Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5607
Revised: July 13, 2013
Accepted: August 5, 2013
Published online: September 14, 2013
Processing time: 94 Days and 9.8 Hours
Advances in understanding the interaction between the human immune system and the microbiome have led to an improved understanding of the function of the vermiform appendix as a safe-house for beneficial bacteria in the colon. These advances have been made despite long standing clinical observations that the appendectomy is a safe and effective procedure. However, more recent clinical data show that an appendectomy puts patients at increased risk for recurrent Clostridium difficile (C. difficile)-associated colitis, and probably other diseases associated with an altered microbiome. At the same time, appendectomy does not apparently put patients at risk for an initial onset of C. difficile-associated colitis. These clinical observations point toward the idea that the vermiform appendix might not effectively protect the microbiome in the face of broad spectrum antibiotics, the use of which precedes the initial onset of C. difficile-associated colitis. Further, these observations point to the idea that historically important threats to the microbiome such as infectious gastrointestinal pathogens have been supplanted by other threats, particularly the use of broad spectrum antibiotics.
Core tip: Although the function of the appendix has remained an enigma for centuries, recently emerging advances in the fields of immunology and gut microbiology have merged with observations made in the clinic to form a coherent picture. Although the appendix is apparently a safe-house for beneficial bacteria, it seems likely that this safe-house does not satisfactorily protect the microbiome from broad spectrum antibiotics. In this view, selection pressures which threatened the microbiome and likely drove the evolution of the appendix have been supplanted in post-industrial society by new threats to the microbiome that the human body is not adapted for.
-
Citation: Sanders NL, Bollinger RR, Lee R, Thomas S, Parker W. Appendectomy and
Clostridium difficile colitis: Relationships revealed by clinical observations and immunology. World J Gastroenterol 2013; 19(34): 5607-5614 - URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5607
Appendectomy, like a wide variety of other surgical procedures, is extremely common in industrialized society. However, unlike common surgical procedures that include sterilizations for contraception, Cesarean sections, and inguinal hernia repairs, appendectomies are frequently performed as a prophylaxis for disease. The lifetime risk for appendicitis is only 8.6% for males and 6.7% for females, contrasting to the 12% and 23% lifetime rate of appendectomies performed, respectively[1]. These numbers indicate that approximately half of all appendectomies, including more than 60% in females, are incidental procedures, aimed at averting future episodes of appendicitis. This approach is generally successful, but 36 incidental appendectomies are required to prevent one case of appendicitis[1]. Given the large number of appendectomies currently performed, many of them elective, recently emerging evidence regarding the apparent function of the vermiform appendix has justifiably garnered much interest.
The idea that the vermiform appendix is a vestige of evolution was developed more than 150 years ago by Darwin[2]. The proposal was simple and made sense in the light of available data: the appendix and small cecum present in humans and some primates is the remainder of a larger cecum used for fermentation in a human ancestor with a diet much higher in fiber[2]. However, recent studies using current methods employed in the field that Darwin[2] founded have disproved that idea. In summary, a modern cladistics-based approach demonstrates that the appendix has evolved repeatedly in a wide range of animals, that some clades have a propensity to evolve an appendix, and that the evolution of the appendix is usually not associated with a decrease in the size of the cecum. In fact, a recent analysis of 361 mammalian species found a significant direct correlation between appendix and cecum size[3]. In other words, the appendix tends to be associated with a large cecum, not a smaller one. At present, many questions regarding the evolution of the appendix remain unanswered: it is not even known whether the first appendix evolved before or after the first cecum[4], or how often which precedes the other in evolution (given the rise of the appendix more than once during evolution). Although the absolute disproof of Darwin’s views of the appendix is recent, the idea that the appendix is a vestige of evolution has been disputed effectively for more than a century. For example, Berry[5] concluded in 1900 that, based on anatomical and phylogenetic data, “The vermiform appendix of man is not, therefore, a vestigial structure. On the contrary, it is a specialized part of the alimentary canal”. Keith[6] supported Berry’s views and argued further that that the appendix, rather than being a flawed structure which gives rise to appendicitis, is a victim of changes in the environment due to industrialization: “When we come to realize how slowly evolutionary processes have affected man’s body in past times, we can hardly expect our internal digestive system to adapt itself to the rapid pace demanded by the ever-accumulating resources of civilization”.
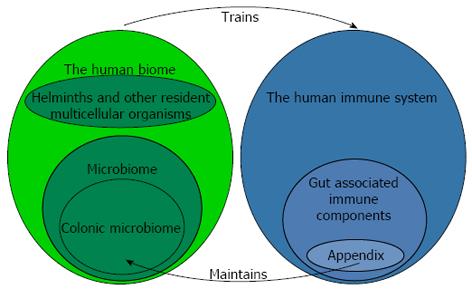
When Keith[6] recorded his views in 1912, the incidence of appendicitis had profoundly increased in the lifetime of many practicing physicians, and it was therefore correctly surmised that something environmental was causing the disease. The opinion of the day was that changing diet following industrialization was in some way responsible for appendicitis. Although the view that appendicitis was due to an environmental factor or factors in industrial and post-industrial environments was solidified by numerous epidemiologic studies[7-11], it was not until the 1980s that Barker et al[12-14] determined that factors associated with indoor plumbing were somehow responsible for appendicitis[14]. These intriguing findings by Barker as well as additional work by Strachan on allergic disease[15] eventually gave rise to the currently held view that factors within post-industrial culture, including sanitation practices (e.g., toilets and water treatment facilities) and modern medicine, lead to depletion of species normally associated with the ecosystem of the human body, or the “human biome” (not to be confused with the “microbiome”, Figure 1). The resulting state, termed “biome depletion” is associated with a profoundly over-reactive immune system that is prone to a variety of immune related diseases, including appendicitis. Barker’s personal view is that the introduction of running hot water into a home might be the single most telling factor associated with an increased incidence of appendicitis (personal communication to Parker W). Since hot water is necessary for the effective use of soap, and given the effectiveness of soap in biological decontamination, this view makes sense. Here it should be noted that approaches which deal with the consequences of biome depletion are expected to one-day make appendicitis a rare disease. These approaches involve reconstitution of the human biome without abandoning the modern technology, including soap, water treatment facilities and medicine, which so effectively prevents the spread of water-borne disease[16-18].
Despite proof that the appendix is not a vestige of evolution and that appendicitis is not the result of a faulty structure, the idea that the appendix is a vestige seems attractive simply because removal of the appendix does not, to the practicing physician or to the patients concerned, seem to have deleterious effects. This observation, apparent to everyone, presents a quandary: how can the appendix have some function, but yet appendectomy has no negative side effects? The answer to this quandary is readily apparent if one considers that actual function of the appendix.
In 2003 it was observed that the immune system apparently supports growth of mutualistic biofilms in the mammalian gut[19]. This view, although surprising at the time due to prevailing views in the field of immunology, now seems rather obvious in hindsight based on current knowledge regarding microbial ecology and host-microbe relationships[20-22]. This new view led to the evaluation of biofilm distribution in the human gut, and biofilms were indeed found to be most abundant in the appendix, where immune tissue had long been known to be the most abundant within the gut. This biofilm distribution in the gut set the stage for a deductive proof regarding the function of the appendix: Since the appendix is a structure harboring microbial biofilms, and since biofilms are protective of bacteria (a long standing observation in the field of microbiology), the appendix is, in essence, a safe house for bacteria (Figure 1). Given the shape and location of the appendix, it would indeed be difficult to imagine how the appendix might not be protective of bacteria.
Given the apparent function of the appendix, it has been proposed that an evolutionary driving force for the emergence of the appendix may be as an aid in the recovery from diarrheal illness associated with gastrointestinal (GI) infection. In this view, fragments of biofilms routinely shed from the appendix would serve as “seeds” for inoculation of the colon with a normal microbial flora following a diarrheal purge[23]. This explanation makes sense in light of (1) the relative seclusion of the apex of the appendix from the fecal stream, which presumably affords some protection from pathogenic organisms that might temporarily infect the GI tract; and (2) the pronounced role of diarrheal illness in human survival. Indeed, water-borne diseases followed by dysentery are frequently the leading cause of death during war and natural disasters[24-27], have affected both the rich and the poor[28] and are still one of the leading causes of death in developing cultures[29,30]. These observations are consistent with the view that that rapid reconstitution of the microbiome and restoration of a normal bowel following diarrheal illness might be adaptive in many circumstances. In fact, the relatively low mortality rate associated with diarrheal illness, less than one percent[31], is possibly a testament to the effectiveness of natural recovery mechanisms such as those that might involve the appendix. Adding further weight to this view, a very recent study by Guanine et al[32] found that “the human appendix contains a wealth of microbes, including members of 15 phyla”. Species identified included members of phyla which constitute more than 98% of the normal colonic microbiome (Firmicutes, Proteobacteria, Bacteroidetes Actinobacteria, and Fusobacteria), indicating that the appendix possesses a microbial diversity sufficient to reconstitute the microbiome of the colon.
If this inductive rationale is correct, the paradoxical removal of the functional appendix without immediate and substantial harm is readily explained: Although water-borne disease is one of the leading causes of death in developing countries, the use of modern water treatment facilities and sanitation prevents widespread outbreaks of pathogens which might deplete the normal flora from a substantial portion of the population. Further, the absence of starvation and the presence of modern medicine in developed countries minimize the effects of diarrheal illness on the population.
Approximately 50% of cases of appendicitis are generally considered to be enigmatic in origin, with the remainder being attributed to a blockage of the appendix. However, work from David Barker during the 1980’s first identified clues which eventually pointed toward the underlying cause of appendicitis. Barker noticed that epidemics of appendicitis followed the introduction of indoor plumbing into various communities. This observation was followed by epidemiologic studies showing that appendicitis is associated with developed but not with developing countries. Almost at the same time, another epidemiologist, Strachan[15], found that a hyper-active immune system is a consequence of the hygienic environment following the industrial revolution[15]. Strachan’s observations point toward the idea that appendicitis, like many other allergic, autoimmune, and inflammatory diseases, is a result of biome depletion, a consequence of industrialization[16-18]. This culture-related basis for appendicitis explains why the appendix was not selected against during the course of evolution. Many components of the immune system, such as the appendix, are made obsolete by post-industrialized society, and these have also not been selected against during evolutionary history. Not only are these components now obsolete, but these components often become overly sensitive due to an absence of stimulation and cause detrimental health effects, such as ulcerative colitis that is exacerbated by the appendix[33]. Another example of a maladapted immune component is the immune compartment that produces immunoglobulin E (IgE). High levels of IgE lead to allergies and other destructive side effects in industrialized societies, but levels significantly higher than those found in industrialized countries are present in developing countries as a result of productive (beneficial) responses to parasitic infections[34-36].
Although an appendectomy is a relatively simple surgical procedure, the effects of removing the appendix are not necessarily straightforward. The appendix is associated with the highest concentration of gut associated lymphoid tissue (GALT) in the gut, and the function of the GALT is vastly complex and incompletely understood. Thus, an appendectomy is expected to profoundly alter the immune system with its hundreds or possibly thousands of interconnected components. Numerous functions have been attributed to the GALT, and it remains unknown how appendectomy alters many of those functions. However, some effects are established. First, appendectomy does have a moderating effect on pathogenic inflammatory immune responses of the gut. The observation that patients without an appendix tend to be at less risk for ulcerative colitis is more than 10 years old[33]. More recently, Bolin et al[37] used appendectomy as a treatment for ulcerative proctitis, a form of colitis, and showed an improvement of symptoms in 90% of patients, with complete remission in 40% of patients[37]. Possibly the most straight-forward explanation for this result is that removal of a substantial amount of GALT from the intestinal tract led to decreased immune reactivity in the gut. Whether the “safe-house” function of the appendix had anything to do with the result seems more speculative.
Perhaps the most intriguing effects of appendectomy involve its effects on the incidence of Clostridium difficile (C. difficile) colitis. C. difficile colitis is a pathogenic state associated with overgrowth of the bacterium C. difficile, a gram-positive, spore-forming, anaerobic bacillus[38,39], and is generally not seen in individuals with a normal microbiome. However, alteration of the normal flora (generally by antibiotic use) can lead to overgrowth of C. difficile and subsequent disease. Recurrent C. difficile colitis is not a minor problem in modern medical practice, with one study showing nosocomial C. difficile diarrhea present in 3.4 to 8.4 cases per 1000 hospital admissions[40], and an increase in in-hospital mortality from 2.4% to 13.5%[41].
It might at first glance be expected that the appendix, if present, would be protective against C. difficile overgrowth. There is, however, at least one central problem with this supposition: it remains unknown if the appendix can effectively protect mutualistic bacteria against the modern antibiotics which generally precede C. difficile colitis. It seems reasonable that the appendix has evolved in the presence of enteric pathogens and thus that it may be effective in helping the body to recover from infectious disease. However, the use of high dose antibiotics is a very recent development in human history, and thus it is not reasonable to assume that the appendix may be protective under these conditions. To our knowledge, no studies have addressed this issue. Our laboratory has assessed the protection from antibiotics afforded by immune-mediated biofilms in vitro, and found that immune mediated biofilms formed by one species (Escherichia coli) are poorly protected from antibiotics. However, much additional work needs to be done in this field using a wide range of microbial species as well as whole animal models before any sort of answer which might have clinical implications can be obtained.
The supposition that the appendix, if indeed it is a safe-house for bacteria, should be protective against C. difficile colitis has a second potential flaw: If indeed the appendix does not protect mutualistic bacteria from antibiotic use, the appendix could hypothetically protect those organisms which are resistant to antibiotics, such as C. difficile, from a diarrheal purge. Thus, if the appendix performs its function perfectly, it could hypothetically increase the incidence of C. difficile colitis in the face of antibiotic use. Fortunately, this does not appear to be the case. At present, clinical data point toward the idea that the presence or absence of an appendix does not strongly affect the propensity for the initial onset of C. difficile colitis. In a study by Im et al[42], 80% of their patients with C. difficile colitis (203 out of 253) had an appendix, which is only slightly lower than the percentage found in the total population[1]. Another study, by Merchant et al[41], obtained essentially identical results, with 80% of their patients with C. difficile colitis (109 out of 136) having an appendix. Merchant et al[41] found that 82% of “normal” individuals (in their study, patients without GI complaints) had an appendix, as would be expected based on larger studies[1]. However, these observations do not directly address the actual effect of the appendix on the propensity for C. difficile colitis following antibiotic use, since they do not address the effect of appendectomy on the use of antibiotics. In other words, the data indicate that appendectomy does not affect the risk for C. difficile colitis, but it does not indicate whether an appendectomy might affect the risk for C. difficile colitis following antibiotic treatment. Since Merchant et al[41] did not control for antibiotic treatment, increased antibiotic use in those with an appendix, if it exists, would have confounded the study. Nevertheless, the observations do clearly indicate that the loss of an appendix is not associated with a dramatically increased risk for an initial onset of C. difficile colitis.
As stated above, it is possible that a perfectly functional appendix, if indeed it did not protect the normal flora from antibiotics, might selectively protect antibiotic resistant organisms such as C. difficile from a diarrheal purge. This possibility has been previously proposed by Merchant et al[41]. However, since the relative number of patients with and without an appendix in patient groups with C. difficile colitis is essentially the same as that in the normal population, the possibility that the appendix preferentially protects C. difficile seems extremely unlikely. Further, appendectomy itself affords a much lower risk of C. difficile colitis (0.2%) compared to colectomy (1.11%) small-bowel resection (1.17%) and gastric resection (1.02%)[41], further suggesting that the appendix may be relatively uninvolved in the initial onset of C. difficile colitis. In addition, the fact that an intact appendix protects against recurrent (as opposed to the initial onset of) C. difficile colitis (see below) argues strongly against this view. However, again, it is not known to what extent the presence or absence of an appendix might affect antibiotic use, the major trigger for C. difficile colitis. This factor probably needs to be examined before any firm conclusions can be drawn.
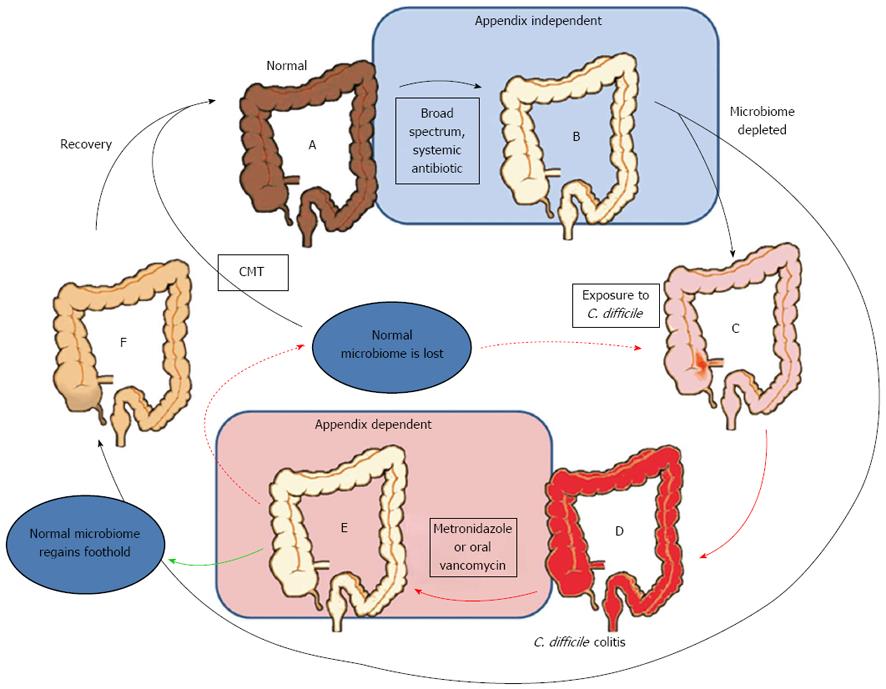
Strong evidence from Im et al[42] study indicates that the appendix may play a protective role in recurrent C. difficile colitis. Im et al found a 2.5-fold increased risk of recurrent C. difficile colitis in patients without an appendix compared to those with an appendix. Figure 2 illustrates a possible scenario that potentially explains the connection between the appendix, the initial onset of C. difficile colitis, and recurrent C. difficile colitis. The central issue revolves around the use of broad spectrum antibiotics which initiate the initial C. difficile colitis, and the more limited antibiotic treatments used after the first C. difficile infection. The standard of care for recurrent and severe C. difficile colitis is oral vancomycin, a treatment that is limited to the lumen of the bowel. Given the position of the appendix out of the main flow of the bowel, it seems likely that it may indeed be effective at protecting the normal flora from oral vancomycin, just as it putatively protects the normal flora from contamination by pathogens in the main fecal stream.
Consistent with the idea that the connection between recurrent C. difficile colitis and the appendix involves the bacterial safe-house function of the appendix, recurrent C. difficile colitis can be rapidly resolved using fecal microbiome transplants[43-45]. This observation indicates that C. difficile colitis is indeed an issue involving a depleted gut microbiome, thus adding support to the idea that an appendix might help restore the gut microbiome in times of stress. Indeed, proof of a depleted biome in recurrent C. difficile colitis patients has been provided by phylogenetic analyses of stool samples in patients with recurrent C. difficile colitis: decreased bacterial diversity[46] as well as a deficiency of Firmicutes and Bacteriodetes[47] have been demonstrated in those patients.
Although the function of the appendix as a safe-house for the colonic microbiome explains the clinical observations illustrated in Figure 2, an alternative, although not mutually exclusive, explanation also exists: as noted above, appendectomy probably lowers the immunoreactivity of the gut, and thus may lower the ability of the gut to respond to C. difficile. Thus, the loss of the appendix may, hypothetically, reduce the ability of the immune system to mount an immune response to C. difficile, which is known to be important in the resolution of the colitis. Thus, a second explanation for the connection between appendectomy and recurrent C. difficile colitis shown in Figure 2 may be that the immunosuppressive effect of appendectomy impedes the immune response to C. difficile, thus putting the patient at risk for recurrent C. difficile colitis. Consistent with this view, Im’s data also indicated that increasing age (> 60 years), which is associated with reduced immune function, was also a risk factor for recurrent C. difficile colitis[42]. In this view, the lack of a connection between the initial onset of C. difficile colitis and appendectomy may be due to the lack of time necessary to mount an immune response that would be dependent on the immune tissue of the appendix.
A potentially alarming observation was made in the study by Merchant et al[41]: 31 percent (39 out of 121) of their patients which were tested for C. difficile colitis but which were found negative for C. difficile colitis had a previous appendectomy. This number is very significantly greater than is expected if the presence or absence of an appendix was not related in some way: The probability (binomial test) of observing 38 out of 121 patients with an appendectomy is < 0.0001 given a null hypothesis of 0.18 (a population-wide rate of 18% appendectomy). If this observation is confirmed by additional studies, it would indicate an association between appendectomy and complications which resemble C. difficile colitis (and thus induce clinicians to order a test for C. difficile), but which are in fact not associated with C. difficile. This idea deserves further attention before any firm conclusions can be drawn, but the observations made by Merchant et al[41] nevertheless have great potential importance, and certainly raise a sense of urgency for further study of this topic.
The strongest connection between appendectomy and inflammatory diseases unrelated to C. difficile colitis of the bowel is provided by the Merchant et al study[41]. However, additional indirect evidence for this connection is provided by the effectiveness of colonic microbiota transplants in treating some patients whose disease has resisted other therapeutic options[43,44]. The effectiveness of microbiota transplants in some patients strongly indicates that a loss of the normal microbiome is at the root of the symptoms experienced by these patients. Thus, to the extent that the appendix assists in maintenance of the microbiome, the lack of an appendix may influence the incidence of these idiopathic cases. At the same time, it is recognized that loss of the microbiome by a wide range of modern medical interventions (e.g., sterile birth practices, broad spectrum antibiotics) may circumvent any protective role of the appendix, and direct assessment of the rate of appendectomy in patients with an altered microbiome should be undertaken.
Acute appendicitis is the widely recognized indication for appendectomy, although alternatives involving medical treatment are being considered. Medical treatment alone has the substantial disadvantages that (1) heavy use of antibiotics must be employed, which is not without its own side effects; and (2) recurrence of appendicitis following antibiotic use is possible. A controlled study by Eriksson et al[48] compared the outcomes of patients treated with a 10 d antibiotic regimen (cefotaxime and tinidazole in the hospital for two days followed by eight days of oral antibiotics) versus patients who underwent appendectomy. They found that patients on the antibiotic regimen used significantly less morphine, had lower white blood cell counts, and had less pain at follow up. Two surgical patients underwent post-operative antibiotic therapy for complications, and there was an appendicitis recurrence rate of 35% in the antibiotic group. Another study by Styrud et al[49] saw an 86% success rate with antibiotics with only a 14% recurrence rate within one year. The complication rate in the surgical group was 14%. These studies suggest that acute non-perforated appendicitis can be treated conservatively with an antibiotic regimen; however, the risk of recurrence should be compared to the risk of surgical complication in the patient.
Antibiotics have also proven effective at delaying appendectomy. Nine sailors who were diagnosed with appendicitis while serving at sea received various antibiotic regimens until the men could be taken to a hospital, and all achieved positive outcomes[50]. A study of 695 children showed that an antibiotic regimen in children allowed the appendectomy to be delayed up to 18 h after admission without an increase in complications[51].
It seems highly likely that the appendix, evolved in a time before sewer systems and water treatment facilities existed, is somewhat out of place in post-industrial society. Removal of the appendix and its associated GALT does afford some degree of immune suppression, which can be advantageous in a post-industrial environment rampant with inflammatory diseases of the bowel. However, removal of the appendix may also impede the ability of the body to replenish helpful bacteria, and/or appendectomy might hinder helpful immune responses, such as those directed at C. difficile. Whatever the cause, appendectomy appears to be associated with an increased risk for recurrent C. difficile colitis, which is not a minor problem in modern medical practice. Indeed, one study found nosocomial C. difficile diarrhea present in 3.4 to 8.4 cases per 1000 hospital admissions[40], and an increase in in-hospital mortality from 2.4% to 13.5%[41]. With this in mind, further studies aimed at biome reconstitution, which are predicted to eliminate the vast majority of appendicitis cases, and thus the need for most appendectomies, are warranted. Further, studies regarding the long term effects of incidental appendectomies should be carefully considered.
P- Reviewers Burdette SD, Hokama A S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-925. [PubMed] |
| 2. | Darwin C. The Descent of Man and Selection in Relation to Sex. London: John Murray 1871; . |
| 3. | Smith HF, Parker W, Kotze SH, Laurin M. Multiple independent appearances of the cecal appendix in mammalian evolution and an investigation of related ecological and anatomical factors. Comptes Rendus Palevol. 2013;In press. [DOI] [Full Text] |
| 4. | Smith HF, Fisher RE, Everett ML, Thomas AD, Bollinger RR, Parker W. Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J Evol Biol. 2009;22:1984-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Berry RJ. The True Caecal Apex, or the Vermiform Appendix: Its Minute and Comparative Anatomy. J Anat Physiol. 1900;35:83-100.9. [PubMed] |
| 6. | Keith A. THE FUNCTIONAL NATURE OF THE CAECUM AND APPENDIX. Br Med J. 1912;2:1599-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hutt MS. Epidemiology of chronic intestinal disease in middle Africa. Isr J Med Sci. 1979;15:314-317. [PubMed] |
| 8. | Ajao OG. Abdominal emergencies in a tropical African population. Br J Surg. 1981;68:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Walker AR, Richardson BD, Walker BF, Woolford A. Appendicitis, fibre intake and bowel behaviour in ethnic groups in South Africa. Postgrad Med J. 1973;49:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Janssens PG, De Muynck A. Appendicular pathology in the African Negro. Trop Geogr Med. 1966;18:81-96. [PubMed] |
| 11. | Burkitt DP. Related disease--related cause? Lancet. 1969;2:1229-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 179] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Barker DJ, Osmond C, Golding J, Wadsworth ME. Acute appendicitis and bathrooms in three samples of British children. Br Med J (. Clin Res Ed). 1988;296:956-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Barker DJ, Morris JA, Simmonds SJ, Oliver RH. Appendicitis epidemic following introduction of piped water to Anglesey. J Epidemiol Community Health. 1988;42:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Barker DJ, Morris J. Acute appendicitis, bathrooms, and diet in Britain and Ireland. Br Med J (. Clin Res Ed). 1988;296:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3392] [Cited by in RCA: 3273] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 16. | Parker W, Ollerton J. Evolutionary Biology and Anthropology Suggest Biome Reconstitution as a Necessary Approach toward Dealing with Immune Disorders. Evolution, Medicine, and Public Health. 2013;2013:89-103. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Parker W, Perkins SE, Harker M, Muehlenbein MP. A prescription for clinical immunology: the pills are available and ready for testing. A review. Curr Med Res Opin. 2012;28:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Bilbo SD, Wray GA, Perkins SE, Parker W. Reconstitution of the human biome as the most reasonable solution for epidemics of allergic and autoimmune diseases. Med Hypotheses. 2011;77:494-504. [PubMed] |
| 19. | Bollinger RR, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;109:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3533] [Article Influence: 176.7] [Reference Citation Analysis (5)] |
| 21. | Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R. Evolution of mammals and their gut microbes. Science. 2008;320:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2974] [Cited by in RCA: 2535] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 22. | Thomas AD, Parker W. Cultivation of epithelial-associated microbiota by the immune system. Future Microbiol. 2010;5:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. 2007;249:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Tucker SC. The encyclopedia of the Spanish-American and Philippine-American wars: a political, social, and military history. Santa Barbara: ABC-CLIO 2009; 189. |
| 25. | McCallum JE. Military medicine. Santa Barbara: ABC-CLIO 2008; . |
| 26. | Mandry OC. Bacillary dysentery in Puerto Rico. J Public Health Trop Med. 1935;10:308-341. |
| 27. | World Health Organization. Acute watery diarrhoeal syndrome in Ethiopia. Geneva: Global Alert and Response (GAR) 2006; . |
| 28. | The Health Of The Presidents: The 41 United States Presidents Through 1993 From A Physicianâs Point Of View: McFarland and Company Incorporated Pub, 2004. . |
| 29. | Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614-627. [PubMed] |
| 30. | World Health Organization. Acute watery diarrhoeal syndrome in Afghanistan. Geneva: Global Alert and Response (GAR) 2002; . |
| 31. | Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651-666. [PubMed] |
| 32. | Guinane CM, Tadrous A, Fouhy F, Ryan CA, Dempsey EM, Murphy B, Andrews E, Cotter PD, Stanton C, Ross RP. Microbial composition of human appendices from patients following appendectomy. MBio. 2013;4. [PubMed] |
| 33. | Askey D. Transient ischaemic attack. Nurs Stand. 2007;21:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Devalapalli AP, Lesher A, Shieh K, Solow JS, Everett ML, Edala AS, Whitt P, Long RR, Newton N, Parker W. Increased levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scand J Immunol. 2006;64:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Scaglia M, Tinelli M, Revoltella R, Peracino A, Falagiani P, Jayakar SD, Desmarais JC, Siccardi AG. Relationship between serum IgE levels and intestinal parasite load in African populations. Int Arch Allergy Appl Immunol. 1979;59:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Bolin TD, Wong S, Crouch R, Engelman JL, Riordan SM. Appendicectomy as a therapy for ulcerative proctitis. Am J Gastroenterol. 2009;104:2476-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 873] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 39. | Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am. 2001;30:753-777, ix-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 295] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 41. | Merchant R, Mower WR, Ourian A, Abrahamian FM, Moran GJ, Krishnadasan A, Talan DA. Association Between Appendectomy and Clostridium difficile Infection. J Clin Med Res. 2012;4:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Im GY, Modayil RJ, Lin CT, Geier SJ, Katz DS, Feuerman M, Grendell JH. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol. 2011;9:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Floch MH. Fecal bacteriotherapy, fecal transplant, and the microbiome. J Clin Gastroenterol. 2010;44:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 469] [Article Influence: 33.5] [Reference Citation Analysis (1)] |
| 45. | Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 679] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 46. | Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 793] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 47. | Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 48. | Eriksson S, Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, Rex L, Badume I, Granström L. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg. 2006;30:1033-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 50. | Adams ML. The medical management of acute appendicitis in a nonsurgical environment: a retrospective case review. Mil Med. 1990;155:345-347. [PubMed] |
| 51. | Surana R, Quinn F, Puri P. Is it necessary to perform appendicectomy in the middle of the night in children? BMJ. 1993;306:1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |