Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5500
Revised: July 7, 2013
Accepted: July 23, 2013
Published online: September 7, 2013
AIM: To evaluate the protective effects of fucoidan on oxidative stress-induced barrier disruption in human intestinal epithelial cells.
METHODS: In Caco-2 cell monolayer models, the disruption of barrier function by oxidative stress is mediated by H2O2. The integrity of polarized Caco-2 cell monolayers was determined by measuring the transepithelial resistance (TER) and permeability was estimated by measuring the paracellular transport of FITC-labeled 4-kDa dextran (FD4). The protective effects of fucoidan on epithelial barrier functions on polarized Caco-2 cell monolayers were evaluated by TER and FD4 flux. The expression of tight junction (TJ) proteins was assessed using reverse-transcription polymerase chain reaction (RT-PCR) and immunofluorescence staining.
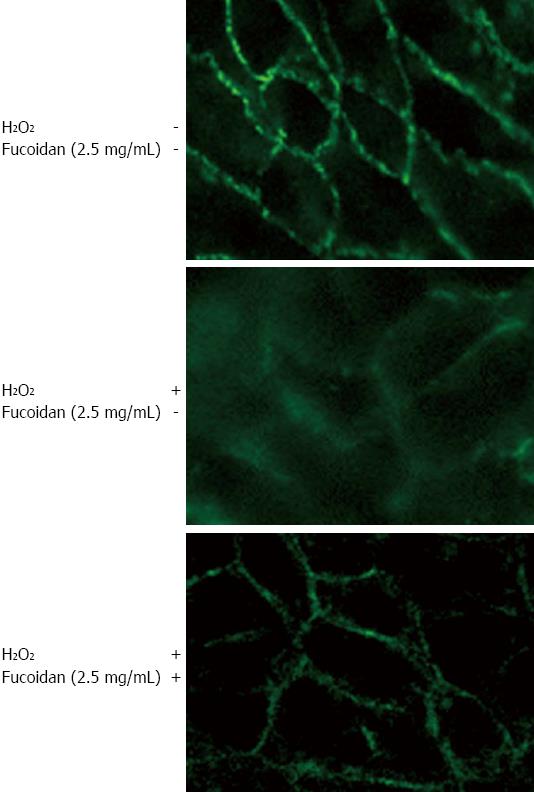
RESULTS: Without H2O2 treatment, fucoidan significantly increased the TER compared to control (P < 0.05), indicating a direct enhancement of intestinal epithelial barrier function. Next, H2O2 disrupted the epithelial barrier function in a time-dependent manner. Fucoidan prevented the H2O2-induced destruction in a dose-dependent manner. Fucoidan significantly decreased H2O2-induced FD4 flux (P < 0.01), indicating the prevention of disruption in paracellular permeability. RT-PCR showed that Caco-2 cells endogenously expressed claudin-1 and -2, and occludin and that H2O2 reduced the mRNA expression of these TJ proteins. Treatment with fucoidan attenuated the reduction in the expressions of claudin-1 and claudin-2 but not occludin. Immunofluorescence staining revealed that the expression of claudin-1 was intact and high on the cell surface. H2O2 disrupted the integrity of claudin-1. Treatment with fucoidan dramatically attenuated the expression of claudin-1.
CONCLUSION: Fucoidan enhanced intestinal epithelial barrier function by upregulating the expression of claudin-1. Thus, fucoidan may be an appropriate therapy for the treatment of inflammatory bowel diseases.
Core tip: The oxidative stress-induced disruption of the intestinal epithelial cells and subsequent increased paracellular permeability are critically important in the pathogenesis of inflammatory bowel diseases (IBD). A growing body of experimental evidence indicates that fucoidan, a dietary substance of fucose-enriched sulfated polysaccharides, display a wide variety of pharmacological anti-inflammatory activities. This study demonstrates that fucoidan protected the epithelial barrier function from oxidative injury of the tight junction as well as barrier disruption by upregulating the expression of claudin-1. Thus, fucoidan may be an appropriate therapy for the treatment of IBD.
- Citation: Iraha A, Chinen H, Hokama A, Yonashiro T, Kinjo T, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Higa F, Tateyama M, Kinjo F, Fujita J. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J Gastroenterol 2013; 19(33): 5500-5507
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5500
Although the gastrointestinal (GI) tract is constantly exposed to bacterial microflora, an excess immune response against the bacterial microflora does not occur in the normal state, as a result of some type of immunological tolerance underlying the GI immune system. However, disruption of this immunological tolerance against intestinal microbial antigens may cause abnormal intestinal inflammation and the development of chronic inflammatory diseases, such as inflammatory bowel diseases (IBD)[1,2]. IBD can be classified into two distinct diseases, ulcerative colitis (UC) and Crohn’s disease (CD). Although the precise etiology of these diseases remains unclear, several reports have indicated that intestinal microflora is responsible for the pathogenesis of both UC and CD[3,4]. Intestinal epithelial cells (IEC) play a role as the first line of defense and act as a functional barrier. IECs separate the host’s internal milieu from the external environment. In addition to functioning as a barrier, it has become evident that IECs also play an important role in the maintenance of immune homeostasis[5]. IECs produce anti-microbial peptides, such as defensins, and protect the host from the attachment of luminal bacteria[6]. Not only do IECs function in a direct bacteriocidal role, but IEC-derived factors can also promote the differentiation of anti-inflammatory types of dendritic cells and macrophages to induce mucosal tolerance against luminal bacteria[7,8]. Furthermore, in intestinal inflammation, IECs can produce several chemokines and pro-inflammatory cytokines in response to luminal bacteria to induce the migration of granulocytes, lymphocytes, and dendritic cells, resulting in the induction of host immunity. Thus, IECs function as a defensive frontline of host mucosal immunity. Accordingly, direct epithelial cell damage, induced by mucosal irritants or cytotoxic agents, results in a marked loss of barrier function[9]. The epithelial barrier consists of several essential elements, including an intact epithelial monolayer and the tight junction (TJ). The TJ consists of four integral membrane proteins: occludins, claudins, tricellulin and the junctional adhesion molecule. A large body of evidence indicates that disruption of the TJ and increased paracellular permeability are critically important in the pathogenesis of IBD[10]. The oxidative stress-induced opening of the TJ barrier is an important mechanism contributing to the TJ barrier defect present in IBD[11].
Caco-2, a human intestinal epithelial cell line, is the most well studied cell line for investigations of in vivo intestinal epithelial barrier integrity and function[12]. Hydrogen peroxide (H2O2), a highly toxic oxidizing agent, is constantly generated within intestinal epithelial cells and must quickly be detoxified by antioxidant enzymes[13]. It has been established that H2O2 is involved in oxidative stress-induced cell injury and disrupts intestinal epithelial barrier function, thus leading to enhanced paracellular permeability and the promotion of marked changes in the expression and/or localization of a number of TJ proteins, including claudins and occludins. In Caco-2 cell monolayer models, the disruption of barrier function by oxidative stress is mediated by H2O2[13].
Fucoidan, a dietary substance, represents a class of fucose-enriched sulfated polysaccharides found in the extracellular matrix of brown algae. A growing body of experimental evidence indicates that fucoidans display a wide variety of pharmacological activities, including anti-inflammatory, anti-angiogenic, anti-coagulant, and anti-adhesive effects, in experimental models[14-16]. Thus, great interest has been generated in investigating the potential pharmacological effects of fucoidan on H2O2-induced TJ destruction in IECs.
In this study, we examined the protective effect of fucoidan on H2O2-induced TJ destruction in human IECs, which may provide a novel approach for the treatment of IBD.
The brown algae Cladosiphon okamuranus Tokida was cultivated in Okinawa, Japan. Purified fucoidan derived from C. o. Tokida was provided by Uruma Bio Co. Ltd., (Okinawa, Japan). Fucoidan was dissolved in Dulbecco’s Vogt modified Eagle’s media (DMEM) (Sigma-Aldrich Co., St. Louis, MO).
A human intestinal epithelial cell line, Caco-2 cells (RBRC-RCB0988 RIKEN Bio Resource Center, Ibaraki, Japan), were cultured in DMEM supplemented with 10% (v/v) heat-inactivated FBS (Nichirei Biosciences Inc., Tokyo, Japan), 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies Gibco, France), and 10 ml GlutaMAXTM (100 ×) (Life Technologies Gibco, France). The cell cultures were incubated on collagen-coated tissue culture plates Transwell® (Corning, New York, NY) in a humidified atmosphere of 5% CO2 at 37 °C.
The integrity of polarized Caco-2 cell monolayers was determined by measuring the transepithelial resistance (TER), which reflects the tightness of the TJ between epithelial cells[17,18]. The TER was measured in Ωcm2 using a Millicell ERS-2 Epithelial Volt-Ohm Meter (Millipore, Bedford, MA). Caco-2 cells were cultured on 24 mm Transwell® polycarbonate inserts (0.4 μmol/L pore size) for 14 to 21 d. To examine the direct effect of fucoidan on well-polarized Caco-2 cell monolayers, confluent polarized Caco-2 cell monolayers were incubated in the presence or absence of fucoidan (2.5 mg/mL) in apical medium for 24 h. To evaluate the protective effects of fucoidan on epithelial cell injury, serial doses of fucoidan (0, 0.1, 1.0, or 2.5 mg/mL) were added to the apical medium 30 min prior to the administration of H2O2 (500 μmol/L) to the basolateral side of the Transwell®. Changes in the TER during the experimental periods were calculated as the percentage of the corresponding basal values. TER of unseeded inserts was subtracted.
Permeability was estimated by measuring the paracellular transport of FITC-labeled 4-kDa dextran (FD4) (Molecular Probes, Netherland). Once the cells were grown to confluence (TER > 350 Ωcm2), sterilized FD4 was added into the apical well at 1 mg/mL. H2O2 (500 μmol/L) was administered to the basolateral side of the Transwell®. Fucoidan (2.5 mg/mL) was added to the apical medium 30 min prior to H2O2 administration. After 6 h of incubation, the basolateral medium was collected, and the fluorescence was measured using a fluorescence spectrometer at an excitation of 485 nm and emission of 535 nm. The permeability was expressed as the percentage of fluorescence of the H2O2-treated group. Flux of unseeded inserts was subtracted.
Caco-2 cells were cultured for 14 to 21 d. Once grown to confluence (TER > 350 Ωcm2), H2O2 (500 μmol/L) was administered to the basolateral side of the Transwell®. Fucoidan (2.5 mg/mL) was added to the apical medium 30 min prior to H2O2 administration. After 24 h of incubation, the cells were harvested, and total RNA was isolated using the RNeasy Mini kit (Qiagen, KJ Venlo, the Netherlands). Isolated RNA was treated with RNase-free DNase I (Qiagen) to prevent any carry-over of genomic DNA. The cDNA was synthesized from 2 μg of total RNA with Quantitect reverse transcriptase (Qiagen). Reverse-transcription polymerase chain reaction (RT-PCR) was performed using a PCR master mix (Takara Biosystems, Foster City, CA). Primers were listed 5’-3’ as follows: Claudin-1: F, GCG CGA TAT TTC TTC TTG CAG G; R, TTC GTA CCT GGC ATT GAC TGG. Claudin-2: F, CTC CCT GGC CTG CAT TAT CTC; R, ACC TGC TAC CGC CAC TCT GT. Occludin: F, TCA GGG AAT ATC CAC CTA TCA CTT CAG; R, CAT CAG CAG CAG CCA TGT ACT CTT CAC.
Caco-2 cells were cultured for 14 to 21 d on a Lab-Tek chamber plate (Corning). H2O2 (500 μmol/L) was administered to the basolateral side of the Transwell®. Fucoidan (2.5 mg/mL) was added to the apical medium 30 min prior to H2O2 treatment. After 6 h of incubation, the cells were washed twice with cold PBS and fixed with cold acetone (Wako Pure Chemical Industries, Osaka, Japan) for 10 min. The cells were then removed from the Transwell® and mounted on slides. Next, the cells were incubated with mouse anti-human claudin-1 (Zymed Laboratories, San Francisco, CA) at 4 °C overnight. After washing with PBS, the cells were incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Netherland) then subsequently washed in PBS. The immunofluorescence was examined and imaged using fluorescence microscopy (Nikon Eclipse 80i).
Statistical analysis was performed using the GraphPad Prism software program, version 4.0 (GraphPad Software Inc., San Diego, CA). Differences with P < 0.05 were considered significant. All of the data were expressed as the mean ± SEM.
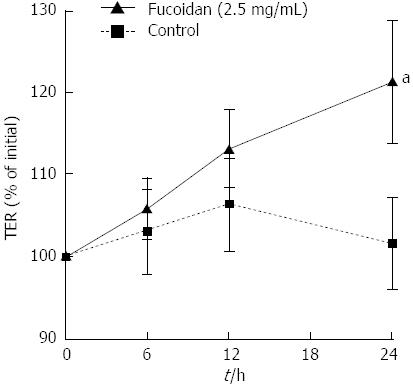
First, we determined the effect of fucoidan on the protective functions of Caco-2 cell monolayers. To determine whether fucoidan directly induced the increase in epithelial resistance or TER was upregulated by the promotion of epithelial cell proliferation, we examined the effect of fucoidan on well-polarized Caco-2 cell monolayers. Completely polarized Caco-2 cell monolayers showed approximately 600 Ωcm2 TER. Because polarized Caco-2 cells could not further proliferate, the direct effect of fucoidan on intestinal epithelial barrier functions could be examined. Interestingly, fucoidan significantly increased the TER (P < 0.05 compared with control), indicating an enhancement of intestinal epithelial barrier function (Figure 1).
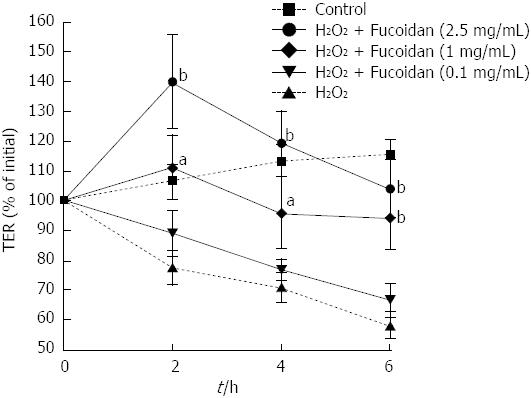
Next, we focused on the preventive effects of fucoidan on epithelial cell injury. To assess the effect of fucoidan on intestinal epithelial barrier function, completely polarized Caco-2 cell monolayers were injured using H2O2 (500 μmol/L). H2O2 was added into the lower well of the Transwell® and changes in intestinal epithelial barrier function were monitored by measuring the TER. As shown in Figure 2, H2O2 disrupted the epithelial barrier function in a time-dependent manner. In contrast, treatment with fucoidan prevented H2O2-induced intestinal epithelial injury at an early time point (P < 0.05, P < 0.01 compared with cells exposed to H2O2 alone at respective time point). However, low dose (0.1 mg/mL) of fucoidan did not protect the intestinal epithelium against H2O2 injury after 4 h of exposure; however, high doses (1 and 2.5 mg/mL) of fucoidan prevented the disruption of the epithelial barrier to some extent even at the late phase. Thus, fucoidan prevented H2O2-induced destruction of the intestinal epithelial barrier in a dose-dependent manner.
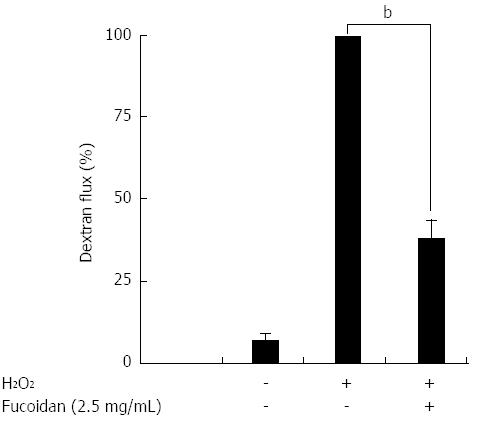
Next, we examined whether H2O2 increased the paracellular permeability of Caco-2 cell monolayers following epithelial injury and whether fucoidan could prevent this effect. For this experiment, an FD4 flux assay was performed. H2O2 markedly increased FD4 flux into the lower well (Figure 3). As expected, pretreatment with fucoidan 30 min prior to H2O2 administration significantly suppressed the increase in FD4 flux into the lower well across the Caco-2 cell monolayers (P < 0.01) (Figure 3). These results suggested that H2O2 functionally injured the Caco-2 cell monolayers and that fucoidan prevented the disruption of intestinal epithelial barrier function.
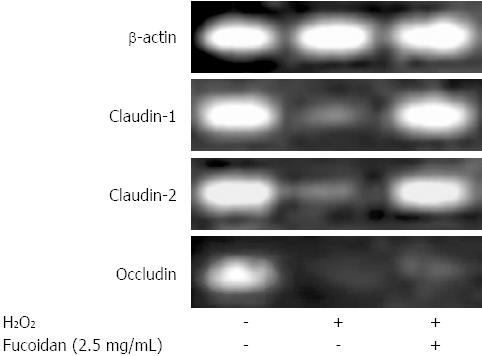
To determine how fucoidan treatment promotes an increase in intestinal epithelial barrier function, we examined the effect of fucoidan on the mRNA expression of major TJ-associated proteins. As shown in Figure 4, Caco-2 cells endogenously expressed claudin-1 and -2, and occludin. H2O2 reduced the mRNA expression of these proteins. In addition, pretreatment with fucoidan attenuated the reduction in the expressions of claudin-1 and claudin-2 mRNA but not occludin mRNA. These results suggested that fucoidan treatment strongly induced the expression of claudin-1 and -2 that promote intestinal epithelial barrier function.
We further examined the effect of fucoidan on the intercellular localization of claudin-1 using immunofluorescence microscopy. We found that the expression of claudin-1 was intact and high on the cell surface in control cells. H2O2 strongly disrupted the integrity of claudin-1, resulting in lower expression. Furthermore, pretreatment with fucoidan dramatically attenuated the H2O2-induced injury, restoring cell integrity and promoting the expression of claudin-1 (Figure 5).
IBD is associated with an epithelial barrier defect characterized by impaired absorptive function and increased mucosal barrier defects, which are caused by impaired TJ complexity, particularly affecting claudins[19-21]. Whereas claudin-1, -3, -4, -5 and -8 demonstrate sealing functions, claudin-2, -10b and -15 act as paracellular channels and promote the charge-selective passage of small ions[10]. Recent studies have revealed that the expression of barrier-forming claudin-1 and -4 and occludin are downregulated in the intestinal epithelia of patients with UC[22], and downregulation of claudin-3, -5 and occludin have been observed in CD[23]. However, the pore-forming protein claudin-2 is upregulated in both UC and CD, resulting in leaky TJ strands[22,23]. Amasheh et al[24] recently established an experimental IBD model of native colon in vitro, which showed an impairment of epithelial barrier function via downregulation of claudin -1, -5, and -7 after exposure to tumor necrosis factor (TNF)-α and interferon gamma (IFN)-γ. Because the present study showed the impaired expression of claudin-1 and occludin by oxidative stress, our model mimicked the intestinal inflammation observed in IBD.
Fucoidans represent an intriguing group of naturally occurring polysaccharides that might have promising therapeutic applications in various clinical situations. Algal fucoidans are characterized by a wide variety of biological functions and by a highly complex and heterogeneous structure, which varies within algal species. Fucoidans from various algal species might differentially affect inflammation. Although numerous biological activities of fucoidan have attracted attention, only a few studies have examined the pharmacological activity of fucoidan in intestinal inflammation[25]. Matsumoto et al[26] have shown that the oral administration of fucoidan ameliorated murine chronic colitis by downregulating the synthesis of interleukin-6 (IL-6), a key pro-inflammatory cytokine in IBD, in colonic epithelial cells. They concluded that fucoidan derived from C. o. Tokida might be useful as a dietary substance for the treatment of IBD. In addition, Zhang et al[27] revealed that intravenous administration of fucoidan reduced colonic mucosal damage and crypt destruction of dextran sodium sulfate-induced murine chronic colitis by reducing colonic myeloperoxidase activity and abolishing TNF-α-induced venular leukocyte rolling and extravascular recruitment. Moreover, Tanoue et al[28] established an in vitro model of a co-culture system using intestinal epithelial Caco-2 cells and macrophage RAW264.7 cells to treat intestinal inflammation by fucoidan. They clearly showed that fucoidan suppressed IL-8 gene expression in epithelial cells via reduction in TNF-α production from macrophages stimulated with lipopolysaccharide. For gastric inflammation, fucoidan has been found to protect against aspirin-induced gastric ulceration by inhibiting IL-6, TNF-α, and IFN-γ[29,30]. However, to the best of our knowledge, our study is the first to report that fucoidan protects and strengthens epithelial barrier function, both under physiological and pathological conditions via induction of the expression of claudin-1 in human IECs. The mechanisms how fucoidan regulates the TJ proteins in this study are unknown. We next plan to investigate cytokine studies and signaling pathways which may regulate the expression of claudins and occludin by the treatment of fucoidan with a consistent time course experiments.
Pro-inflammatory cytokines, such as TNF-α, IFN-γ, and IL-13, affect the expression of TJ proteins in IECs and induce epithelial cell apoptosis, resulting in the disruption of intestinal epithelial barrier function[22,31,32]. Because IECs function as a defensive frontline of host mucosal immunity in the intestine, disruption of barrier function of IECs causes an excessive immune response to intestinal bacteria[5]. Thus, dysfunction of IECs strongly contributes to the pathogenesis of bacteria-triggered chronic inflammation of the intestine in IBD. However, defects in TJ barrier function are insufficient to cause disease. Increased paracellular permeability can increase mucosal immune activity and enhance disease progression and severity. Thus, restoration of TJ barrier function might be effective, either alone or in combination with other agents, in preventing disease in at-risk individuals or maintaining remission in patients with IBD[9]. Although recent advances in anti-TNF-α antibody therapy can dramatically inhibit intestinal inflammation, strengthening the intestinal epithelial barrier is still challenging and has been eagerly investigated. It is well known that zinc, a trace element, assists with the maintenance of intestinal barrier integrity. Glutamine, an essential amino acid, supports recovery from a loss in TER. Moreover, the expression of claudin-1 and occludin proteins were decreased when Caco-2 cells were deprived of glutamine through inhibition of glutamine synthetase[33]. Furthermore, a direct influence on TJ protein expression has been observed from several plant components, including the flavonoid quercetin and the isoquinoline alkaloid berberine[34]. Quercetin, which is obtained from fruits, enhances barrier function by upregulating claudin-4 expression[35], whereas berberine, a herbal agent, prevented the barrier impairment induced by TNF-α and IFN-γ[36]. We have demonstrated that fucoidan directly induced the expression of some TJ proteins and might contribute to the enhancement of epithelial barrier functions. Thus, we believe that the activity of fucoidan, which increases the epithelial protective function and promotes epithelial regeneration, might serve as an appropriate therapy for the treatment of IBD.
Although dietary components may regulate TJ permeability by directly targeting the signal transduction pathways involved in TJ regulation, specific dietary components have been identified that influence cytokine signaling, thereby modifying TJ permeability[34]. The intestinal barrier is a complex environment, and the regulation of barrier function cannot be elucidated using in vitro models alone. Interactions between dietary components and microbiota are also crucial in the regulation of barrier integrity[34]. It is important to consider the interactions between different components of the intestinal barrier when establishing strategies to enhance barrier integrity using dietary compounds. The present study may provide insight for the development of novel agents with low toxicity in the treatment of intestinal inflammation. Because the healing of intestinal inflammation is a complex process involving numerous factors, further work is required to elucidate the therapeutic effect of fucoidan.
The oxidative stress-induced disruption of the intestinal epithelial cells and subsequent increased paracellular permeability are critically important in the pathogenesis of inflammatory bowel diseases (IBD). Although recent advances in anti-tumor necrosis factor-α antibody therapy can dramatically inhibit intestinal inflammation, strengthening the intestinal epithelial barrier is still challenging and has been eagerly investigated.
Recent studies have revealed that some dietary components may regulate tight junction (TJ) permeability by directly targeting the signal transduction pathways involved in TJ regulation. A growing body of experimental evidence indicates that fucoidan, a dietary substance of fucose-enriched sulfated polysaccharides with low toxicity, display a wide variety of pharmacological anti-inflammatory activities. However, only a few studies have examined the protective effects of fucoidan for intestinal inflammation.
The authors investigated the effect of fucoidan on oxidative stress-induced barrier disruption in a Caco-2 cell monolayer model, with an emphasis on the alterations of TJ proteins. This study demonstrates that fucoidan protected the epithelial barrier function from oxidative injury of the TJ as well as barrier disruption by upregulating the expression of claudin-1.
Fucoidan may be an appropriate therapy to control the expression of claudin-1 for the treatment of IBD.
Tight junctions: TJ forms a network of close contacts between membranes of adjacent cells. TJ consists of four integral membrane proteins: claudins, occludins, tricellulin and the junctional adhesion molecule. A large body of evidence indicates that disruption of the TJ and increased paracellular permeability are critically important in the pathogenesis of IBD. Fucoidan: fucoidan is a dietary substance, which represents a class of fucose-enriched sulfated polysaccharides found in the extracellular matrix of brown algae. Numerous experimental evidences indicate that fucoidans display a wide variety of pharmacological activities, including anti-inflammatory, anti-angiogenic, anti-coagulant, and anti-adhesive effects.
The manuscript described that fucoidan is effective to improve intestinal epithelial barrier function. This is an interesting study that could be worth publishing.
P- Reviewers Kuo SM, Leach ST, Liu ZJ, Tobacman JK S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3114] [Article Influence: 183.2] [Reference Citation Analysis (8)] |
| 2. | Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1195] [Article Influence: 66.4] [Reference Citation Analysis (1)] |
| 4. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 992] [Cited by in F6Publishing: 956] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 5. | Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 733] [Cited by in F6Publishing: 751] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 6. | Wehkamp J, Stange EF. Paneth cells and the innate immune response. Curr Opin Gastroenterol. 2006;22:644-650. [PubMed] [Cited in This Article: ] |
| 7. | Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 622] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 552] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 9. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2385] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 10. | Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood). 2012;237:474-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736-749. [PubMed] [Cited in This Article: ] |
| 13. | Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, Jiang H. Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci. 2012;57:1792-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Mar Drugs. 2011;9:1731-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J. 2010;9:54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 16. | Holtkamp AD, Kelly S, Ulber R, Lang S. Fucoidans and fucoidanases--focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol. 2009;82:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Maher S, Feighery L, Brayden DJ, McClean S. Melittin as an epithelial permeability enhancer I: investigation of its mechanism of action in Caco-2 monolayers. Pharm Res. 2007;24:1336-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126-3135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 19. | Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 332] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 367] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 888] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 24. | Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 765] [Cited by in F6Publishing: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 26. | Matsumoto S, Nagaoka M, Hara T, Kimura-Takagi I, Mistuyama K, Ueyama S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin Exp Immunol. 2004;136:432-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Zhang XW, Liu Q, Thorlacius H. Inhibition of selectin function and leukocyte rolling protects against dextran sodium sulfate-induced murine colitis. Scand J Gastroenterol. 2001;36:270-275. [PubMed] [Cited in This Article: ] |
| 28. | Tanoue T, Nishitani Y, Kanazawa K, Hashimoto T, Mizuno M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem Biophys Res Commun. 2008;374:565-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Raghavendran HR, Srinivasan P, Rekha S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int Immunopharmacol. 2011;11:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Choi JI, Raghavendran HR, Sung NY, Kim JH, Chun BS, Ahn DH, Choi HS, Kang KW, Lee JW. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem Biol Interact. 2010;183:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Willemsen LE, Hoetjes JP, van Deventer SJ, van Tol EA. Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol. 2005;142:275-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] [Cited in This Article: ] |
| 33. | Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 776] [Article Influence: 59.7] [Reference Citation Analysis (1)] |
| 35. | Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr. 2008;138:1067-1073. [PubMed] [Cited in This Article: ] |
| 36. | Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145-4155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |