper gastrointestinal glutathione system. Gut. 2002;50:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Strassburg CP, Nguyen N, Manns MP, Tukey RH. UDP-glucuronosyltransferase activity in human liver and colon. Gastroenterology. 1999;116:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Nijhoff WA, Grubben MJ, Nagengast FM, Jansen JB, Verhagen H, van Poppel G, Peters WH. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis. 1995;16:2125-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology. 2010;139:1699-1710, 1710.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Hoensch H, Peters WH, Roelofs HM, Kirch W. Expression of the glutathione enzyme system of human colon mucosa by localisation, gender and age. Curr Med Res Opin. 2006;22:1075-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |