INTRODUCTION
Despite progresses in our biological and clinical knowledge, colorectal cancer (CRC) remains one of the major public health problems throughout the world[1]. By its frequency, CRC ranks third in men and women worldwide[1]. In addition, CRC continues to be one of the most common fatal types of cancer. Originally depicted as a multi-step dynamical disease, CRC develops slowly over several years and progresses through cytologically distinct benign and malignant states, from single crypt lesions through adenoma, to malignant carcinoma with the potential for invasion and metastasis[2,3]. According to the theory of multi-step carcinogenesis, colorectal epithelial cells accumulate a number of molecular changes to eventually become fully malignant[4,5]. In spite of unifying theories, genetic and epigenetic events during the carcinogenesis process differ considerably from tumor to tumor. Thus, CRC is not a single disease; rather it encompasses different molecular and pathological entities with a wide range of clinical behaviors[6]. At the molecular level, CRC is the tip of an iceberg the basis of which encloses a complex array of gene alterations, affecting supra-molecular processes. Essentially, like individual fingerprints, each tumor arises and behaves in a unique fashion that is unlikely to be exactly recapitulated by any other tumor. Nevertheless, molecular changes allows for a categorization of CRC, which is largely accepted, although likely to over-simplistic. It has been demonstrated that main genetic and epigenetic features, such as microsatellite instability (MSI), chromosomal instability, CpG island methylator phenotype (CIMP) or global DNA hypomethylation, lead to alterations of gene function on a genome-wide scale. It is known that activation of oncogenes, including KRAS, BRAF, PIK3CA and TP53, affects intracellular signaling pathways[6,7]. The suppressor pathway is disrupted in CRC with chromosomal instability occurring in the majority of CRCs (approximately 85%), which have a molecular profile characterized by specific chromosomal amplifications and transformations, aneuploidy, and loss of heterozygosity[6-8]. Differently, CRCs of the mutator pathway (approximately 15%) have a defective DNA mismatch repair system, which leads to accumulation of thousands of unrepaired mutations[8]. This inability to repair DNA slippage errors and mismatches can easily be demonstrated because it results in variability in the length of DNA microsatellites, formed by repetitive sequences, that is MSI. It is accepted that MSI CRCs have a heterogeneous histological appearance, better prognosis due to a reduced metastatic potential, and a different response to chemotherapy[9-14].
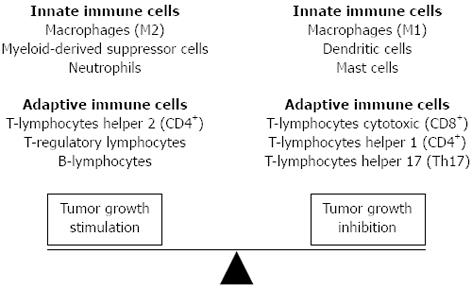
Histopathological examination reveals that likely other solid tumors, CRC are associated with diverse immune cell infiltrates[15-20], and that in the cancer context, epithelial cells coexist with extracellular matrix components and non-neoplastic cell types, including fibroblasts, myofibroblasts, adipocytes, endothelial cells, pericytes, which collectively form the tumor stroma. Several lines of evidence support the concept that tumor stromal cells, are not merely a scaffold, but rather they influence growth, survival, and invasiveness of cancer cells, dynamically contributing to the tumor microenvironment, together with immune cells[20-25]. The types of immune system cells that are found infiltrating CRC consist of cells of the innate immune system i.e., macrophages, neutrophils, mast cells and natural killer cells, as well as cells associated with an adaptive immune response i.e., T and B lymphocytes. Although it is commonly thought that an immune response localized to the tumor inhibit cancer growth, it is clear that some types of tumor-associated inflammation may also exert an opposite action, at least at some point of CRC natural history[26] (Figure 1). Here we sought to briefly review these two contradictory aspects of the immune response to CRC.
Figure 1 Immune cells have variable behavior controlled by complex interactions in the tumor microenvironment.
Although it is commonly thought that an immune response localized to the tumor inhibit cancer growth, it is now clear that some types of tumor-associated inflammation can exert an opposite action.
INNATE IMMUNITY AND COLORECTAL CANCER
It is well known that innate immunity represents the body’s first defense or “gut reaction” to an abnormal situation, such as cancer, and does not involve specific recognition of immunogenic peptides, or antigens[20]. Aside T and B lymphocytes , innate immune cells orchestrate an inflammatory environment that may function to either stimulate or inhibit cancer growth[20]. Various innate immune cells have been implicated in CRC development and progression[15,27,28]. Among these, macrophages, are a primary source of secreted pro-inflammatory cytokines, and are generally distinguished as type 1 (M1) or type 2 (M2)[29-31]. M1s generally have an interleukin (IL) 12lowIL-10high phenotype, show impaired expression of reactive nitrogen intermediates, poor antigen presentation and have tumoricidal capacity, while show high expression of angiogenic factors [including Vascular-endothelial growth factor (VEGF) epidermal-growth factor (EGF) and semaphorin 4D], metalloproteases (MMPs) and cathepsins as well as of the growth arrest-specific protein 6 (GAS6)[29-32]. Additionally, M1s can support T-helper 1 (Th1) adaptive immunity[33]. Conversely, M2s secrete immunosuppressive cytokines and promote tumor growth[22,34]. It has been shown that cancer cells shape their interaction with macrophages by escaping phagocytosis and by promoting a M2-like polarization throughout chemokines and polarizing cytokines including chemokine (C-C motif) ligand 2 (CCL2), colony stimulating factor 1 (CSF1), macrophage slowing factor (MSF), tumor necrosis factor-alpha (TNF-α, IL-10 and transforming growth factor-β (TGF-β).
Among the cells with M2 phenotype, the tumor-associated macrophages (TAM) have been shown as capable of secreting proteases that enhance invasion and metastases, together with a range of cytokines inhibiting an adaptive tumor-specific immune response, and angiogenic factors that increase neovascularity. In patients with CRC, macrophages are usually found located around necrotic areas of tumor and the advancing tumor margin[28]. Their role in CRC still remains controversial. While, it was originally thought that the main function of TAMs was a direct cytotoxic effects on tumor cells, phagocytosis apoptotic/necrotic cell debris, and present tumor-associated antigens to T lymphocytes, current evidences suggest that inflammation and TAMs can also promote tumor growth and metastasis. Kang et al[35] have recently highlighted an association between intra-tumoral TAM densities with CRC malignant aggressiveness. Additionally, increased frequencies of intra-tumoral TAM have been associated with high levels of MMP type 2 and 9 expression in CRC cells[36,37]. These findings are in accord with a previous cell-line study showing that co-culturing of tumor cells with macrophages enhances cancer cell migration, invasiveness, and MMP-2 and MMP-9 secretion[35].
Kaler et al[38] have recently established that macrophages promote Wnt signaling pathway in CRC cells and thus enhance their proliferation, and demonstrated that macrophages exert their pro-tumorigenic activity mainly through the release of IL-1β. The same authors demonstrated that tumor necrosis factor related apoptosis inducing ligand (TRAIL) induced apoptosis of CRC cells is inhibited by macrophage derived IL-1β, and showed that macrophages and recombinant IL-1β counteract TRAIL-induced apoptosis through activation of Wnt signaling and stabilization of the nuclear transcription factor Snail in tumor cells.
A number of studies have also shown that macrophages can release a vast diversity of cytokines, proteolytic enzymes, growth factors, and inflammatory mediators that may directly influence and stimulate the growth and migration of tumor cells[29-33]. Li et al[39] first reported that IL-6 released by macrophage directly promotes CRC cell progression. They showed that monocyte/macrophage-derived IL-6 enhances migration of HT-29 CRC cells in vitro[39]. Although, how IL-6 enhances HT-29 cell migration still remains unknown, it has been suggested that TGF-β may be involved in this process. Using monoclonal antibodies to neutralize IL-10 in macrophage supernatant, Li et al[39] found that the IL-6-mediated effects on HT-29 CRC cells were all enhanced. Therefore, the interaction between IL-6 and IL-10 released from macrophage is indeed involved in CRC progression and prognosis. The above findings support the fact that TAMs play a regulatory role in the tumor microenvironment by modulating secretion of cytokines such as IL-6 and IL-10, thereby causing cancer cells to manipulate their microenvironment to facilitate cancer growth[40,41].
TAM activation and maturation is under the influence of the tumor microenvironment. TAMs retain a relatively immature phenotype, characterized by a low expression of differentiation-associated antigens in hypoxic microenvironments[35,42,43]. Conversely to an M2 phenotype with pro-tumoral functions in CRC[35], it has been shown that the macrophages, especially those secreting IL-12 and IL-23, infiltrating the tumor front are positively correlated with a favorable outcome, which implicates TAMs as possessing anti-tumor functions. Forssell et al[44] stained with the pan-monocyte/macrophage marker CD68 a series of CRC specimens and showed that the higher macrophage infiltration along the tumor invasive front correlated with improved survival in colon cancer compared to rectal cancer. They concluded that a dense macrophage infiltration at the tumor front positively influences prognosis in colon cancer and that the degree of cell-to-cell contact may influence the balance between pro-tumorigenic and anti-tumorigenic properties of macrophages. High levels of tissue macrophages have been also associated with earlier disease stage, absence of nodal and lymphovascular metastases and an overall better prognosis. Zhou et al[45] by analyzing the relationship between the density of TAMs and the potential of hepatic metastasis and survival have shown that a higher density of macrophages along the invasive front of CRC was associated with a higher 5-year survival rate. In addition, according to Forssell’s scoring system that defines CD68 hot-spots as small areas among which the infiltration of macrophages was considerably above the average level of CD68-positive cells, the highest CD68 hot-spot was associated with both the incidence of hepatic metastasis and the interval between colon resection and the occurrence of hepatic metastasis[45].
The mechanisms behind the anti-tumor effects of TAMs have still not been fully elucidated, and seem potentially be ascribed to the M1 phenotype, which is in part controlled by the CD4+T lymphocytes and the death of cancer cells[45-47]. It has been ascertained that recruitment of TAMs contributes to the development of an adaptive immune response against cancer, and the balance between antigen availability and clearance through phagocytosis and subsequent degradation of senescent or apoptotic cells.
It is undeniable that the discrepancies in results between different studies may be due to a number of factors related to the location of the TAMs and the assessment methods employed. Recent studies have reported that different macrophage phenotypes localized to different regions of the carcinoma have variable effects on tumor cells[18,19]. Furthermore, evidences have shown that the relationship between TAMs and tumor progression is tumor type-dependent.
Other innate immune system cells can be detected in CRC microenvironment, including mast cells (MCs), neutrophils, natural killer (NK) cells, and eosinophils.
Nagtegaal et al[48] have shown that peritumoral MCs prevent local and distant recurrence, with improved survival as a consequence. The significant benefit of MCs on tumor progression in CRC was also highlighted by Gounaris et al[49] who reported that depletion of MCs, either by drugs either in MC-deficient mice, led to remission of existing polyps. Similar to other immune cell types, high numbers of MCs are associated with earlier CRC disease stage and have been proposed as an independent prognostic marker for improved survival[50]. It has also been reported that the number of MCs progressively decreases from normal mucosa through premalignant conditions and the lowest numbers are seen in cancers. Because of their location, MCs may prevent the metastasis of carcinomas that are restricted to the sub-mucosa. In vivo studies have observed that destruction of lymphatic vessels in the peritumoral infiltrate is always accompanied by MCs. This led to the hypothesis that MCs degranulate in the sub-mucosa when they come into contact with the inflammatory infiltrate or CRC cells, thus leading to the destruction of lymphatic vessels and thereby preventing further metastasis. Gulubova and Vlaykova[51] proposed the MCs density along the invasive front of the primary CRC as a helpful tool for prognosis of patients after surgical therapy. In their study, it has been shown that patients with low MCs density had significantly better prognosis compared to those with high MCs density. In addition, Blatner et al[52] reported that in CRC, MCs contribute to systemic regulatory T-cell dysfunction. MCs have an intricate interaction with T-regulatory cells that controls the functions of both cell types in a reciprocal manner. MCs play also an important role in allograft acceptance, where they are required to sustain the peripheral tolerance mediated by T-regulatory cells. These latter can inhibit MCs differentiation and hinder degranulation by contact-dependent mechanisms and production of soluble factors, such as IL-10. Conversely, the activation and subsequent degranulation of MCs breaks peripheral tolerance. MCs degranulation or direct cell contact and secretion of IL-6 promote Th-17 conversion of T-regulatory cells with loss of both forkhead box P3 transcription factor (Foxp3) expression and T-cell-suppressive properties.
Neutrophils may form up to 15% of the inflammatory infiltrate associated with CRCs and this proportion increases within areas of tumor necrosis[28]. In patients with rectal cancer, high concentrations of neutrophils have been shown as independent predictors of improved prognosis especially when microscopic abscesses form[28]. However, an elevated neutrophil/lymphocyte ratio was, however, found by Halazun et al[53] led to a poorer survival time and higher rate of recurrence in CRC patients undergoing surgery for liver metastasis.
NK cells are granular lymphocytes that form part of the innate cellular immune response[28]. In CRC, high numbers of NK cells in the inflammatory infiltrate has been associated with better prognosis[28]. The number of NK cells decreases with increasing cancer stage. Additionally, it has been shown that the ratio of NK cells in the peripheral blood is an important prognostic indicator in CRC patients and it is of interest to note that 5-fluorouracil-based chemotherapy increases the number of NK cells[54,55].
Aside the above innate immune cell types, dendritic cells (DCs), antigen-presenting cells that are critical to the stimulation of effective anti-tumor adaptive immune responses, can become defective in the tumor microenvironment and aid in tumor immune evasion by failing to stimulate T lymphocytes. It has been suggested that the presence of DCs may be of significant benefit in patients with CRC. Xie et al[56] also demonstrated that the presence of DCs was found predominantly in early compared to later disease stages and mostly located in tumor surrounding tissue. Suzuki et al[57] showed the presence of mature CD83+ DCs at the cancer invasive front and by light and electron microscopy demonstrated their aggregation into clusters with lymphocytes, the majority of which were CD45RO+ T lymphocytes. They concluded that mature CD83+ DCs at the invasive margin promote T-cell activation for the generation of tumor specific immunity.
Although, there is growing evidence that the host innate immune system has a critical role in regulating carcinogenesis, the specific receptors involved and the importance of their interaction with commensal bacteria remain to be elucidated. Two major classes of innate immune receptors, the Toll-like receptors and Nod-like receptors, many of which are upstream of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), have been investigated[58,59]. Particularly, the toll-like receptors have been implicated in promoting colon tumorigenesis. Fukata et al[60] have shown that toll-like receptor-4 (TLR4) is over-expressed in human colitis-associated cancer and, that mice deficient in TLR4 are markedly protected against colitis-associated neoplasia. A high TLR4 expression in the tumor microenvironment has recently been reported as a possible marker of disease progression in CRC[60]. Conversely, the role of nod-like receptors in regulating colorectal tumorigenesis remains unclear. Chen et al[61] reported an increased intestinal permeability associated with enhanced inflammatory cytokine production and epithelial cell proliferation in Nod1-deficient mice. As the depletion of the gut microbiota suppressed tumor development in Nod1-deficient mice, a link should exist between commensal bacteria and host innate Nod1 signaling pathway involved in the development of inflammation-mediated CRC.
ADAPTIVE IMMUNITY AND COLORECTAL CANCER
It is today well recognized that cells of the adaptive immune system are recruited in CRC and colitis-associated tumours (CAC), where they have either pro- and anti-tumorigenic roles. T lymphocytes participate in inflammation, cancer development and progression, as well as in anticancer immunity[20-22]. In CAC the adaptive immune system seems to have mainly a pro-tumorigenic role, while in CRC it may play a double-faced role, being the balance between immune-surveillance (carried out by CD8+ and CD4+ T lymphocytes) and tumour-promoting inflammation (by various sub-types of T lymphocytes) to change over time, and eventually dictating disease progression. It has been suggested that immune-surveillance might mediate the early recognition and elimination of transformed cells and aberrant crypt foci, keeping small tumours in a dormant state[62]. Immune-surveillance is retained important during the metastatic process, when small numbers or isolated tumoral cells travel and can be attacked by antitumor immune cells not inhibited by factors in the tumour microenvironment. Galon et al[18] suggested that once human CRC become clinically detectable, the adaptive immune response plays a role in preventing tumour recurrence and metastasis. Independently, Chiba et al[63] and Banerjea et al[64] reported that intra-tumoral T cells modify the tumour stroma or CRC cells in a way that attenuate the metastatic potential.
Cytotoxic T lymphocytes (CD8+ T cells, or CTL) constitute one of the leading effector of antitumor immunity. In order for CD8+ T cells to recognize antigens, these need to be exposed on the tumour cells in association with the human leukocyte antigen (HLA) class I proteins[65]. Upon encounter of a tumour cell antigen/HLA I complex for which their T cell receptor (TCR) is specific, CD8+ T lymphocytes clonally expand and differentiate[65]. Once activated, cytotoxic T lymphocytes can mediate specific destruction of tumour cells through the release of lytic components via cell-cell interaction[65,66]. Perforin, a cytolytic protein found in the granules of CD8 T-cells and NK cells, and enzymatic proteases, including granzyme B, are secreted determining cell death by disruption of the cell membrane and activation of the apoptotic pathway respectively. CD4+ T cells, which only respond to antigens presented by the HLA class II proteins expressed by DCs, are important for antitumor immunity. On the peculiar cytokine profile induced, CD4+ T lymphocytes are mainly subdivided in Th1 or Th2 lymphocytes[67]. Th1 cells secrete cytokines such as interferon-gamma (IFN-γ) and TNF-α, and support cytotoxic T lymphocytes by producing IL-2, required for CD8+ T cells proliferation. Conversely, Th2 cells principally secrete IL-10, IL-4, and IL-5, and limit cytotoxic T lymphocytes proliferation.
Regulatory T cells (Treg cells) have been defined as a T-cell population that functionally suppresses an immune response by influencing the activity of another cell type[68]. Treg cells have been categorized into two main classes based on their ontogeny: naturally occurring Treg (nTreg), which develop in the thymus and are present in mice and healthy humans from an early postnatal period, and Treg which can arise in the periphery (or in vitro)[69]. nTreg are characterized by their high expression of CD25 (CD4+CD25+) and co-expression of the FOXP3[69]. Needham et al[70] reported that depletion of intra-tumoral Tregs enhances antitumor immunity and tumour rejection in mouse models. Clarke et al[71] have shown that depletion of Tregs in the peripheral blood of patients with CRC was recently shown to unmask CD4+ T-cell responses to tumour antigens. It is known that self-tumour antigens may also induce the preferential proliferation of CD4+CD25+FOXP3+ Treg cells. Tregs function to maintain immune homeostasis and limit acute inflammation. These cells interfere with T cell priming and can affect the antitumor function of effector cells via secretion of TGF-β and IL-10. A number of investigations suggest that Tregs infiltrating the tumour may adversely affect prognosis[72-75]. Increased levels of Treg cells have been, however, associated with a favourable prognosis in CRC[76-79]. The mechanisms of this dual effect, which seems dependent on the tissue type, are not well understood, although it is hypothesized that Tregs might suppress inflammation induced by growth promoting innate immune system cells.
Although the role of B lymphocytes in cancer has been overshadowed by the interest in developing T-cell-mediated cellular responses, it is now apparent that B lymphocytes can play a complementary role in the host response against tumour. B lymphocytes represent a cell population that express clonally diverse cell surface Ig receptors recognizing specific antigenic epitopes. In addition to the role of B lymphocytes in antibody production, these cells mediate/regulate several other functions fundamental for immune homeostasis. Of significant importance is the antigen-presenting role of B lymphocytes in the initiation of T-cell immune responses. Moreover, B lymphocytes can play a significant role in infection and autoimmunity as regulatory cells (indicated as Bregs) via the elaboration of suppressive cytokines, such as IL-10, TGF-β, or IL-4. The role played by B cells in cancer immunology remains still complex and somewhat controversial. Depending upon their state of activation, B lymphocytes have had divergent roles on T-cell differentiation and effector function. Oversimplifying, resting B lymphocytes have been reported to suppress T-cell-mediated antitumor immunity, by acting on both CD4+ and CD8+ T lymphocytes. In contrast, a number of reports suggest the efficacy of activated B lymphocytes in cellular immunotherapy of malignancies. In particular, activated B lymphocytes have been reported to enhance the ability to generate tumour-infiltrating lymphocytes in vitro involving anti-CD3 and IL-2.
The therapeutic targeting of tumours or components of the immune system with molecule-specific monoclonal antibodies (mAb) is now considered a viable treatment option for cancer patients. One of the currently applied antibodies in clinics is represented by rituximab (Rituxan) that targets B cells for elimination by binding the B cell-associated marker CD20. Interestingly, it has been recently developed a C57BL/6 TRAIL-sensitive tumour model with the aim of being able to use gene-targeted mice to better evaluate the innate and adaptive immune cells contributing to the tumoricidal activity of the MD5-1 mAb in more clinically relevant established tumours. C57BL/6 gene-targeted or immune cell-depleted mice were used to examine the antitumor activity of MD5-1 against the TRAIL-sensitive mouse MC38 colon adenocarcinoma. It has been shown that an intact B cell compartment is critical for the therapeutic activity of MD5-1 against established tumours. B cells were confirmed to trigger tumour cell apoptosis by FcR-mediated cross-linking of the MD5-1 mAb in vitro and in vivo B lymphocytes were critical for directly triggering MD5-1-mediated tumour cell apoptosis.
Although the role of B-cells in human CRCs is still not completely characterized, B-cell-deficient mice exhibit spontaneous regression of MC38 colon carcinoma cells. Studies involving BCR transgenic mice indicated that B lymphocytes might inhibit antitumor T lymphocytes responses by antigen-nonspecific mechanisms. Shah et al investigated the role of B lymphocytes in tumour immunity by studying immune responses of mice genetically lacking B lymphocytes to primary tumours. They highlight that although the effects of B lymphocytes on anti-tumour response warrant further study, adoptive transfer of CD40(-/-) B lymphocytes into B lymphocytes-deficient mice resulted in restored growth of MC38 colon carcinoma cells suggesting additional factors other than CD40 are involved in dampening anti-tumour responses.
PROGNOSTIC INFORMATION OF IMMUNE CELL INFILTRATE
In contrast to infiltration of cells responsible for chronic inflammation, the presence of high numbers of T lymphocytes has been reported to be a positive prognostic factor in several cancers. The first reports on the beneficial effect of lymphocytic infiltration in CRC appeared already in the Eighties. They were later confirmed until recent most studies highlighting a prominent function for memory T lymphocytes and CD8+ T lymphocytes in predicting disease-free survival and overall survival. In general terms, it has been suggested that prognosis in patients with cancer is positively affected by (1) the presence of a tumour gene signature consistent with a type I adaptive immune response (i.e., increased antigen presentation, IFN-γ signalling, and TCR signalling); and (2) the presence of T cells that penetrate through tumour stroma and deeply infiltrate the parenchyma to become intra-tumoral T cells[20]. Thus, besides a Th-1 response signature, the other key feature of an effective immune response is the ability of T cells to reach the site of the tumour and to infiltrate it. Because T-cell infiltration is not spatially homogeneous in CRC, attention has been focused on the predictive values of T lymphocytes located in the center of the tumor (CT), along the invasive margin (IM) and in lymphoid aggregate mainly detectable in proximity of the tumor (these aggregates are called tertiary lymphoid structures)[80,81].
In a large series of CRCs, Pagès et al[82] assessed the immune component of the tumoral microenvironment by a combination of high-throughput gene expression and immunophenotypic analyses, and evaluated its possible influence on tumour dissemination. They found an association between evidence of an immune reaction within the tumour and the absence of tumour local invasion of vascular, lymphatic, and neural structures (collectively referred to as VELIPI). CRCs without VELIPI were associated with an enhanced immune cell infiltration and an increase of mRNA expression of adaptive Th1 effector T-cell markers [CD8, T-box transcription factor 21 (T-bet), IFN regulatory factor-1 (IRF-1), IFN-γ, granulysin, and granzyme B]. The immunohistochemical analysis of adaptive immune markers i.e., CD3, CD8, granzyme B, and CD45RO, by tissue microarrays prepared from the CT and from its IM revealed a statistically significant correlation between the density of these immune cells and outcome for all patients but those with metastatic disease at diagnosis. Further, the combined analysis of both tumour regions improved the accuracy of survival prediction compared with single-region analysis[18].
Independently, Deschoolmeester et al[83] showed that the presence of a pronounced lymphocytic infiltration within the tumour is associated with improved survival. They found that CD3+ and CD8+ T lymphocytes within tumour nests and of CD3+ in the stroma had a major impact on the patients’ overall survival. The improved survival associated with infiltration of T lymphocytes has been suggested to result from the effective suppression of micrometastases. Therefore, the densities of CD8+ T cells within the primary tumour might be a potential marker of the presence of a systemic immunosurveillance mechanism. In addition, tumour cells secrete substances in the stromal compartment, which might be recognized by the immune system that subsequently attack the tumour. A weak adaptive immune reaction correlated with a very poor prognosis even in patients with early tumour invasion. Conversely, a high density of adaptive immune cells correlated with a highly favourable prognosis whatever the local extent of the tumour and the regional lymph node invasion.
In mice, targeted disruptions of genes that encode critical components of the immune system (i.e., mice lacking: IFN-γ or its receptor, signal transducer and activator of transcription-1 mediating IFN-γ receptor signalling, perforin, recombination activating gene-2, or IL-12) induce an increased susceptibility of the host to tumours[84]. These findings make it possible to hypothesize an immune-mediated control of tumour development by the adaptive compartment.
A number of studies have reported that MSI, CIMP, BRAF mutation, PIK3CA mutation, and tumour LINE-1 hypomethylation are associated with CRC prognosis and that lymphocytic infiltration is associated with many of these molecular variables. The associations of a prognostic biomarker with a given disease, strongly suggests its stage-dependency as outcome predictor. This is best exemplified by MSI CRC, whose overall prognostic advantage is associated with a low frequency of stage III and IV cases at diagnosis as compared to microsatellite stable counterpart. Most MSI CRCs show a pronounced intra-tumoral inflammatory reaction (in fact a criterion for MSI testing), the mechanistic explanation of which, however, is still incompletely understood. Within these tumours, infiltrating lymphocytes have been identified as predominantly activated CD8+ T cells. The presence of these cytotoxic T lymphocytes has been attributed to the inherently greater production of abnormal peptides as a result of unreliable DNA repair in MSI-positive tumours. It is known that truncated peptides produced by frameshift mutations due to MSI may be immunogenic and contribute to the host immune response. However, little is still known about the interrelationship between tumour-infiltrating T lymphocytes, MSI status, and other tumour molecular features. It is indubitable that to define the prognostic effect of tumour-infiltrating T-cells independently of those potential confounders, large studies of CRC with extensive molecular characterization are needed. Additionally, caution is needed before incorporating tumour-infiltrating T cells into tumour staging. To minimise the risk of inappropriate tumour down-staging at diagnosis, survival data need to be confirmed in independent series of patients studied in the past decade. Moreover, the association has to be conclusively proven between low densities of tumour-infiltrating T cells and the clinical detection of metachronous metastases, which remains the most appropriate outcome measure for recognising a role of the local immune response in micrometastasis suppression. Recently, Laghi et al[85] investigated the relationship between the density of CD3+ T infiltrating lymphocytes along the tumour invasive margin, and the occurrence of metachronous distant-organ metastases after potentially curative resection, in a large, consecutive series of patients with deeply invading (pT3 or pT4) MSI-typed CRC, and no evidence of distant organ metastasis at diagnosis. They found that large areas of CD3+ cells at the invasive front of pT3 or pT4 CRCs are associated with a low risk of metachronous metastasis and consequently a survival advantage, only in patients with node-negative cancers, but not in patients whose cancers involved lymphnodes. Of interest, the prognostic advantage conferred by a high density of CD3+ cells was independent of tumour microsatellite status in patients with stage II CRC. CD3-immunostaining of CRC tissue might therefore be useful for selecting stage II patients who, because they are at very low risk for cancer progression, could be spared adjuvant treatments.
Nosho et al[86] examined the prognostic role of tumour-infiltrating T-cell subsets in a database of 768 CRCs from two prospective cohort studies. They concurrently assessed the densities of CD3+, CD8+, CD45RO+, and FOXP3+ lymphocytes as well as other relevant molecular and pathological features, therefore making possible to evaluate the independent effect of each T-cell subset density on patient survival. They found that the density of CD45RO+ cells, but not that of CD3+, CD8+, or FOXP3+ cells, was an independent prognostic biomarker of longer survival in CRC patients. In contrast, Salama et al[76] by analysing T-cell infiltrates in 967 CRCs including 593 stage II and 374 stage III cases, reported that FOXP3+ lymphocytes density had stronger prognostic significance than CD8+ and CD45RO+ cells, and predicted a better outcome. FOXP3+ lymphocytes were found not associated with any histopathologic features. At multivariate analysis, stage, vascular invasion, and FOXP3+ cell density in tumoral tissue were independent prognostic indicators. These results led Salama et al[76] to conclude that the inclusion of FOXP3+ cell density may help to improve the prognostication of early-stage CRC, although these authors do not explored this parameter with tumor stage.
CONCLUSION
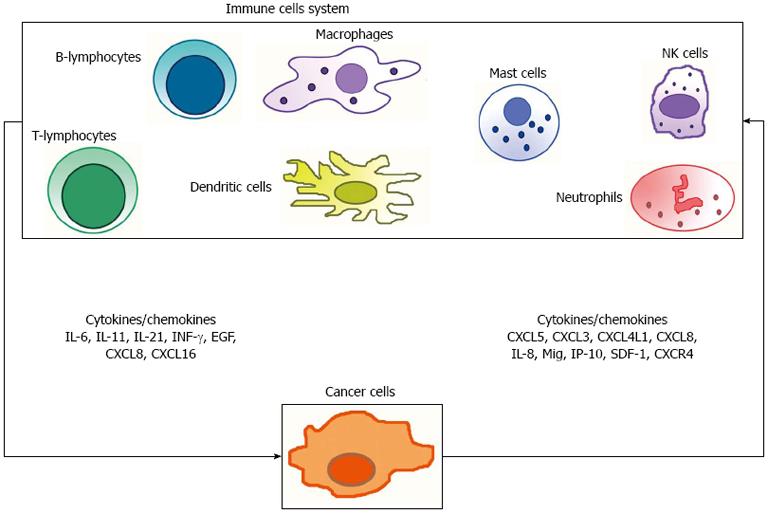
It is accepted that human cancer is a complex dynamical disease[87,88]. It is also now well recognized that cancers are not just composed of malignant cells, but that they are microenvironment consisting of many cell types, including a range of immune cells. It is indubitable that the antitumor immune response involves the interaction of several cell types and products (Figure 2), of the adaptive as well as of the innate immune system. It is also clear that CRC can escape immune surveillance using several strategies. The molecular profiles of the function and interaction of innate and adaptive immune cells, and the definition of tumor antigens have all led to build the basis of the knowledge of how the immune system modulates tumor growth and inhibition. The challenge remains to determine not only how the “rejection” pathway initiates in human malignancy, but also how that rejection is maintained. It is indubitable that the analysis of the type, quantity, location and the functions of the immune infiltrate becomes a primary step in understanding CRC natural history, and, in a clinical perspective, its prognostic determinants. A comprehensive analysis of all components of the lymphocytic infiltrates in the context of their localization, organization and impact at various steps of tumor progression remains largely, if not entirely, to be reported to prospective studies. In parallel, understanding the mechanisms of efficient immune reactions, the place where they are initiated, the cells and key cytokines and chemokines involved (Figure 2), and their impact at different stages of the disease should provide new tools and goals for more effective and less toxic targeted therapies.
Figure 2 Anti- and pro-tumor immune response involves the interaction of several cell types of the adaptive as well as of the innate immune system, and an intricate network of products (i.
e., cytokines and chemokines). IL: Interleukin; INF-γ: Intracellular interferon-γ; EGF: Epidermal-growth factor; NK cells: Natural killer cells.