Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3150
Revised: February 3, 2013
Accepted: February 28, 2013
Published online: May 28, 2013
AIM: To investigate the effects of Lactobacillus plantarum (L. plantarum) CAI6 and L. plantarum SC4 on hyperlipidemic mice.
METHODS: Male Kunming mice were fed a high-cholesterol diet for 28 d to construct hyperlipidemic models. Hyperlipidemic mice and normal mice were assigned to 3 groups which were separately treated with L. plantarum CAI6, L. plantarum SC4, and physiological saline through oral gavage for 28 d. Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured by commercially available enzyme kits. FACS Calibur flow cytometry was used to examine hepatic and renal nuclear factor-erythroid 2-related factor 2 (Nrf2) expression. The morphology of livers was checked by hematoxylin and eosin staining and optical microscope observation.
RESULTS: Compared with normal mice, hyperlipidemic mice possessed significantly higher TC (3.50 ± 0.43 vs 2.89 ± 0.36, P < 0.01), TG (1.76 ± 0.07 vs 1.10 ± 0.16, P < 0.01), and LDL-C (1.72 ± 0.20 vs 0.82 ± 0.10, P< 0.01) levels, resulting in an increase of atherogenic index (AI) (2.34 ± 1.60 vs 0.93 ± 0.55, P < 0.05) and LDL-C/HDL-C ratio (1.43 ± 0.12 vs 0.51 ± 0.16, P < 0.05). After treatment with L. plantarum CAI6/L. plantarum SC4, TG (1.43 ± 0.27/1.54 ± 0.10 vs 1.76 ± 0.07, P < 0.01/P < 0.05) and LDL-C (1.42 ± 0.07/1.47 ± 0.12 vs 1.72 ± 0.20, P < 0.01/P < 0.01) in hyperlipidemic mice significantly decreased. In addition, TC, HDL-C, AI, and LDL-C/HDL-C ratio were all positively changed. Meanwhile, the treatment markedly alleviated hepatic steatosis and significantly stimulated Nrf2 expression (73.79 ± 0.80/72.96 ± 1.22 vs 54.94 ± 1.84, P < 0.01/P < 0.01) in hepatocytes of hyperlipidemic mice.
CONCLUSION: L. plantarum CAI6 and L. plantarum SC4 may protect against cardiovascular disease by lipid metabolism regulation and Nrf2-induced antioxidative defense in hyperlipidemic mice.
Core tip: Protective effects of Lactobacillus plantarum (L. plantarum) CAI6 and L. plantarum SC4 strains on hyperlipidemic mice were found, including regulating lipid metabolism, alleviating hepatic steatosis and reducing cardiovascular disease (CVD) risk. The mechanism of intracorporal antioxidation of Lactobacillus strains may be related to stimulation of nuclear factor-erythroid 2-related factor 2 (Nrf2) expression. Hypolipidemic effect and Nrf2-induced antioxidative defense may contribute to the reduction of CVD risk. We suggest that food fermented by the strains be used as part of the diet to relieve lipid metabolism related metabolic syndrome and to reduce the risk of CVD.
-
Citation: Wang LX, Liu K, Gao DW, Hao JK. Protective effects of two
Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol 2013; 19(20): 3150-3156 - URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3150
Metabolic syndrome is a cluster of cardiovascular and type 2 diabetic risk factors[1]. The International Diabetes Federation defines metabolic syndrome as central obesity plus any two of raised triglycerides (TG), reduced high-density lipoprotein cholesterol (HDL-C), raised blood pressure, and raised fasting plasma glucose[2]. Disturbances of lipid homeostasis lead to metabolic syndrome and increase the risk of cardiovascular disease. A 1% increase in plasma cholesterol levels can increase the risk of coronary events up to 3%[3]. Elevated low-density lipoprotein cholesterol (LDL-C) accompanied with insufficient HDL-C is a risk predictor of atherosclerosis[4]. Hypercholesterolemia stimulates formation of free radicals, decreases activity of antioxidant enzymes, and eventually leads to elevated lipid peroxides including oxidized low density lipoprotein (Ox-LDL), which is an independent atherogenic factor[5].
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a transcription factor that binds to antioxidant response elements (ARE) in the promoter regions of many antioxidative enzymes and phase II detoxifying enzymes[6]. Oxidative stress results in accumulation of Nrf2 in the cytoplasm and initiation of transcription of target genes after translocating into the nucleus[7]. Inhibition of LDL oxidation can protect against oxidative stress linked atherosclerosis. Belghitha found that bacterial levan was beneficial for antiarteriosclerosis hypolipidemic and antioxidant effects[8]. We suppose that both exogenous antioxidants and endogenous antioxidant enzymes can strengthen antioxidative defense. Therefore, antioxidant defense triggered by improving Nrf2 expression can be envisaged as a promising strategy in reducing the incidence of atherosclerosis.
Some strains of Lactobacillus have been studied as probiotics. They may protect against mutagens and carcinogen exposure, gastrointestinal disease, skin disorders, yeast infections, urinary tract infection, diabetes[9], and immune dysfunction[10]. Hypolipidemic effects of LAB were shown in mice[11,12] and rabbits[13]. Antioxidative activity of LAB, a potential application valued in prevention of atherosclerosis, was also reported in vitro[14] and in vivo[15]. Extracorporeal antioxidative activity of Lactobacillus casei KCTC 3260 may be caused by chelating metal ions[14]. However, an intracorporal antioxidative mechanism of LAB has not been defined. In the present study, we investigated the change in Nrf2 expression in mice after the application of Lactobacillus plantarum (L. plantarum) CAI6 or L. plantarum SC4 to discuss the possible mechanism.
Probiotic strains may have acid or bile salt tolerance to colonize the gastrointestinal tract of the host. Several tests have been taken to screen strains tolerating low pH and high concentration of cholate[16]. Cells have been coated with sodium alginate to increase viscosity and stability of strains[17].
The primary aim of this study was to investigate the effects of L. plantarum CAI6 and L. plantarum CS4 on alleviation of metabolic syndrome and prevention of cardiovascular disease (CVD) in high-cholesterol fed mice.
L. plantarum CAI6 (GenBank accession number: KC470704) and L. plantarum SC4 (GenBank accession number: KC470705) were isolated from Chinese pickled cabbage and raw milk, respectively. The two strains could survive in MRS medium (pH 3.0) supplemented with 0.3% sodium taurocholate (date not shown). The strains were cultured in MRS broth and incubated at 37 °C for 24 h. Each activated culture was centrifuged and diluted with 0.9% saline water to obtain a preparation of 2.0 × 109 cfu/mL. 0.1% (w/v) sodium alginate was added to make the strains have better acid resistance, bile resistance, and stability in digestive tract.
Thirty-Six adult male Kunming mice with a weight of 20 ± 2 g were obtained from the Academy of Military Medical Sciences (Beijing, China). The mice were housed in a controlled animal room at 20 ± 2 °C and 60% relative humidity with 12 h light/dark photoperiod. Diet and water could be accessed freely. The care and handling of the animals were in compliance with the internationally accepted standard guidelines for use of animals, and was approved by Science and Technology Committee of Yanshan University on Animal Care and Use.
Half of the mice were fed a high-cholesterol diet (75%basic diet, 10%lard, 10%soybean meal, 5%egg yolk) for a period of up to 28 d to construct a hyperlipidemic model (HM). For comparison, the other mice were maintained on standard laboratory diet for 28 d to develop a normal model (NM). Then the mice of HM and NM were randomly assigned to three groups (n = 6), respectively.
Group I (NM): normal mice fed a standard laboratory diet and physiological saline (10 mL/kg); Group II (NM/CAI6): normal mice fed a standard laboratory diet and L. plantarum CAI6 (10 mL/kg); Group III (NM/SC4): normal mice fed a standard laboratory diet and L. plantarum SC4 (10 mL/kg); Group IV (HM): hyperlipidemic mice fed a high-cholesterol diet and physiological saline (10 mL/kg); Group V (HM/CAI6): hyperlipidemic mice fed a high-cholesterol diet and L. plantarum CAI6 (10 mL/kg); and Group VI (HM/SC4): hyperlipidemic mice fed a high-cholesterol diet and L. plantarum SC4 (10 mL/kg).
The mice were treated by intragastric administration for 28 d before being anesthetized with chloral hydrate and sacrificed. Food and water consumption and body weight were recorded daily. Blood samples were drawn from the ophthalmic venous plexus. After centrifugation (2000 rmp, 10 min, 4 °C), the serum samples were collected and stored at -20 °C. The liver and kidney were excised, rinsed in ice-cold physiological saline, weighed, and then stored at -20 °C.
Serum TC, TG, HDL-C, and LDL-C levels were measured using commercially available enzyme kits (Beihuakangtai clinical reagent Ltd, Beijing, CHN). The TC level was measured with an enzymatic method based on the conversion to a chromogen with maximum absorption at 500 nm by cholesterol ester hydrolase, cholesterol oxidase, and peroxidase[18]. The determination of TG was based on an enzymatic method coupled with lipase, glycerokinase, glycerol oxidase and peroxidase[19]. The HDL-C level was assayed by the same enzymatic method based on specific precipitation of VLDL-C and LDL-C in the presence of phosphotungstic acid. The LDL-C value was calculated by following formula: LDL-C = TC-HDL-C-TG/5[20].
Fresh livers of mice were fixed with 4% paraformaldehyde for 24 h, dehydrated gradually in a graded series of ethanol, clarified in xylene, and embedded in paraffin wax. Hematoxylin and eosin stained liver was observed by an optical microscope (X5Z-G, Chongqing Optical and Electrical Instrument Co., Ltd, CHN).
Liver and kidney tissues suspensions were prepared in ice-cold 0.1 mol/L PBS. Antibodies were diluted in 0.1 mol/L PBS containing 0.1%NaN3. 106 cells/mL were incubated with primary anti-Nrf2 antibodies for 30 min on ice, mixed with 200 μL of 4 % paraformaldehyde, and incubated for 30 min at 4 °C. After washing twice with 0.1 mol/L PBS containing 0.1%NaN3, the supernatant was discarded by centrifugation. 3%BSA, 0.1%NaN3 and 10% saponin (Sigma Co., United States) were added to cell pellets and blended for 15 min, then mixed with 500 μL PBS (pH 7.4). After removal of the supernatant, 0.5 μg of secondary FITC-conjugated rabbit anti-mouse antibody were added and incubated on ice for 60 min. Finally, the cells were resuspended in 1 mL of 0.1 mol/L PBS. The hepatocytes and nephrocytes were scanned using a FACSCalibur (Becton-Dickinson, United States) respectively, and fluorescence of Nrf2 positive cells were quantified. Nonspecific binding of secondary antibody was excluded by incubating the cells only with the FITC-labelled secondary antibody. For reproducibility, the experiment was repeated three times. The software BD CellQuest Pro (Becton Dickinson Biosciences, United States) was used and the data were calculated by fluorescence intensity formula [I = Log (x-mode) × 340].
All data were expressed as mean ± SD. Statistical analysis was performed using SPSS 13.0 software. Differences between the groups were analyzed by One-Way ANOVA followed by Duncan’s multiple range tests. Statistical significance was considered at the P < 0.05 level.
As shown in Table 1, the mice subjected to a high cholesterol diet had a significant increase in body weight (BW) compared with mice on a normal diet (P < 0.01). In HM mice, oral administration of CAI6 or SC4 daily did not have any significant effect on BW, while CAI6-treated mice and SC4-treated mice had a significant (P < 0.01 and P < 0.05, respectively) increase vs the NM group. In NM mice, CAI6 or SC4 had no significant effect on BW compared with NM mice receiving neither CAI6 or SC4. None of the groups showed a significant difference in total dietary intake or liver index. However, an increase in liver index in the HM group compared to the NM group was found, and CAI6 and CS4 decreased this.
| Group | NM | NM/CAI6 | NM/SC4 | HM | HM/CAI6 | HM/SC4 |
| Body weight, food intake, and liver index of normal model and hyperlipidemic model mice after treatment for 30 d | ||||||
| IBW (g) | 26.53 ± 2.96 | 25.47 ± 2.71 | 27.03 ± 2.09 | 33.17 ± 1.44b | 31.32 ± 1.06b | 30.78 ± 1.66b |
| FBW (g) | 33.43 ± 4.78d | 28.50 ± 3.56d | 32.30 ± 2.73d | 39.05 ± 2.50b | 36.68 ± 2.22 | 38.08 ± 1.87a |
| TFI (g) | 44.76 ± 6.94 | 47.07 ± 4.04 | 47.05 ± 2.51 | 52.29 ± 11.97 | 51.09 ± 6.34 | 53.32 ± 13.14 |
| 1LI (%) | 4.11 ± 0.30 | 4.08 ± 0.15 | 4.10 ± 0.49 | 4.65 ± 0.50 | 4.41 ± 0.58 | 4.54 ± 0.64 |
| Serum lipid levels in the different groups of mice | ||||||
| TC (mmol/L) | 2.89 ± 0.36d | 2.60 ± 0.24c | 2.85 ± 0.28a | 3.50 ± 0.43b | 3.16 ± 0.35 | 3.27 ± 0.36 |
| TG (mmol/L) | 1.10 ± 0.16d | 1.06 ± 0.15d | 1.08 ± 0.13d | 1.76 ± 0.07b | 1.43 ± 0.27d | 1.54 ± 0.10a |
| HDL-C (mmol/L) | 1.62 ± 0.52 | 1.76 ± 0.48a | 1.67 ± 0.14 | 1.20 ± 0.42 | 1.36 ± 0.55 | 1.31 ± 0.55 |
| LDL-C (mmol/L) | 0.82 ± 0.10d | 0.75 ± 0.07 | 0.81 ± 0.09 | 1.72 ± 0.20b | 1.42 ± 0.07d | 1.47 ± 0.12d |
| 2AI | 0.93 ± 0.55a | 0.57 ± 0.41d | 0.71 ± 0.09d | 2.34 ± 1.60a | 1.65 ± 0.99 | 1.94 ± 1.47 |
| LDL-C/HDL-C | 0.51 ± 0.16a | 0.43 ± 0.19d | 0.49 ± 0.04d | 1.43 ± 0.12a | 1.04 ± 0.16 | 1.12 ± 0.14 |
Effect of the treatment on serum lipid levels was shown in Table 1. The TC, TG and LDL-C levels of mice in the HM group showed a significant increase (P < 0.01) compared with the NM group. Meanwhile, HDL-C in the HM group decreased by 26% compared to the NM group. Moreover, a significantly increased (P < 0.05) atherogenic index (AI) accompanied with a significantly reduced (P < 0.05) LDL-C/HDL-C ratio was observed in the HM group. The HM mice orally administrated with strains (CAI6/SC4) showed different degrees of decline in serum TG (-19%, P < 0.01/-13%, P < 0.05), TC (-10%/-7%), LDL-C (-17%, P < 0.01/-15%, P < 0.01) levels, and an increase in HDL-C (+13%/+9%) level as compared to the HM group that did not receive CAI6 or SC4. As a result, those mice had a lower AI (-29%/-17%) and a higher LDL-C/HDL-C ratio (+23%/+11%) than the HM group receiving no treatment. As for the NM mice, the administration had no significant effect on serum TC, TG, HDL-C and LDL-C levels, but could reduce AI (-39%/-24%) and increase LDL-C/HDL-C ratio (+21%/+5%).
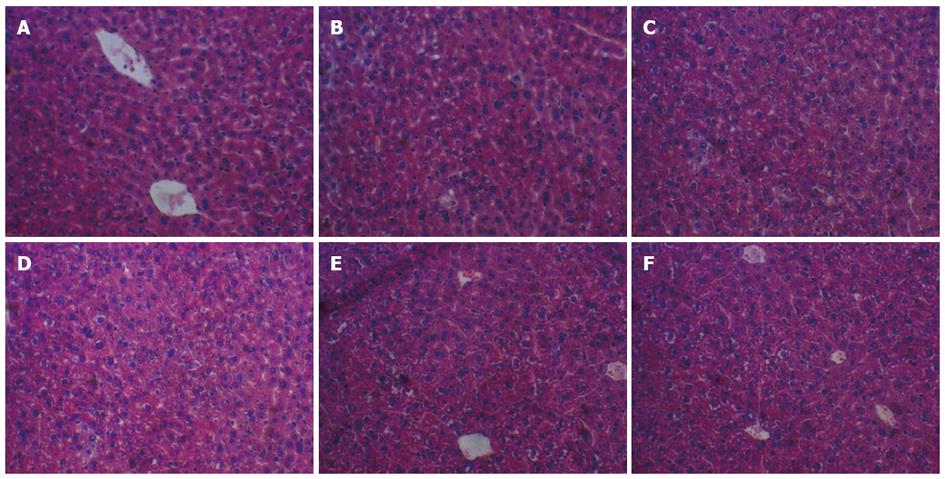
The livers of the HM group were larger compared with those in the NM group and became beige. The hepatic cells with clear cytoplasm, nucleus, nucleolus and central vein (A) in the NM, NM/CAI6 and NM/SC4 group, showed normal histology in Figure 1A-C[21]. The liver sections of the HM mice exhibited massive fatty changes and severe steatosis with cytoplasmic vacuoles confirmed by histopathological examination in Figure 1D. In addition, the size of lipid droplets in the HM/CAI6 and NM/SC4 groups were remarkably smaller than those of the HM group (Figure 1E, F), suggesting that the L. plantarum strains could reduce the build-up of lipid droplets and keep hepatocytes normal.
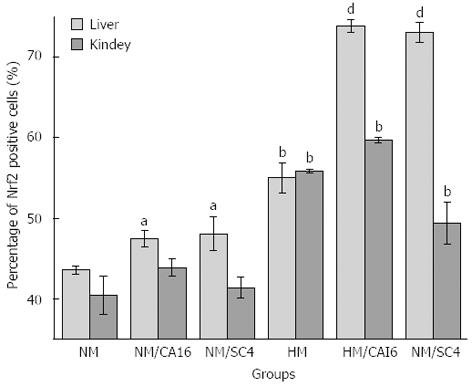
Nrf2 expression in liver and kidney tissues was shown in Figure 2. Liver/kidney Nrf2 levels were significantly higher (+26%, P < 0.01/+38%, P < 0.01) in the HM group than the NM group. Liver Nrf2 increased significantly in the HM/CAI6 (+34%, P < 0.01) and HM/SC4 (+33%, P < 0.01) groups respectively, compared with the HM group. A significant increase in liver Nrf2 level was also found in the NM/CAI6 (+9%, P < 0.05) and NM/SC4 (+10%, P < 0.05 ) groups as compared to the NM group. Contrary to the situation in the liver, the HM/CAI6 or HM/SC4 groups showed no significant increase in kidney Nrf2 level.
The mice in the HM group possessed raised TG, reduced HDL-C, and elevated BW resulting in a high risk of developing metabolic syndrome. Accumulation of TG and TC in the bloodstream has been proven related to atherosclerosis, and by extension, the risk of coronary heart disease and stroke. Elevated concentrations of oxidized LDL-C are associated with artherosclerotic plaque formation on the walls of arteries, but increased HDL-C levels may reduce the risk due to the ability of HDL transporting cholesterol back to the liver for excretion or to other tissues[22]. Therefore, the HM mice had an increased risk of CVD. The results showed that both AI, an indicator for severity of atherosclerosis[23], and LDL-C/HDL-C, a valuable tool to evaluate coronary heart disease (CHD) risk[24], were increased in HM mice, which is in agreement with the above view.
Serum TG, TC and LDL-C levels decreased in the HM/CAI6 and HM/SC4 groups when compared with the HM group while serum HDL-C level increased revealing that L. plantarum CAI6 and L. plantarum SC4 exerted a hypolipidemic effect and could relieve lipid related metabolic syndrome. AI and LDL-C/HDL-C ratios were also lower in the HM/CAI6 and HM/SC4 groups, suggesting that L. plantarum CAI6 and L. plantarum SC4 reduced the risk of CVD. Non-HDL-C, representing the total amount of cholesterol in the potentially atherogenic lipoproteins, was regarded as a risk predictor of cardiovascular outcomes on drug treatment[25]. After strains (CAI6/SC4) treatment, non-HDL-C decreased by 22% (1.80 vs 2.30) and 15% (1.96 vs 2.30) respectively. Additionally, NM mice treated with strains (CAI6/SC4) had positive changes in AI (-39%/-24%) and LDL-C/HDL-C ratio (-16%/-4%). So, we suppose that the strains have preventive and therapeutic effects for CVD.
The liver is the hub of fat synthesis and transportation. The liver can use fatty acids hydrolyzed from fat in food to synthetize cholesterol and TG. High fat intake of HM mice disequilibrated lipid metabolism, resulting in accumulation of TG in liver and an increased increment of the liver index, and hepatic steatosis occurred[26]. The result of liver tectology proved that the L. plantarum strains had important potential in alleviating hepatic steatosis attributed to mediation of lipid metabolism and had protective effects on hepatic structure.
Oxidative stress is thought to be linked to atherogenesis[27]. Therefore, much attention has been paid to the antioxidant hypothesis in prevention and treatment of CVD[28,29]. In recent times, antioxidant activity of many hypolipidemic substances has been detected and the activities of SOD, CAT and GSH-Px are usually used as test standards[15,30]. To suffer ROS-mediated injury, cells initiate the Nrf2/ARE signaling pathway stimulated by Nrf2. Giovanni. E reported that the pathway plays an important role in vascular homeostasis and the defense of endothelial and smooth muscle cells against sustained oxidative stress associated with diseases such as atherosclerosis and preeclampsia[31]. Our results showed that liver and kidney Nrf2 expression was significantly (P < 0.01) increased in the HM group compared with the NM group, revealing that a cholesterol-rich diet may bring about remarkable modifications in antioxidant defense mechanisms in vivo. Furthermore, oral administration of L. plantarum CAI6 and L. plantarum SC4 both could significantly (P < 0.01) increase liver Nrf2 levels compared with the HM group. So we envisage that spontaneous and strain-stimulated complex mechanisms conjointly promote Nrf2 expression in the liver. Accumulation of Nrf2 in the liver may initiate anti-oxidative stress and result in inhibition of Ox-LDL production, reducing the risk of atherosclerosis. Combining all the analyses above, the anti-CVD mechanism of the strains may be a regulation of lipid metabolism to alleviate metabolic syndrome and a stimulation of Nrf2 expression to initiate antioxidant defense. The actual mechanisms which account for strain-stimulated Nrf2 expression and the transcription and expression of antioxidase genes in Nrf2/ARE pathway need further study.
Dietary management has been shown to be an effective way to alleviate metabolic syndrome[32,33]. Some LAB-fermented probiotic products are commercially available[34]. Jeun et al[11] found that live L. plantarum was more effective than dead L. plantarum in reduction of plasma lipid levels and supposed that the active compounds may be derived from metabolic activity of live L. plantarum. Adding fermented foods seems like a good way to increase the number of live bacteria in a diet. LAB-fermented food, including both live LAB and active metabolin, seems the best form to apply LAB in our daily lives. The strains L. plantarum CAI6 and L. plantarum SC4, which can be regarded as probiotics for their beneficial impacts, were isolated from milk and pickle, respectively. Therefore, L. plantarum CAI6-fermented milk or L. plantarum SC4-fermented pickle may also exert those effects. We suppose that the strains-fermented food may be used as a dietary approach to alleviate metabolic syndrome and prevent CVD.
Hyperlipidemia, an integral part of metabolic syndrome, is a major risk factor for cardiovascular diseases (CVD). Oxidation of low-density lipoprotein (LDL) cholesterol is linked to atherogenesis, and antioxidant drugs become a therapeutic approach. Lactobacillus regarded as probiotics have been proven to possess hypolipidemic and antioxidant effects. A probiotic diet, a kind of dietary management, for example fermented food, is a promising way to prevent and treat CVD.
CVD remains a major public health problem. The relationship between cardiovascular risk and serum lipid and related factors has been extensively studied to find valuable predictors of cardiovascular risk. Oxidative modification of LDL plays an important role in the genesis of arteriosclerosis. Based on the antioxidant hypothesis that suboptimal levels of principal antioxidant micronutrients are hitherto underrated risk factors for CVD, much research has focussed on the effects of antioxidant substances on CVD. Some Lactobacillus strains are probiotics, so novel Lactobacillus strains potentially used as probiotics have been continuously selected, and their applications have been investigated. Dietary approaches to alleviate metabolic syndrome have been shown in many studies.
Recent research has highlighted the antioxidant hypothesis in prevention and treatment of CVD and demonstrated the antioxidant ability of Lactobacillus strains. However, the antioxidative mechanism of Lactobacillus in vivo has not been known. In the study, the authors investigated the effects of Lactobacillus plantarum (L. plantarum) CAI6 and L. plantarum SC4 on nuclear factor-erythroid 2-related factor 2 (Nrf2) expression in hyperlipidemic mice. This is the first study to report the mechanism of intracorporal antioxidation of Lactobacillus strains may be the stimulation of Nrf2 expression. Furthermore, these studies suggest that L. plantarum CAI6 and L. plantarum SC4 could reduce the risk of CVD by a hypolipidemic effect and Nrf2-induced antioxidative defense.
As L. plantarum CAI6 and L. plantarum SC4 have shown obviously protective effects on hyperlipidemic mice, food fermented by the strains may be used as dietary management to alleviate disorders of lipid metabolism related metabolic syndrome and to reduce the risk of CVD.
Metabolic syndrome is a combination of medical disorders, such as disturbances of glucose and lipid homeostasis, which increase the risk of developing CVD. Coronary heart disease, a type of CVD, is a narrowing or blockage of the coronary arteries, usually caused by atherosclerosis in a condition where an artery wall thickens as a result of the accumulation of fatty materials. Nrf2 is a transcription factor that regulates the expression of anti-oxidative stress enzyme genes. Stimulating the Nrf2 related signal pathway has been studied for treating diseases caused by oxidative stress.
This manuscript is worth publishing.
P- Reviewer Verran DJ S- Editor Song XX L- Editor O’Neill M E- Editor Lu YJ
| 1. | Fonseca VA. The metabolic syndrome, hyperlipidemia, and insulin resistance. Clin Cornerstone. 2005;7:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5130] [Cited by in F6Publishing: 5058] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 3. | Kannel WB. Range of serum cholesterol values in the population developing coronary artery disease. Am J Cardiol. 1995;76:69C-77C. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Lusis AJ. Atherosclerosis. Nature. 2000;407:233-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4039] [Cited by in F6Publishing: 3983] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 5. | Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A-11A. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 894] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 6. | Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 270] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758-2770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Belghith KS, Dahech I, Hamden K, Feki A, Mejdoub H, Belghith H. Hypolipidemic effect of diet supplementation with bacterial levan in cholesterol-fed rats. Int J Biol Macromol. 2012;50:1070-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 10. | Kaburagi T, Yamano T, Fukushima Y, Yoshino H, Mito N, Sato K. Effect of Lactobacillus johnsonii La1 on immune function and serum albumin in aged and malnourished aged mice. Nutrition. 2007;23:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Jeun J, Kim S, Cho SY, Jun HJ, Park HJ, Seo JG, Chung MJ, Lee SJ. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26:321-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Donchevaa NI, Antova GP, Softovaa EB, Nyagolovb YP. Experimental and clinical study on the hypolipidemic and antisclerotic effect of Lactobacillus Bulgaricus strain GB N 1 (48). Nutrition Res. 2002;22:393-403. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Canzi E, Zanchi R, Camaschella P, Cresci A, Greppi GF, Orpianesi C, Serrantoni M, Ferrari A. Modulation by bactic-acid bacteria of the intestinal ecosystem and plasma cholesterol in rabbitas fed a casein diet. Nutrition Res. 2000;20:1329-1340. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Lee J, Hwang KT, Chung MY, Cho DH, Park CS. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): role for a metal ion chelating effect. J Food Sci. 2005;70:m388–m391. [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Gao DW, Zhu GH, Gao ZR, Liu ZW, Wang LX, Guo W. Antioxidative and hypolipidemic effects of lactic acid bacteria from pickled Chinese cabbage. J Medicinal Plants Res. 2011;5:1439-1446. [Cited in This Article: ] |
| 16. | Succi M, Tremonte P, Reale A, Sorrentino E, Grazia L, Pacifico S, Coppola R. Bile salt and acid tolerance of Lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiol Lett. 2005;244:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Mandal S, Puniya AK, Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int Dairy J. 2006;16:l190-l195. [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470-475. [PubMed] [Cited in This Article: ] |
| 19. | McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538-542. [PubMed] [Cited in This Article: ] |
| 20. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] [Cited in This Article: ] |
| 21. | Kumar S, Kumar V, Prakash O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4:347-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221-1232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 23. | Aissaoui A, Zizi S, Israili ZH, Lyoussi B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J Ethnopharmacol. 2011;137:652-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Fernandez ML, Webb D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J Am Coll Nutr. 2008;27:1-5. [PubMed] [Cited in This Article: ] |
| 25. | Rubenfire M, Brook RD, Rosenson RS. Treating mixed hyperlipidemia and the atherogenic lipid phenotype for prevention of cardiovascular events. Am J Med. 2010;123:892-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest. 2012;42:1189-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Vogiatzi G, Tousoulis D, Stefanadis C. The role of oxidative stress in atherosclerosis. Hellenic J Cardiol. 2009;50:402-409. [PubMed] [Cited in This Article: ] |
| 28. | Gey KF. Ten-year retrospective on the antioxidant hypothesis of arteriosclerosis: Threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. J Nutr Biochem. 1995;6:206–236. [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kamiya K, Satake T. Chemical constituents of Baeckea frutescens leaves inhibit copper-induced low-density lipoprotein oxidation. Fitoterapia. 2010;81:185-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Ramachandran S, Rajasekaran A, Manisenthilkumar KT. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic mice. Asian Pac J Trop Biomed. 2012;2:262-268. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Mann GE, Niehueser-Saran J, Watson A, Gao L, Ishii T, de Winter P, Siow RC. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Shengli Xuebao. 2007;59:117-127. [PubMed] [Cited in This Article: ] |
| 32. | Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19:71-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 33. | Jones JL, Fernandez ML, McIntosh MS, Najm W, Calle MC, Kalynych C, Vukich C, Barona J, Ackermann D, Kim JE. A Mediterranean-style low-glycemic-load diet improves variables of metabolic syndrome in women, and addition of a phytochemical-rich medical food enhances benefits on lipoprotein metabolism. J Clin Lipidol. 2011;5:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Linda C, Douglas , Sanders ME. Probiotics and prebiotics in dietetics practice. J Am Diet Assoc. 2008;108:510-521. [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |