Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4363
Revised: March 4, 2013
Accepted: March 15, 2013
Published online: July 21, 2013
Processing time: 351 Days and 19.9 Hours
AIM: To investigate large tumor suppressor 1 (LATS1) expression, promoter hypermethylation, and microsatellite instability in colorectal cancer (CRC).
METHODS: RNA was isolated from tumor tissue of 142 CRC patients and 40 colon mucosal biopsies of healthy controls. After reverse transcription, quantitative polymerase chain reaction (PCR) was performed, and LATS1 expression was normalized to expression of the ACTB and RPL32 housekeeping genes. To analyze hypermethylation, genomic DNA was isolated from 44 tumor CRC biopsies, and methylation-specific PCR was performed. Microsatellite instability (MSI) status was checked with PCR using BAT26, BAT25, and BAT40 markers in the genomic DNA of 84 CRC patients, followed by denaturing gel electrophoresis.
RESULTS: Decreased LATS1 expression was found in 127/142 (89.4%) CRC cases with the average ratio of the LATS1 level 10.33 ± 32.64 in CRC patients vs 32.85 ± 33.56 in healthy controls. The lowest expression was found in Dukes’ B stage tumors and G1 (well-differentiated) cells. Hypermethylation of the LATS1 promoter was present in 25/44 (57%) CRC cases analyzed. LATS1 promoter hypermethylation was strongly associated with decreased gene expression; methylated cases showed 162× lower expression of LATS1 than unmethylated cases. Although high-grade MSI (mutation in all three markers) was found in 14/84 (17%) cases and low-grade MSI (mutation in 1-2 markers) was found in 30/84 (36%) cases, we found no association with LATS1 expression.
CONCLUSION: Decreased expression of LATS1 in CRC was associated with promoter hypermethylation, but not MSI status. Such reduced expression may promote progression of CRC.
Core tip: Searching for new colorectal cancer (CRC) molecular markers is a very important objective, because CRC is one of the most common malignancies in the world and one of the most fatal of human neoplasms. Decreased expression of large tumor suppressor 1 in CRC was associated with promoter hypermethylation, but not microsatellite instability status. Such reduced expression may promote progression of CRC.
-
Citation: Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I, Celinski K, Gach T, Kulig J, Korybalski B, Kmiec Z. Underexpression of
LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol 2013; 19(27): 4363-4373 - URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4363.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4363
Colorectal cancer (CRC) is one of the most common malignancies in the world and one of the most fatal human neoplasms. Almost 1.2 million new cases occur annually, accounting for 608700 related deaths in 2008[1]. Although nearly 90% of patients may be successfully cured with surgery in early stages, CRC is frequently diagnosed in late stages, i.e., Dukes’ C and D, when the prognosis is poor[2,3]. Therefore, the search for CRC molecular markers, as well as elucidation of epigenetic factors that are responsible for variability in the expression of putative markers, is very important.
Human large tumor suppressor 1 (LATS1, also known as WARTS) was discovered in 1999[4] as a highly conserved homolog of the Drosophila melanogaster (D. melanogaster) lats gene[5]. LATS1 encodes a serine/threonine kinase, which is involved in the regulation of various cellular processes. Before mitotic division, the presence of LATS1 is crucial for control of the R1 tetraploidy checkpoint[6]. During the early phase of mitosis, LATS1 associates with cell division control protein 2 homolog[7], and the progress of cytokinesis occurs only after association of the MOB kinase activator 1A cytoplasmic protein with LATS1[8,9].
More recently, genetic studies in Drosophila have identified LATS as a central mediator in a tumor suppressing pathway called the Salvador-Warts-Hippo (SWH) pathway[10,11]. The SWH pathway is also a critical factor in the regulation of organ size in D. melanogaster and mammals[12,13]. Moreover, deregulation of SWH pathway activity has been implicated in the genesis of multiple human cancers[11,14-16]. Several mammalian factors are involved in signal transduction in the SWH pathway, including the tumor suppressor proteins neurofibromin 2, Ras association family member 1-6, serine/threonine kinase 3, LATS1, and an oncogene called Yes-associated protein (YAP). YAP, a transcription coactivator that associates with various transcription factors, is overexpressed in human carcinomas including ovarian, liver, and prostate cancers[13]. LATS1 kinase is a main negative regulator of YAP. LATS1 inhibits the transcriptional activity/function of YAP via phosphorylation of Ser 127 in YAP[17]. Moreover, LATS1-phosphorylated YAP is involved in a p53-independent apoptosis pathway in which phosphorylated YAP plays a role in transcriptional activation of the proapoptotic gene, p53 up-regulated modulator of apoptosis[18]. Overexpression of LATS1 in LATS1-/- mouse cells (by introducing human LATS1 with adenovirus-mediated gene transfer) and HeLa cells suppresses tumorigenicity in vivo and in vitro by inducing apoptosis[18,19].
LATS1 is considered to play a suppressor role in some tumors. Decreased LATS1 expression is found mainly in soft tissue-derived tumors, including sarcomas[20] and astrocytomas[21]. However, LATS1 quiescence was also observed in breast[22], cervical[23] cancers and head and neck squamous cell carcinoma[24]. In the gastrointestinal tract, decreased LATS1 expression has been recently observed in gastric cancer[25], but in a small sample of CRCs, LATS1 overexpression was found[26].
Hypermethylation of CpG islands (GC-rich sequences) in regulatory portions of a gene is an important epigenetic mechanism responsible for decreased gene expression or gene silencing[27-30]. Aberrant methylation of CpG islands in the promoter region of LATS1 has been found in breast and ovarian cancers[4,22,31] and soft-tissue sarcomas[20]. Our preliminary results suggested that LATS1 expression is decreased in CRC and is associated with promoter hypermethylation[32]. In the present study, we used quantitative polymerase chain reaction (QPCR) to determine the expression profile of LATS1 in a relatively large group of CRC patients. We also examined the hypermethylation status of the LATS1 promoter as a putative epigenetic mechanism affecting gene expression.
The study was approved by the local ethics committees, and informed, written consent regarding the use of tissue was obtained before surgery or colonoscopy from all CRC and control patients, respectively. The specimens were obtained from four gastrointestinal endoscopic units and surgical clinics located in geographically different parts of Poland from 2008 to 2011. Clinical and demographic data were collected at the time of enrollment (Table 1). The study included 142 patients with CRC (87 males and 55 females; mean age 68 ± 10.8 years; range, 37-90 years). No CRC patients had a second neoplastic disease. None of the patients had undergone previous chemo- or radiotherapy. Tumors located in the anal canal and anus were not included in this study. The control group comprised 40 healthy individuals (17 males and 23 females; mean age 53 ± 14.2 years; range, 21-76 years) who underwent colonoscopy as part of routine surveillance for CRC. None of the CRC patients or controls suffered from inflammatory bowel disease or had a family history of CRC. Patients were not on medication at the time of investigation. Before medical examination, blood samples were collected for routine testing from all CRC patients.
| Clinical parameter | Blood parameters (mean ± SD) | MSI results (n = 84) | QPCR results | |||||||||
| RBC (106/μL) | Ht | Hb (g/dL) | WBC (103/μL) | BAT 26 | BAT 25 | BAT 40 | MSI-L | MSI-H | Downregulated cases vs control | |||
| CRC 142 cases | 4.26 ± 4.58 | 36% ± 5.2% | 12 ± 2.1 | 7.41 ± 2.71 | 28/84 (33) | 25/84 (30) | 26/84 (31) | 30/84 (36) | 14/84 (17) | 127/142 (88)1 | ||
| Sex and age (mean ± SD, range, yr) | M (n = 87) | 67 ± 10.4 (37-89) | 4.36 ± 0.502 | 36% ± 6.0% | 12 ± 2.4 | 7.26 ± 2.91 | 21/54 (39) | 18/54 (33) | 16/54 (30) | 22/54 (41) | 9/54 (17) | 78/87 (90) |
| F (n = 55) | 69 ± 11.4 (44-90) | 4.18 ± 0.41 | 35% ± 4.6% | 11 ± 1.8 | 7.53 ± 2.58 | 7/30 (23) | 7/30 (23) | 10/30 (33) | 8/30 (37) | 5/30 (17) | 49/55 (88) | |
| Tumor location | Right side | 55 (39) | 4.09 ± 0.462 | 33% ± 4.7%2 | 10 ± 1.92 | 7.18 ± 3.45 | 9/32 (28) | 8/32 (25) | 7/32 (22) | 8/32 (25) | 4/32 (12.5) | 51/55 (93) |
| Left side | 87 (61) | 4.36 ± 0.432 | 38% ± 4.8%2 | 12 ± 1.82 | 7.56 ± 2.17 | 19/52 (36.5) | 17/52 (33) | 19/52 (36.5) | 22/52 (42) | 10/52 (19) | 76/87 (81) | |
| Ascending colon | 46 (32) | 4.08 ± 0.46 | 33% ± 5.0%2 | 11 ± 2.02 | 7.26 ± 3.42 | 9/26 (35) | 8/26 (31) | 6/26 (23) | 7/26 (27) | 4/26 (15) | 43/46 (93)1 | |
| Transverse colon | 11 (8) | 4.12 ± 0.43 | 33% ± 4.2%2 | 10 ± 1.52 | 7.43 ± 3.47 | 0/6 (0) | 0/6 (0) | 1/6 (17) | 1/6 (17) | 0/6 (0) | 10/11 (91)1 | |
| Descending/sigmoid colon | 41 (29) | 4.29 ± 0.53 | 36% ± 5.2%2 | 12 ± 2.12 | 7.49 ± 2.35 | 6/23 (26) | 7/23 (30) | 9/23 (39) | 9/23 (39) | 4/23 (17) | 32/41 (78)1 | |
| Rectum | 44 (31) | 4.43 ± 0.33 | 39% ± 4.5%2 | 13 ± 1.62 | 7.47 ± 2.08 | 13/29 (45) | 10/29 (34) | 10/29 (34) | 13/29 (45) | 6/29 (21) | 42/44 (95)1 | |
| Dukes’ stage | A | 27 (19) | 4.23 ± 0.49 | 38% ± 5.3% | 12 ± 2.0 | 7.58 ± 2.08 | 2/19 (11) | 2/19 (11)2 | 1/19 (6)2 | 4/19 (22) | 0/19 (0) | 22/27 (81)12 |
| B | 41 (29) | 4.30 ± 0.47 | 36% ± 5.5% | 11 ± 2.1 | 7.38 ± 1.99 | 9/23 (39) | 5/23 (22)2 | 7/23 (30)2 | 8/23 (35) | 4/23 (17) | 40/41 (98)1 | |
| C | 54 (38) | 4.22 ± 0.47 | 36% ± 5.0% | 12 ± 2.0 | 7.42 ± 3.41 | 12/34 (35) | 14/34 (41)2 | 15/34 (44)2 | 14/34 (41) | 8/34 (24) | 49/54 (91)1 | |
| D | 20 (14) | 4.34 ± 0.26 | 32% ± 4.4% | 10 ± 1.9 | 7.13 ± 2.26 | 5/8 (62.5) | 4/8 (50)2 | 3/8 (37.5)2 | 4/8 (50) | 2/8 (25) | 18/22 (82)1 | |
| Lymph node metastasis | Negative | 68 (48) | 4.28 ± 0.46 | 36% ± 5.5% | 12 ± 2.1 | 7.44 ± 1.97 | 11/42 (26) | 7/42 (17)2 | 8/42 (19)2 | 12/42 (29)2 | 4/42 (10)2 | 62/68 (91)1 |
| Positive | 74 (52) | 4.23 ± 0.45 | 35% ± 5.0% | 11 ± 2.1 | 7.39 ± 3.28 | 17/42 (40) | 18/42 (43)2 | 18/42 (43)2 | 18/42 (43)2 | 10/42 (24)2 | 65/74 (88)1 | |
| Histological differentiation (G stage) | Well (G1) | 3 (2) | 4.17 ± 0.35 | 36% ± 4.7% | 12 ± 1.4 | 7.44 ± 2.19 | 1/3 (33) | 1/3 (33) | 1/3 (33) | 0/3 (0) | 1/3 (33) | 3/3 (100)1 |
| Moderate (G2) | 48 (34) | 4.26 ± 0.47 | 36% ± 5.0% | 11 ± 2.0 | 7.17 ± 2.29 | 8/31 (26) | 6/31 (19) | 8/31 (26) | 9/31 (29) | 4/31 (13) | 46/48 (96)1 | |
| Poorly (G3) | 88 (62) | 4.16 ± 0.45 | 35% ± 5.5% | 11 ± 2.2 | 7.62 ± 3.54 | 18/47 (38) | 18/47 (38) | 17/47 (36) | 20/47 (43) | 9/47 (19) | 84/88 (95)1 | |
| Undifferentiated (G4) | 3 (2) | 4.36 ± 0.79 | 37% ± 6.6% | 12 ± 2.3 | 9.16 ± 4.74 | 1/3 (33) | 0/3 (0) | 0/3 (0) | 1/3 (33) | 0/3 (0) | 2/3 (66)1 | |
All steps of material collection, including patients’ clinical data, tissue collection, storage, shipment, and laboratory processing, followed The Cancer Genome Atlas (TCGA) instructions and were standardized in all collaborative clinics[33,34]. Briefly, CRC samples were obtained during surgical hemicolectomy, and control group specimens were collected during colonoscopy. For histopathologic examination and molecular studies, samples (5 mm × 5 mm × 5 mm) from macroscopically altered tumor tissue were taken within 20 min after tumor resection. For control patients, one biopsy (2 mm × 2 mm × 2 mm) was fixed in 10% neutral buffered formalin, and two specimens from the adjacent location to the biopsy site were collected for nucleic acid analyses. The formalin-fixed samples were obtained for the routine histological survey; if the result of histological examination showed pathological condition of the patient’s tissue, the adjacent biopsies were excluded from the control group analyzed in this study. Both tumor samples and mucosal biopsies were immediately placed in sterile vials containing RNAlater (Ambion-Life Technologies, Grand Island, NY, United States), incubated for 6 h at 4 °C, and then stored at -25 °C until further analysis.
Total RNA was extracted from a portion of the tumor samples (ca. 3 mm × 5 mm × 5 mm) and the entire mucosal biopsies of control patients using a Total RNA kit (A&A Biotechnology, Gdynia, Poland). Isolated RNA was quantified with spectrophotometry (Nanodrop ND 1000, Thermo Fisher Scientific, Fitchburg, WI, United States). DNA was digested with RNase-free DNase I (Fermentas-Thermo Fischer Scientific, Fitchburg, WI, United States) for 30 min at 37 °C. Then, the DNase was inactivated by adding EDTA and incubating at 65 °C for 10 min. Before storing at -85 °C, RNA integrity was analyzed with agarose gel electrophoresis. Total RNA (2 μg) was reverse transcribed using 0.5 μg oligo(dT)18 primers (Sigma-Aldrich, Munich, Germany) and 200 U RevertAid M-MuLV Reverse Transcriptase (Fermentas-Thermo Fischer Scientific, Fitchburg, WI, United States) in a total volume of 20 μL, and the resulting cDNA was stored at -25 °C. In 84 of the CRC cases, 1 mL venous blood that was collected in sterile K2-EDTA vials was used for DNA isolation using a Blood Mini DNA kit (A&A Biotechnology, Gdynia, Poland). From these same patients, DNA was also extracted from a portion of the tumor samples (ca. 3 mm × 5 mm × 5 mm) adjacent to the tumor fragments used for the RNA study using the Genomic Mini AX Tissue kit (A and A Biotechnology, Gdynia, Poland) and stored at -25 °C.
Quantification of LATS1 gene expression was carried out using iQ Cycler (Bio-Rad, Hercules, CA, United States) with Sybr®Green I as a fluorophore. LATS1 expression was determined with Livak’s comparative method 2-ΔΔCt[35] relative to the geometric mean of the expression levels of two housekeeping genes: β-actin (ACTB; GenBank acc. No. NM_001101.3) and ribosomal protein L32 (RPL32; NM_000994.3). These genes showed very stable expression in CRC in our previous studies[36,37] and studies of other investigators[38]. Except for the ACTB assay[39], all primers were designed by us using GenBank data. QPCR conditions were validated and showed 90%-100% efficiency for all assays. The amplification primer pairs were 5’-TGCACTGGCTTCAGATGGACAC-3’ and 5’-ATGTGCTAGACATCGCTGGTGC-3’; for LATS1 (functional transcript, ENSEMBL No. ENST00000543571, GenBank No. NM_004690.2), 5’-TGTGCCCATCTACGAGGGGTATGC-3’ and 5’-GGTACATGGTGGTGCCGCCAGACA-3’ for ACTB[39], and 5’-TGACAACAGGGTTCGTAGAAGAT-3’ and 5’-GTTCTTGGAGGAAACATTGTGAG-3’ for RPL32. The reaction mixture (17 μL) included 0.4 μL cDNA, 0.2 μmol/L each forward and reverse primers, and real-time PCR iQ SYBR Green SuperMix (Bio-Rad). All reactions were performed in duplicate. The amplification parameters were denaturation for 5 min at 95 °C, followed by 38 cycles of denaturation for 15 s at 95 °C, annealing for 20 s at 55 °C for RPL32, 57 °C for LATS1, and 60 °C for ACTB, extension for 15-25 s at 72 °C, and fluorescence reading for 5 s at 77 °C-80 °C. Dynamic melting curve analysis was performed for all reactions. Data were automatically collected and analyzed with iCycler iQ Optical Software ver. 3.0a (Bio-Rad).
Microsatellite instability (MSI) status was determined according to the National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition[40] and was based on polymorphism analysis of three markers: BAT26 for MSH2, BAT25 for the c-kit oncogene, and BAT40 for the HSD3B2 suppressor gene. BAT sequences were obtained from the UniSTS database (http://www.ncbi.nlm.nih.gov/unists), and the methodology was based on our previous results[41]. Briefly, the PCR reaction contained 0.5 U Taq polymerase, PCR buffer (Fermentas-Thermo Fischer Scientific, Fitchburg, WI, United States), 200 nmol/L each primer pair, 0.1 mmol/L each dNTP, and 30 ng DNA in a final volume of 15 μL and was performed using the following parameters: denaturation for 5 min at 95 °C, 35 cycles of denaturation for 15 s at 95 °C, annealing for 20 s at 49 °C (BAT25, BAT26) or 54 °C (BAT40), and extension for 30 s at 72 °C. Denaturing acrylamide gel electrophoresis was performed in a Sequi-Gen II Sequencing Cell (Bio-Rad) followed by silver staining (AgNO3; POCH, Gliwice, Poland) for identification of extra DNA bands, which were considered mutations in the selected BAT markers. Low- and high-grade MSI (MSI-L and MSI-H, respectively) were confirmed by 1-2 and all 3 mutated markers, respectively. If no mutation was observed in the paired tumor and blood DNA samples, the sample was confirmed as microsatellite stable (MSS).
Because the method of bisulfite conversion of DNA requires at least 1 μg DNA, we performed this analysis with only 44 tumor samples with sufficient material using the EZ DNA MethylationTM kit (Zymo Research, Orange, CA, United States). Briefly, 1 μg tissue DNA was denatured using 0.2 mol/L NaOH and subsequently incubated with a sodium salt of bisulfite ion (HSO3-) at 50 °C for 16 h. Next, the mixture was desulfonated, and DNA was purified on silica-membrane columns to a final volume of 10 μL. Bisulfite-modified DNA was stored at -25 °C. The methylation status of the LATS1 promoter region was determined with methylation-specific PCR (MSPCR). Bisulfite-modified DNA was amplified with primers specific for methylated or unmethylated sequences. The methylated DNA was amplified using M primers: sense 5’-TCGTTTTGTCGTTTAGGTTGG-3’ and antisense 5’-CGACGTAATAACGAACGC-3’, and unmethylated DNA was amplified using UM primers: sense 5’-TAGGTTGGAGTGTGGTGGT-3’ and antisense 5’-CCCAACATAATAACAAACACCT-3’. All primer sequences were previously published[20-22] except for the M sense primer, which was redesigned de novo using the GenBank database and methPrimerDB online software. For the methylation assay, Human HCT116 DKO Non-methylated DNA and Human HCT116 DKO Methylated DNA (Zymo Research) after bisulfite modification were used as positive controls in MSPCR. Briefly, 0.6 μL bisulfite-modified DNA was amplified in a total volume of 15 μL containing reagents from the ZymoTaq™ DNA Polymerase kit (Zymo Research) and 400 nmol/L each primer. MSPCR reactions were as follows: denaturation for 5 min at 95 °C, five cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 52 °C, extension for 20 s at 72 °C; 30 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 50 °C, extension for 20 s at 72 °C; final extension for 5 min at 72 °C. PCR products (10 μL) were run on a 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet illumination. Images were stored using a Gel Doc apparatus and software (Bio-Rad).
Normality of the QPCR data was assessed with the Shapiro-Wilk test. Parametric data such as red blood cells, hematocrit, hemoglobin, and LATS1 mRNA levels between various groups were evaluated using the Mann-Whitney U test. Comparison of LATS1 mRNA ratios between CRC subgroups with various histological and MSI grades and methylation status was calculated using the Kruskal-Wallis analysis of variance (ANOVA) test. Fisher’s exact test was used to assess correlations between the methylation status and clinical-pathological variables. The statistical analyses were performed using Statistica ver. 10 program (Stat Soft Inc., Tulsa, OK, United States), and the level of significance was set at P < 0.05.
Clinico-pathological data including tumor stages according to tumor location, Dukes’ classification, and G grade[42,43] are presented in Table 1. We found no statistical differences in geographic location of patients, sex, age, tumor location, and disease progression. We found a relationship between tumor location and erythrocyte counts, hematocrit level, and hemoglobin concentration; patients with a tumor on the right side were characterized by decreased values compared with patients whose tumor was on the left side (P < 0.05, Table 1). No associations between blood parameters and Dukes classification, TNM, and G grading of CRC were found.
Quantification of LATS1 mRNA was performed in colorectal tumor samples from 142 CRC patients and compared with tissue samples from 40 healthy persons. Decreased LATS1 gene expression was found in 127 of 142 (89.4%) tumors in the CRC cases (P < 0.05). Because QPCR data were not normally distributed (mean values: 10.33 ± 32.64 vs 32.85 ± 33.56, P < 0.05), the median expression ratio was 0.075 (range, 0.003-210.672) in CRC patients vs 40.097 (range, 0.004-98.228) in controls (P < 0.05). Thus, the average expression of LATS1 was many times lower in tumor tissue than in normal colon mucosa of controls. No correlations between LATS1 mRNA level and gender, age, or tumor location were found. Also, no statistical differences in the mRNA ratio were observed in patients who lived in different regions of Poland.
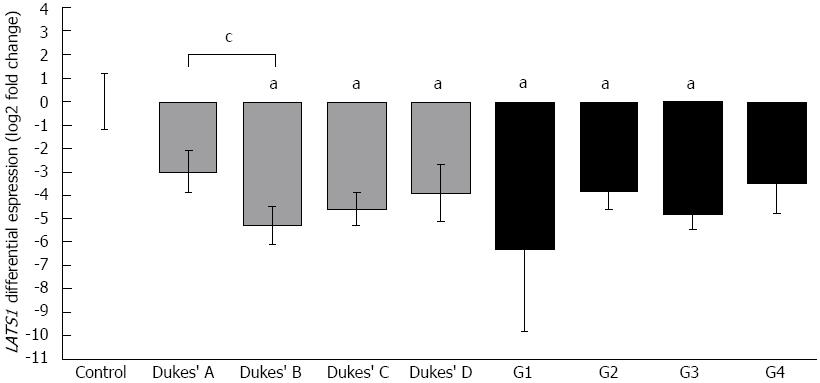
Comparison of LATS1 expression levels with patients’ clinico-pathological data revealed 8 times lower LATS1 levels in Dukes’ A stage compared to controls (Figure 1). The lowest LATS1 expression was observed in Dukes’ B stage, which was 42 times lower than in controls, whereas in more advanced CRC cases described as Dukes’ C and D stage, LATS1 expression was 24 and 14 times lower than in controls, respectively. We found a weak negative correlation between tumor progression and the LATS1 mRNA level (R2 = -0.25, P < 0.05, Spearman’s test, plot not shown). When the histological G grade of cancer cells was considered, LATS1 mRNA levels were significantly decreased in both G2 and G3 grades (Figure 1). However, due to the low number of G1 cases (well-differentiated cells) and G4 (undifferentiated cells) cases (three each), no comparison with grades G2 and G3 was possible.
We analyzed 84 of the 142 CRC cases for MSI status. The highest rate of mutation was found in the BAT26 marker (n = 28/84; 33%), followed by 26 cases for BAT40 (31%) and 25 for BAT25 (30%) (Table 1). Our analysis revealed MSI-L in 30 cases and MSI-H in 14 cases, which equates to 44 (52%) cases with the MSI genotype and 40 (48%) with MSS. The BAT25 and BAT40 markers were different according to the Dukes’ stage (Kruskal-Wallis ANOVA test, P < 0.05), followed by higher occurrence of mutations in CRC cases with positive lymph node metastasis (any T, N1-2, any M) vs negative metastasis (Kruskal-Wallis ANOVA test, P < 0.05). We found no relationship between MSI status and LATS1 expression.
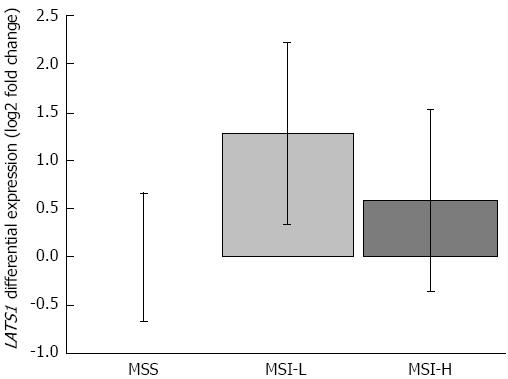
To check if the MSI phenotype influenced the expression pattern of LATS1, we compared QPCR results of LATS1 expression ratios in MSS, MSI-L, and MSH-H cases (Figure 2). Although we found that MSI-L and MSI-H samples were characterized by higher LATS1 levels than MSS cases, we found no statistical differences among those ratios (Figure 2).
To assess the methylation profile of LATS1 during CRC progression, we analyzed 44 CRC cases with different clinico-pathological outcomes. Hypermethylation of the LATS1 promoter was present in 25/44 cases (57%), whereas in 19 CRC tumor samples, no hypermethylation was found. No correlations were observed between LATS1 hypermethylation and gender, age, tumor location, Dukes’ stage, or G grading. Hypermethylation of the LATS1 promoter was found in all patients with Dukes’ A and D stages, and in 31% and 58% cases with Dukes’ B and C stages, respectively (Table 2).
| Clinical parameter | Total | M | Av. LATS1 mRNA fold | UM | Av. LATS1 mRNA fold | Av. LATS1 mRNA fold | P value between | |
| change, control vs M | change, control vs UM | change, UM vs M | UM and M groups | |||||
| Tumor total | 44 | 25 (57) | 597 | 19 | 3.55 | 162 | 0.00005 | |
| Dukes’ stage | A | 4 | 4 (100) | 556 | 0 | No data | No data | - |
| B | 16 | 5 (31) | 699 | 11 | 228 | 3 | 0.041 | |
| C | 19 | 11 (58) | 469 | 8 | 1.53 | 305 | 0.009 | |
| D | 5 | 5 (100) | 1263 | 0 | No data | No data | - | |
| Lymph node metastasis | Negative | 20 | 9 (45) | 632 | 11 | 75 | 8 | 0.015 |
| Positive | 24 | 16 (67) | 586 | 8 | 1.53 | 381 | 0.0002 | |
| Histological differentiation G stage | G1 | 2 | 1 (50) | 801 | 1 | 538 | 1.5 | NS |
| G2 | 11 | 3 (27) | 1216 | 8 | 1.5 | 802 | 0.018 | |
| G3 | 28 | 20 (71) | 538 | 8 | 166 | 3.65 | 0.015 | |
| G4 | 3 | 1 (33) | 699 | 2 | 92 | 8 | NS | |
| MSI status | MSS | 27 | 19 (70) | 7 | 8 | 1.7 | 4 | NS |
| MSI-L | 11 | 7 (64) | 8 | 4 | 2.4 | 3.3 | NS | |
| MSI-H | 6 | 4 (67) | 12 | 2 | 3.5 | 3.4 | NS | |
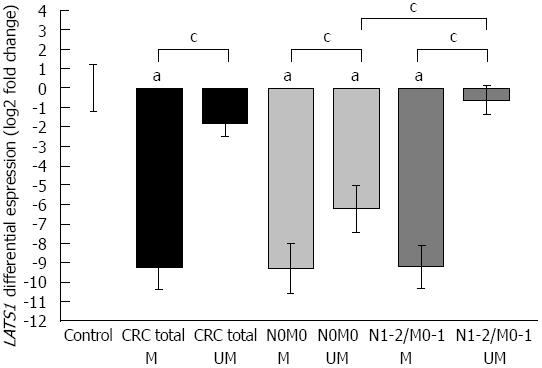
Comparison of LATS1 promoter methylation status with LATS1 mRNA levels in the analyzed tumor specimens (Table 2 and Figure 3) revealed a very significant reduction in LATS1 expression in hypermethylated cases vs non-hypermethylated cases (P < 0.05). The biggest difference was observed in Dukes’ C stage in which the LATS1 expression level was 305 times lower in methylated CRC samples than in unmethylated samples (P < 0.05, plot not shown). The lowest LATS1 ratio was found in Dukes’ D stage (1263 times lower than in control). However, we found that all analyzed samples in this subgroup showed a hypermethylation pattern, and thus, statistical comparison between methylated and unmethylated cases in D stage could not be evaluated. Interestingly, LATS1 expression in unmethylated CRC cases was not statistically different from that in controls.
When the methylation status of the LATS1 promoter was analyzed in patients with and without the presence of metastatic cells in regional lymph nodes and/or distant organs, we found a strong relationship between the metastatic potency of cancer and reduced expression of LATS1 and hypermethylation of its promoter. The LATS1 ratio was 381 times lower in hypermethylated vs unmethylated in metastatic CRC cases (P < 0.05, dark grey boxes in Figure 3, Table 2), and only 8 times lower in hypermethylated vs unmethylated in non-metastatic CRC cases (P < 0.05, light grey boxes in Figure 3, Table 2). Moreover the expression of LATS1 in unmethylated metastatic CRC samples was 49 times reduced as compared to unmethylated non-metastatic CRC cases (P < 0.05, Figure 3, Table 2).
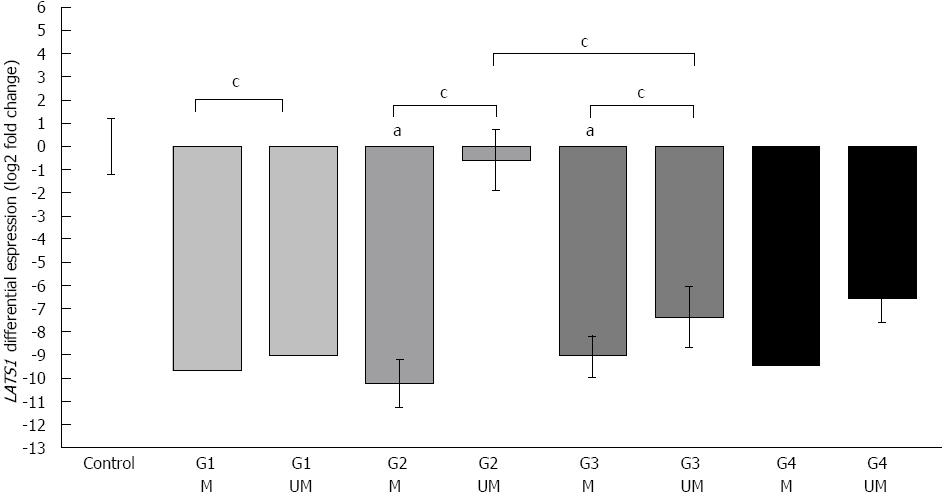
Comparison of the histological grading of CRC (G), methylation status, and LATS1 mRNA levels revealed the highest proportion of hypermethylation (71% of analyzed cases) in poorly differentiated (G3) CRC cases (Table 2). However, the difference in the LATS1 expression level in methylated cases was only ca. 4 times lower than in unmethylated cases in the G3 subgroup (P < 0.05, Table 2, Figure 4). On the contrary, the difference in the LATS1 mRNA level between methylated and unmethylated CRC tissue was much more pronounced in moderate-differentiated G2 cells (P < 0.05, Figure 4). Moreover, the LATS1 ratio in G2 unmethylated cases was not statistically different from that in control healthy patients. Interestingly, G3 unmethylated cases showed much lower (ca. × 100) LATS1 expression than G2 unmethylated biopsies (P < 0.05, Figure 4). Finally, we did not observe any statistically significant correlation between G grading and the LATS1 mRNA level or hypermethylation of its promoter.
When we focused on MSI and the LATS1 methylation status, we did not find any significant relationship because the statistical distribution of the results was very broad (Table 2, figure not shown). Most methylated cases (27/44) were considered MSS with no significant difference between methylated and unmethylated cases. MSI-L and MSI-H samples were also characterized as having relatively small differences in LATS1 expression between methylated and unmethylated cases.
LATS1 is a tumor suppressor gene involved in several important mitotic processes, which are crucial in the development of CRC[7,44]. The most recent data suggest that the SWH pathway may play a very important role in CRC progression[45]. LATS1 is a key transducer of this pathway, and reduced expression of LATS1 is connected with deregulation of SWH, thus activating the YAP oncogene[18]. Moreover, p53, a “genome guardian” protein, is indirectly regulated by LATS1[46]. MDM2, the regulator of p53 ubiquitination, is sequestered by native cellular LATS1, so that in the case of reduced LATS1 expression, degradation of p53 cannot be triggered by MDM2[46]. Those observations suggest that studies on the role of LATS1 in CRC should be intensified. Our investigation provides the first analysis of the LATS1 expression profile in a relatively large group of CRC patients compared with 40 healthy persons as well as analysis of LATS1 promoter hypermethylation as a putative quiescence factor for LATS1 expression in CRC. The results of our quantitative study, which demonstrated decreased LATS1 expression in 89% of CRC patients, are consistent with the decreased LATS1 expression found in other tumors[20-22]. However, Bianchini et al[26] reported 3.11-fold increased expression of LATS1 in 25 CRC patients compared with 13 non-cancerous adjacent tissue samples from the surgical margin. This discrepancy may be due to important methodological differences between the two studies. First, Bianchini et al[26] compared their CRC data to 13 non-cancerous adjacent tissue samples from the surgical margin, whereas in our study of 142 CRC (Dukes’ stages A-D) patients, the histologically normal mucosa of 40 healthy controls was used as a reference sample. Second, Bianchini et al[26] used the microarray technique to generate expression profiles of 19200 different transcripts normalized to glyceraldehyde 3-phosphate dehydrogenase expression in only Dukes’ B and C stage CRC patients. Seven transcripts are generated from LATS1 (Ensembl database), however, Bianchini et al[26] did not specify the isoform they analyzed. Our QPCR assay was designed to amplify the functional isoform of LAST1 that was also analyzed in other tumors[20-22]. Hence, our data cannot be directly compared with the contradictory results of Bianchini et al[26]. Moreover, we are not aware of any other reports suggesting increased LATS1 expression in cancer. Immunohistochemical analysis of LATS1 protein expression in gastric cancer revealed lower expression levels in 40 of 78 tumor lesions compared with normal gastric mucosa. The expression of LATS1 protein was significantly lower in gastric cancer with lymph node metastases than in cases without lymph node involvement[25]. Furthermore, in a group of 117 breast cancer patients, LATS1 mRNA was significantly decreased in the tumor tissue, and its decreased level was associated with a large tumor size, high lymph node metastasis rate, and poor prognosis[22]. In 30 astrocytoma cases, the level of LATS1 was 2-10 times lower as quantified by QPCR compared with 10 samples from normal brain tissue[21]. The most recent data showed that reduced expression of LATS1 was correlated with the occurrence of metastatic glioma and poor survival of patients in a group of 17 cases[47]. Hence, decreased expression of LATS1 in tumor tissue may suggest a suppressor role in CRC and other tumors.
MSI status has been regarded as one of the most important genetic markers and is strongly associated with molecular data, clinical findings, medical treatment, and patient outcome[48,49]. Our finding based on three BAT markers showed that more than half of the CRC patients had MSI tumors. Because we obtained samples from various clinics in different locations in Poland, our findings add to the observations by Smigiel et al[50] who observed MSI-L and MSI-H in 20% and 20.1% of cases, respectively, in a group of 143 CRC patients in the Lower Silesia region, which was not included in our analysis. The MSI phenotype may affect expression patterns of different proteins[51], and thus, we tried to estimate if decreased expression of LATS1 was associated with MSI. Our findings excluded MSI-L and MSI-H as factors that may affect LATS1 expression in the studied sample of CRC patients.
Inactivation of a typical tumor suppressor gene is generally induced by epigenetic factors such as mutation of one allele and/or loss of heterozygosity (LOH) of the other allele[52,53] or hypermethylation of CpG islands in the regulatory region of the gene[27-30]. Such factors may lead to a complete loss of gene function in cancer[54,55]. Expression of the LATS1 transcript can be epigenetically decreased by hypermethylation of CpG islands located within the 5’ upstream regulatory region of the gene[20-22]. Because LATS1 was reduced in several malignancies, we decided to assess the hypermethylation status of the LATS1 CpG island. Our study is the first report of the hypermethylation status of LATS1 in CRC, showing an association between hypermethylation and decreased LATS1 expression. LATS1 hypermethylation was observed in 17/30 (56%) breast cancers and was associated with decreased LATS1 expression; methylated cases showed a 3-fold decreased expression compared with unmethylated cases[22]. LATS1 hypermethylation was found in 13/54 (24%) cases of head and neck cancer[24] and in 64% (56/88) of astrocytomas[21]. Moreover, in astrocytomas, the methylation status was associated with decreased LATS1 expression[21]. A similar relationship between decreased LATS1 expression and its hypermethylation was observed in our study in 57% of analyzed CRC cases. Interestingly, other known epigenetic factors do not seem to be involved in reduced LATS1 expression in cancer. In a group of 25 breast cancers, LOH at 6q24-25.1 (LATS1 locus) was found in only one case (4%), whereas no mutation was found and only two gene polymorphisms were observed. However, neither polymorphism caused amino acid substitution[31]. As further support that hypermethylation may be the major epigenetic factor in LATS1 silencing, the expression of LATS1 in the hypermethylated cell lines U251 (an established glioma cell line) and SHG-44 (a human malignant glioma cell line) was restored by addition of 5-aza-deoxycytidine, and apoptosis of cancer cells results[21].
Decreased expression of LATS1 that is associated with promoter hypermethylation may contribute to suppression of the SWH pathway[10,11,13,18]. This pathway is prone to deregulation because few proteins involved in signal transduction are both tumor suppressors and oncoproteins. Altered expression of YAP, RASSF1A, LATS1, and MST2 in cancer cell lines leads to higher resistance of the cells to apoptosis[10,13,17,18]. Moreover, reduced expression of other genes that are not directly involved in the SWH pathway, such as WW and C2 domain containing 1 (KIBRA) and salvador homolog 1 (SAV1), may contribute to the quiescence of this pathway[11,16,56]. Such suppression of the SWH pathway is related to epithelial-to-mesenchymal transition features and poor prognosis in breast cancer[16].
In conclusion, this is the first study to show decreased expression of LATS1 in CRC, confirming its tumor suppressor function and linking its downregulation to the epigenetic hypermethylation of the LATS1 promoter region.
The authors would like to thank Professor Marian Smoczynski, Chair of the Department of Gastroenterology and Hepatology; Dr. Janusz Chybicki and Dr. Andrzej Babicki from the Department of General Surgery, Hospital Ministry Internal Affairs in Gdansk; and Dr. Sebastian Dobrowolski from the Department of General, Transplant and Endocrinological Surgery for providing tumor samples; the contribution of Dr. Michal Zmijewski and Dr. Tomasz Slebioda from the Department of Histology to the data analysis is also highly acknowledged.
Searching for new colorectal cancer (CRC) molecular markers is a very important objective, because CRC is one of the most common malignancies in the world and one of the most fatal of human neoplasms. The molecular mechanisms of CRC are still unknown, but deregulation of mitotic division as well as apoptosis resistance are clearly associated with CRC progression.
Human large tumor suppressor 1 (LATS1) encodes a serine/threonine kinase, which mediates a tumor suppressor pathway called the Salvador-Warts-Hippo (SWH) pathway. Abnormal expression of LATS1 was observed in some tumors, and its expression in CRC has not been analyzed quantitatively.
This is the first study of a large group of CRC patients that shows quantitatively reduced LATS1 expression at the mRNA level. Decreased levels of LATS1 were strongly associated with hypermethylation of its promoter, particularly in metastatic tumors.
With knowledge regarding the decreased expression of LATS1 in CRC, focusing on its intracellular signaling pathways in CRC and the probable involvement of this gene in CRC pathogenesis as a molecular marker may be possible.
LATS1 is a putative tumor suppressor gene that shows reduced expression in several malignancies. LATS1 is important in karyo- and cytokinesis and is part of the SWH pathway. Hypermethylation of the LATS1 promoter is a common epigenetic factor responsible for downregulation and silencing of this gene.
The authors collected and processed samples of CRC tumors and control colon biopsies from four collaborative clinics from four different regions of Poland. Molecular quantitative assays based on quantitative polymerase chain reaction revealed strongly reduced expression of LATS1 in CRC tumors. Furthermore, this downregulation was strongly associated with the occurrence of hypermethylation of the LATS1 promoter but not with microsatellite instability. This observation confirms the suppressor role of LATS1 in carcinogenesis in this first study on a large group of CRC patients.
P- Reviewer Bujanda L S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11810] [Article Influence: 843.6] [Reference Citation Analysis (4)] |
| 2. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. [PubMed] |
| 3. | Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA. 2003;289:1288-1296. [PubMed] |
| 4. | St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053-1063. [PubMed] |
| 6. | Iida S, Hirota T, Morisaki T, Marumoto T, Hara T, Kuninaka S, Honda S, Kosai K, Kawasuji M, Pallas DC. Tumor suppressor WARTS ensures genomic integrity by regulating both mitotic progression and G1 tetraploidy checkpoint function. Oncogene. 2004;23:5266-5274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Tao W, Zhang S, Turenchalk GS, Stewart RA, St John MA, Chen W, Xu T. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005;65:6568-6575. [PubMed] |
| 9. | Yang X, Yu K, Hao Y, Li DM, Stewart R, Insogna KL, Xu T. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol. 2004;6:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Hergovich A, Hemmings BA. Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors. 2009;35:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Liu AM, Wong KF, Jiang X, Qiao Y, Luk JM. Regulators of mammalian Hippo pathway in cancer. Biochim Biophys Acta. 2012;1826:357-364. [PubMed] |
| 12. | Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011;350:255-266. [PubMed] |
| 13. | Zhang X, Milton CC, Humbert PO, Harvey KF. Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res. 2009;69:6033-6041. [PubMed] |
| 14. | Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182-191. [PubMed] |
| 15. | Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188-192. [PubMed] |
| 16. | Moleirinho S, Chang N, Sims AH, Tilston-Lünel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy C, Faratian D. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene. 2013;32:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496-5509. [PubMed] |
| 18. | Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O’neill E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962-975. [PubMed] |
| 19. | Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001;20:6516-6523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Hisaoka M, Tanaka A, Hashimoto H. Molecular alterations of h-warts/LATS1 tumor suppressor in human soft tissue sarcoma. Lab Invest. 2002;82:1427-1435. [PubMed] |
| 21. | Jiang Z, Li X, Hu J, Zhou W, Jiang Y, Li G, Lu D. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci Res. 2006;56:450-458. [PubMed] |
| 22. | Takahashi Y, Miyoshi Y, Takahata C, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380-1385. [PubMed] |
| 23. | Siam R, Harada R, Cadieux C, Battat R, Vadnais C, Nepveu A. Transcriptional activation of the Lats1 tumor suppressor gene in tumors of CUX1 transgenic mice. Mol Cancer. 2009;8:60. [PubMed] |
| 24. | Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep. 2009;22:1519-1526. [PubMed] |
| 25. | Xu ZP, Zhu JS, Zhang Q, Wang XY. A breakdown of the Hippo pathway in gastric cancer. Hepatogastroenterology. 2011;58:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Bianchini M, Levy E, Zucchini C, Pinski V, Macagno C, De Sanctis P, Valvassori L, Carinci P, Mordoh J. Comparative study of gene expression by cDNA microarray in human colorectal cancer tissues and normal mucosa. Int J Oncol. 2006;29:83-94. [PubMed] |
| 27. | Demokan S, Dalay N. Role of DNA methylation in head and neck cancer. Clin Epigenetics. 2011;2:123-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Dumitrescu RG. Epigenetic markers of early tumor development. Methods Mol Biol. 2012;863:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Draht MX, Riedl RR, Niessen H, Carvalho B, Meijer GA, Herman JG, van Engeland M, Melotte V, Smits KM. Promoter CpG island methylation markers in colorectal cancer: the road ahead. Epigenomics. 2012;4:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Khare S, Verma M. Epigenetics of colon cancer. Methods Mol Biol. 2012;863:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Morinaga N, Shitara Y, Yanagita Y, Koida T, Kimura M, Asao T, Kimijima I, Takenoshita S, Hirota T, Saya H. Molecular analysis of the h-warts/LATS1 gene in human breast cancer. Int J Oncol. 2000;17:1125-1129. [PubMed] |
| 32. | Wierzbicki PM, Dubowska J, Adrych K, Kartanowicz D, Dobrowolski S, Stanislawowski M, Chybicki J, Korybalski B, Godlewski J, Mazurek A. Promoter hypermethylation and underexpression of PLAGL1 and LATS1 tumor suppressor genes in colorectal cancer. Imaging of cell dynamics. Gdansk: Folia Histochem Cytobiol 2008; 78. |
| 33. | Cavalieri D, Dolara P, Mini E, Luceri C, Castagnini C, Toti S, Maciag K, De Filippo C, Nobili S, Morganti M. Analysis of gene expression profiles reveals novel correlations with the clinical course of colorectal cancer. Oncol Res. 2007;16:535-548. [PubMed] |
| 34. | McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, Olson JJ, Mikkelsen T, Lehman N, Aldape K, Yung WK, Bogler O, Weinstein JN,VandenBerg S, Berger M, Prados M, Muzny D, Morgan M, Scherer S, Sabo A, Nazareth L, Lewis L, Hall O, Zhu Y, Ren Y, Alvi O, Yao J, Hawes A, Jhangiani S,Fowler G, San Lucas A, Kovar C, Cree A, Dinh H, Santibanez J, Joshi V, Gonzalez-Garay ML, Miller CA, Milosavljevic A, Donehower L, Wheeler DA, Gibbs RA,Cibulskis K, Sougnez C, Fennell T, Mahan S, Wilkinson J, Ziaugra L, Onofrio R, Bloom T, Nicol R, Ardlie K, Baldwin J, Gabriel S, Lander ES, Ding L, Fulton RS,McLellan MD, Wallis J, Larson DE, Shi X, Abbott R, Fulton L, Chen K, Koboldt DC, Wendl MC, Meyer R, Tang Y, Lin L, Osborne JR, Dunford-Shore BH, Miner TL,Delehaunty K, Markovic C, Swift G, Courtney W, Pohl C, Abbott S, Hawkins A, Leong S, Haipek C, Schmidt H, Wiechert M, Vickery T, Scott S, Dooling DJ,Chinwalla A, Weinstock GM, Mardis ER, Wilson RK, Getz G, Winckler W, Verhaak RG, Lawrence MS, O'Kelly M, Robinson J, Alexe G, Beroukhim R, Carter S,Chiang D, Gould J, Gupta S, Korn J, Mermel C, Mesirov J, Monti S, Nguyen H, Parkin M, Reich M, Stransky N, Weir BA, Garraway L, Golub T, Meyerson M, Chin L,Protopopov A, Zhang J, Perna I, Aronson S, Sathiamoorthy N, Ren G, Yao J, Wiedemeyer WR, Kim H, Kong SW, Xiao Y, Kohane IS, Seidman J, Park PJ,Kucherlapati R, Laird PW, Cope L, Herman JG, Weisenberger DJ, Pan F, Van den Berg D, Van Neste L, Yi JM, Schuebel KE, Baylin SB, Absher DM, Li JZ,Southwick A, Brady S, Aggarwal A, Chung T, Sherlock G, Brooks JD, Myers RM, Spellman PT, Purdom E, Jakkula LR, Lapuk AV, Marr H, Dorton S, Choi YG, Han J, Ray A, Wang V, Durinck S, Robinson M, Wang NJ, Vranizan K, Peng V, Van Name E, Fontenay GV, Ngai J, Conboy JG, Parvin B, Feiler HS, Speed TP, Gray JW, Brennan C, Socci ND, Olshen A, Taylor BS, Lash A, Schultz N, Reva B, Antipin Y, Stukalov A, Gross B, Cerami E, Wang WQ, Qin LX, Seshan VE, Villafania L,Cavatore M, Borsu L, Viale A, Gerald W, Sander C, Ladanyi M, Perou CM, Hayes DN, Topal MD, Hoadley KA, Qi Y, Balu S, Shi Y, Wu J, Penny R, Bittner M,Shelton T, Lenkiewicz E, Morris S, Beasley D, Sanders S, Kahn A, Sfeir R, Chen J, Nassau D, Feng L, Hickey E, Barker A, Gerhard DS, Vockley J, Compton C,Vaught J, Fielding P, Ferguson ML, Schaefer C, Zhang J, Madhavan S, Buetow KH, Collins F, Good P, Guyer M, Ozenberger B, Peterson J, Thomson E; Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5519] [Cited by in RCA: 5951] [Article Influence: 350.1] [Reference Citation Analysis (0)] |
| 35. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] |
| 36. | Wierzbicki PM, Adrych K, Kartanowicz D, Dobrowolski S, Stanislawowski M, Chybicki J, Godlewski J, Korybalski B, Smoczynski M, Kmiec Z. Fragile histidine triad (FHIT) gene is overexpressed in colorectal cancer. J Physiol Pharmacol. 2009;60 Suppl 4:63-70. [PubMed] |
| 37. | Wierzbicki PM, Adrych K, Kartanowicz D, Wypych J, Stanislawowski M, Zwolinska-Wcislo M, Celinski K, Skrodzka D, Godlewski J, Korybalski B. Overexpression of the fragile histidine triad (FHIT) gene in inflammatory bowel disease. J Physiol Pharmacol. 2009;60 Suppl 4:57-62. [PubMed] |
| 38. | Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, Brittner B, Ludwig B, Schilling M. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101-109. [PubMed] |
| 39. | Niu Y, Yeh S, Miyamoto H, Li G, Altuwaijri S, Yuan J, Han R, Ma T, Kuo HC, Chang C. Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ARA70-regulated androgen receptor transactivation. Cancer Res. 2008;68:7110-7119. [PubMed] |
| 40. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] |
| 41. | Wierzbicki PM, Adrych K, Kartanowicz D, Wypych J, Stanislawowski M, Dobrowolski S, Chybicki J, Zwolinska-Wcislo M, celinski K, Korybalski B. Microsatellite instability status in inflammatory bowel disease and colorectal cancer. Ann Acad Med Gedan. 2009;39:163-171. |
| 42. | Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 339] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 43. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS, To KF, Chan FK, Cho CH, Sung JJ, Yu J. Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit Rev Oncol Hematol. 2013;86:251-277. [PubMed] |
| 46. | Matallanas D, Romano D, Al-Mulla F, O’Neill E, Al-Ali W, Crespo P, Doyle B, Nixon C, Sansom O, Drosten M. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol Cell. 2011;44:893-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Ji T, Liu D, Shao W, Yang W, Wu H, Bian X. Decreased expression of LATS1 is correlated with the progression and prognosis of glioma. J Exp Clin Cancer Res. 2012;31:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Laghi L, Malesci A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig Dis. 2012;30:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Manne U, Shanmugam C, Katkoori VR, Bumpers HL, Grizzle WE. Development and progression of colorectal neoplasia. Cancer Biomark. 2010;9:235-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Smigiel R, Stembalska A, Stal A, Jonkisz A, Trusewicz A, Dobosz T, Grzebieniak Z, Sasiadek M. The Microsatellite Instability in Patients with Colon Cancer Treated in Lower Silesia. Adv Clin Exp Med. 2006;15:29-36. |
| 51. | Birkenkamp-Demtroder K, Mansilla F, Sørensen FB, Kruhøffer M, Cabezón T, Christensen LL, Aaltonen LA, Verspaget HW, Ørntoft TF. Phosphoprotein Keratin 23 accumulates in MSS but not MSI colon cancers in vivo and impacts viability and proliferation in vitro. Mol Oncol. 2007;1:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Mizoguchi M, Kuga D, Guan Y, Hata N, Nakamizo A, Yoshimoto K, Sasaki T. Loss of heterozygosity analysis in malignant gliomas. Brain Tumor Pathol. 2011;28:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Beaty MW, Quezado M, Sobel ME, Duray P, Merino MJ. Loss of heterozygosity on chromosome 1 and 9 and hormone receptor analysis of metastatic malignant melanoma presenting in breast. Int J Surg Pathol. 2005;13:9-18. [PubMed] |
| 54. | Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19-27. [PubMed] |
| 55. | Ashktorab H, Schäffer AA, Daremipouran M, Smoot DT, Lee E, Brim H. Distinct genetic alterations in colorectal cancer. PLoS One. 2010;5:e8879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Matsuura K, Nakada C, Mashio M, Narimatsu T, Yoshimoto T, Tanigawa M, Tsukamoto Y, Hijiya N, Takeuchi I, Nomura T. Downregulation of SAV1 plays a role in pathogenesis of high-grade clear cell renal cell carcinoma. BMC Cancer. 2011;11:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |