Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1984
Revised: January 20, 2013
Accepted: February 5, 2013
Published online: March 28, 2013
Processing time: 190 Days and 1.5 Hours
AIM: To study the efficacy of marrow mesenchymal stem cells (MSCs) transplantation combined with interleukin-1 receptor antagonist (IL-1Ra) for acute liver failure (ALF).
METHODS: Chinese experimental miniature swine were randomly divided into four groups (n = 7), and all animals were given D-galactosamine (D-gal) to induce ALF. Group A animals were then injected with 40 mL saline via the portal vein 24 h after D-gal induction; Group B animals were injected with 2 mg/kg IL-1Ra via the ear vein 18 h, 2 d and 4 d after D-gal induction; Group C received approximately 1 × 108 green fluorescence protein (GFP)-labeled MSCs (GFP-MSCs) suspended in 40 mL normal saline via the portal vein 24 h after D-gal induction; Group D animals were injected with 2 mg/kg IL-1Ra via the ear vein 18 h after D-gal induction, MSCs transplantation was then carried out at 24 h after D-gal induction, and finally 2 mg/kg IL-1Ra was injected via the ear vein 1 d and 3 d after surgery as before. Liver function, serum inflammatory parameters and pathological changes were measured and the fate of MSCs was determined.
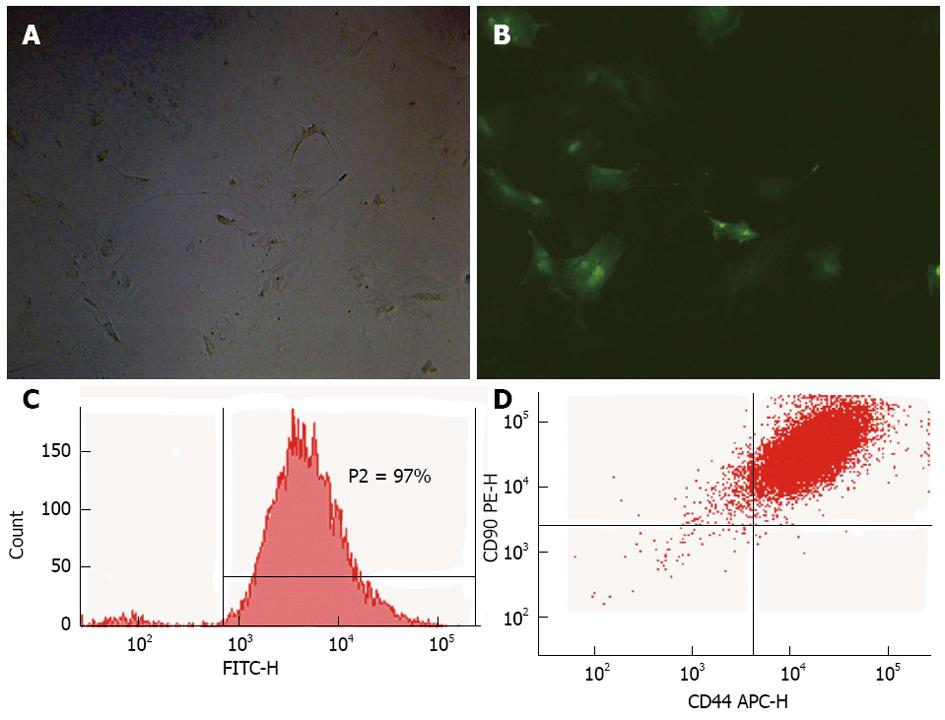
RESULTS: The optimal efficiency of transfection (97%) was achieved at an multiplicity of infection of 80, as observed by fluorescence microscopy and flow cytometry (FCM). Over 90% of GFP-MSCs were identified as CD44+ CD90+ CD45- MSCs by FCM, which indicated that most GFP-MSCs retained MSCs characteristics. Biochemical assays, the levels of serum inflammatory parameters and histological results in Group D all showed a significant improvement in liver injury compared with the other groups (P < 0.05). The number of GFP-MSCs in Group D was also greater than that in Group B, and the long-term cell proliferation rate was also better in Group D than in the other groups.
CONCLUSION: MSCs transplantation is useful in ALF, IL-1Ra plays an important role in alleviating the inflammatory condition, and combination therapy with MSCs transplantation and IL-1Ra is a promising treatment for ALF.
- Citation: Shi XL, Zhu W, Tan JJ, Xiao JQ, Zhang L, Xu Q, Ma ZL, Ding YT. Effect evaluation of interleukin-1 receptor antagonist nanoparticles for mesenchymal stem cell transplantation. World J Gastroenterol 2013; 19(12): 1984-1991
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1984
Acute liver failure (ALF) is defined by the development of coagulopathy and encephalopathy within a short period of time in patients with no previous history of liver disease[1]. The essence of ALF is severe inflammation leading to cell necrosis in a large number of liver cells caused by paracetamol, idiosyncratic drug reactions, hepatitis B, or seronegative hepatitis[2,3]. The key to the treatment of ALF is the reduction of liver cell necrosis and the stimulation of liver cell regeneration.
Liver transplantation is the only efficient treatment for ALF; however, difficulties including severe donor shortage, numerous complications, immunological rejection and high medical costs limit its use[1,4]. Mesenchymal stem cells (MSCs) due to their sufficient source, low immunogenicity and the potential ability for differentiation into hepatocyte-like cells make MSCs transplantation a promising treatment for ALF[5-7]. In order to achieve better results, we transplanted MSCs into a pig model of acute liver failure in addition to interleukin-1 receptor antagonist (IL-1Ra) injection which is used to improve liver inflammation[8,9]. IL-1 is primarily a proinflammatory cytokine due to its ability to stimulate the expression of a number of inflammation-associated genes through the IL-1 signaling cascade[10-12]. IL-1Ra can bind to the IL-1 receptor and blocks IL-1 action through competitive inhibition, but will not initiate the IL-1 signaling cascade due to its IL-1-like structure which will not induce a signal at all[8]. In this study, we evaluated the efficiency of combination therapy with IL-1Ra and MSCs transplantation for the treatment of ALF in swine.
Chinese experimental miniature swine (10 ± 3 kg, aged approximately 5 to 8 mo) were obtained from the Laboratory Animal Centre of the Affiliated Drum Tower Hospital of Nanjing University Medical School. All experiments were approved by the Institutional Animal Care and Use Committee.
MSCs were isolated by density gradient centrifugation from pig bone marrow and cultured in L-Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco BRL, Grand Island, NY, United States). The cells were then transfected with a lentiviral vector carrying the gene encoding green fluorescence protein (GFP), and the multiplicity of infection (MOI) of transfection was determined by fluorescent inverted phase contrast microscopy and flow cytometry (FCM). The surface markers (CD44, CD45, and CD90) of GFP-labeled MSCs (GFP-MSCs) were identified by FCM for their MSCs characteristics.
The animals received a single intravenous injection of D-galactosamine (D-gal) 0.3 g/kg to induce experimental hepatic injury.
Twenty-eight pigs were randomly divided into four groups, Group A (control, n = 7), Group B (IL-1Ra, n = 7), Group C (MSCs transplantation, n = 7), Group D (combined therapy, n = 7). Group A received 40 mL normal saline via the portal vein 24 h after D-gal induction; Group B received 2 mg/kg IL-1Ra (Institute of Process Engineering, Chinese Academy of Sciences, China) via the ear vein 18 h, 2 d and 4 d after D-gal induction; Group C received approximately 1 × 108 GFP-MSCs suspended in 40 mL normal saline via the portal vein 24 h after D-gal induction; Group D received 2 mg/kg IL-1Ra via the ear vein 18 h after D-gal induction, MSCs transplantation was carried out 24 h after D-gal induction, and finally 2 mg/kg IL-1Ra was injected via the ear vein 1 d and 3 d after surgery as before. Liver function, and inflammatory cytokines IL-1β, IL-2 and tumor necrosis factor α (TNF-α) were measured using enzyme-linked immuno sorbent assay Kits (Corbett Life Science, Australia) pre-operatively, intra-operatively, 1-6 d and 1-4 wk after surgery.
Swine were humanely killed for histological examination at 3 d and every week after surgery. Liver tissues were immersion fixed, embedded in paraffin and sectioned at 5 μm, and the slices were submitted for hematoxylin and eosin (HE) and anti-Ki67 (Abcam Ltd., United Kingdom) staining. To determine liver cell proliferation, six high-powered fields of vision were obtained for each slice, and the average number of Ki67+ cells was used for statistical analysis. To trace transplanted MSCs, cells expressing GFP were analyzed by fluorescent microscopy and conventional immunohistochemistry using anti-GFP (Abcam Ltd., United Kingdom).
All statistical analysis were performed using SPSS 16.0. The data are reported as mean ± SD. The statistical significance was analyzed by two-way analysis of variance. P < 0.05 was considered to denote statistical significance.
The optimal efficiency of transfection (97%) was achieved at an MOI of 80, as observed by fluorescence microscopy (Figure 1A and B) and FCM (Figure 1C). Over 90% of GFP-MSCs were identified as CD44+ CD90+ CD45- MSCs (Figure 1D) by FCM, which indicated that most GFP-MSCs retained MSCs characteristics.
Dramatic changes in alanine aminotransferase (ALT), aspertate aminotransferase (AST), total bilirubin (TB), ammonia and prothrombin time (PT) demonstrated that acute liver injury was successfully achieved by D-gal induction in all four groups. Highly significant increases in ALT, AST, TB, ammonia and PT were found in all groups within 48 h after D-gal injection, and then gradually declined. The most significant improvements were found in Group D following combination therapy compared with the other groups; ALT and ammonia in Group D were significantly lower than those in Group A (P < 0.05) within 1 to 4 d after combination therapy, and the low level of TB in Group D lasted longer than that in Group A (P < 0.05) and Group B (P < 0.05) from the first day to 1 wk, the improvement in ALB in Group D appeared the second day after treatment; Group B showed a significant reduction in ALT level within 1 to 3 d after IL-1Ra injection compared with Group A (P < 0.05); MSCs transplantation showed a slight effect on reducing TB and ammonia level in Group C (Table 1).
| Group | Pre-operation | Intra-operation | Time after operation | ||||||||
| D1 | D2 | D3 | D4 | D5 | W1 | W2 | W4 | ||||
| ALT (U/L) | A | 54.7 ± 22.7 | 111.4 ± 44.1 | 167.25 ± 25.8 | 108.8 ± 25.2 | 89.3 ± 32.8 | 63.7 ± 24.6 | 54.1 ± 13.7 | 48.3 ± 17.1 | 38.7 ± 16.3 | 32.4 ± 4.2 |
| B | 48.9 ± 17.3 | 127.5 ± 20.8 | 108.5 ± 21.8a | 77.6 ± 18.9a | 78.5 ± 16.6a | 68.7 ± 10.8 | 55.6 ± 12.9 | 51.6 ± 10.7 | 42.8 ± 11.6 | 45.6 ± 6.9 | |
| C | 52.7 ± 14.7 | 113.1 ± 23.5 | 157.8 ± 31.3 | 118.6 ± 16.7 | 83.6 ± 19.3 | 70.5 ± 11.2 | 58.8 ± 14.9 | 50.6 ± 7.3 | 51.7 ± 9.1 | 44.5 ± 6.9 | |
| D | 45.2 ± 7.7 | 112.6 ± 22.9 | 75.4 ± 10.1ae | 66.1 ± 16.7ae | 48.2 ± 11.9ae | 55.7 ± 9.3 | 51.9 ± 10.8 | 44.7 ± 6.1 | 35.9 ± 2.6 | 33.6 ± 4.2 | |
| TB (μmol/L) | A | 2.9 ± 1.3 | 24.0 ± 8.5 | 32.3 ± 7.4 | 26.2 ± 8.8 | 17.5 ± 5.1 | 12.9 ± 3.8 | 7.9 ± 1.9 | 5.8 ± 1.9 | 3.1 ± 1.2 | 1.9 ± 0.5 |
| B | 2.3 ± 1.5 | 20.8 ± 7.6 | 28.3 ± 7.2 | 19.4 ± 5.7 | 9.7 ± 2.8 | 9.2 ± 1.8 | 8.6 ± 2.2 | 6.5 ± 0.8 | 3.6 ± 0.9 | 2.1 ± 0.5 | |
| C | 1.3 ± 0.9 | 18.9 ± 5.7 | 27.5 ± 5.3 | 15.8 ± 4.1a | 10.7 ± 2.6 | 7.3 ± 0.8 | 5.1 ± 0.8 | 1.9 ± 0.3a | 1.7 ± 0.6 | 0.98 ± 0.1 | |
| D | 1.52 ± 0.73 | 24.2 ± 4.4 | 17.1 ± 2.7ace | 8.9 ± 3.51ac | 5.11 ± 3.3a | 2.68 ± 2.03ac | 1.96 ± 1.51ac | 1.47 ± 0.75ac | 1.25 ± 0.7a | 0.43 ± 0.05 | |
| NH3 (μmol/L) | A | 33 ± 5.2 | 344.5 ± 102.1 | 267.5 ± 134.6 | 179 ± 33.6 | 159.2 ± 41.3 | 111.7 ± 32.6 | 88.5 ± 30.7 | 64.8 ± 7.3 | 99.3 ± 16.4 | 69 ± 24.2 |
| B | 39 ± 10.4 | 318 ± 67.8 | 206.5 ± 22.1 | 160.6 ± 18.2 | 128.8 ± 18.5 | 92.5 ± 11.3 | 114.2 ± 25.6 | 88.5 ± 19.7 | 73 ± 16 | 55.6 ± 11.8 | |
| C | 59 ± 12.4 | 335.8 ± 81.5 | 210.1 ± 21.7 | 163.5 ± 18.2 | 92.8 ± 23.3a | 121.4 ± 24.5 | 110.8 ± 53.2 | 97.2 ± 26.8 | 48.9 ± 10.4a | 57.2 ± 10.5 | |
| D | 38 ± 13.5 | 369.3 ± 104.2 | 148.7 ± 39.4ace | 106.1 ± 23.8ac | 81.3 ± 24.2ac | 84.6 ± 17.5a | 87.7 ± 23.9 | 62 ± 17.8 | 42.7 ± 13.3ac | 41.7 ± 11.3 | |
| ALB (g/L) | A | 30.5 ± 4.3 | 27.7 ± 2.4 | 26.2 ± 3.0 | 25.1 ± 2.1 | 24.4 ± 1.6 | 25.2 ± 1.9 | 25.6 ± 1.4 | 26.3 ± 1.2 | 26.7 ± 1.7 | 28.5 ± 2.5 |
| B | 31.4 ± 3.3 | 28.7 ± 2.6 | 27.1 ± 1.8 | 25.7 ± 2.4 | 25.9 ± 2.7 | 25.6 ± 1.5 | 26.9 ± 0.9 | 26.5 ± 3.2 | 26.8 ± 3.7 | 28.8 ± 2.3 | |
| C | 28.8 ± 2.8 | 27.2 ± 2.2 | 26.6 ± 3.3 | 24.9 ± 1.8 | 25.1 ± 1.1 | 25.8 ± 1.6 | 26.3 ± 0.8 | 27.1 ± 4.2 | 27.9 ± 1.5 | 30.5 ± 2.1 | |
| D | 30.6 ± 3.5 | 26.7 ± 1.2 | 25.1 ± 2.2 | 26.2 ± 2.1 | 27.7 ± 1.5 | 30.1 ± 0.9ace | 30.7 ± 1.4ace | 31.7 ± 2.6 | 32.2 ± 2.1ace | 33.5 ± 2.5 | |
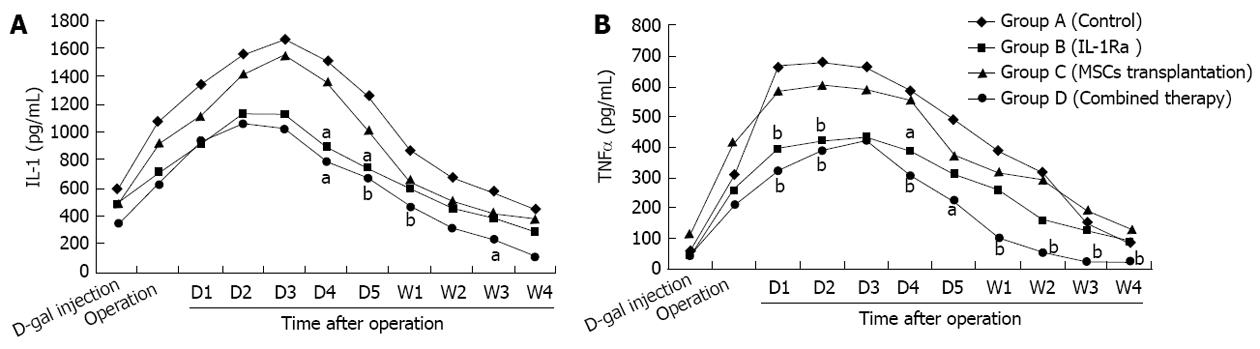
Following D-gal induction, the levels of inflammatory cytokines, IL-1, IL-2 and TNF-α, in all groups increased significantly and reached a peak within 3 d, which lasted for several days before gradually declining. Group B and Group D had a faster improvement in IL-1 and TNF-α than the other groups, Group D had the lowest inflammatory level of all, with IL-1 and TNF-α levels significantly lower than Group A (P < 0.05) from the first injection of 2 mg/kg IL-1Ra; serum inflammatory cytokine levels were better in Group C than in Group A, however, no statistically significant difference between the groups was observed (Figure 2).
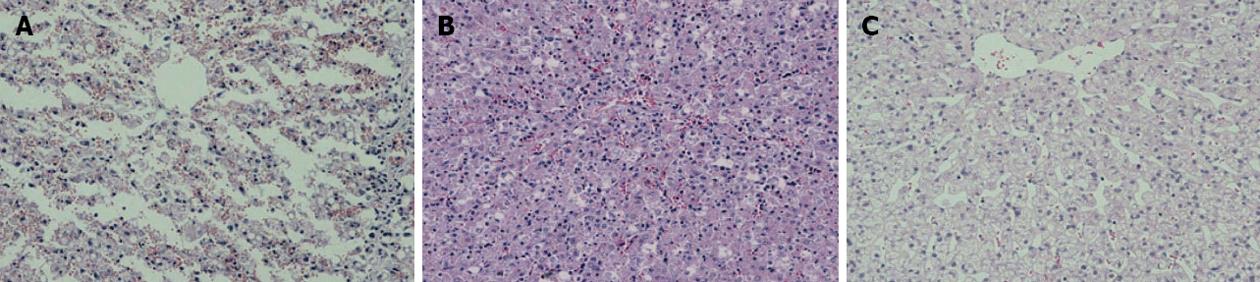
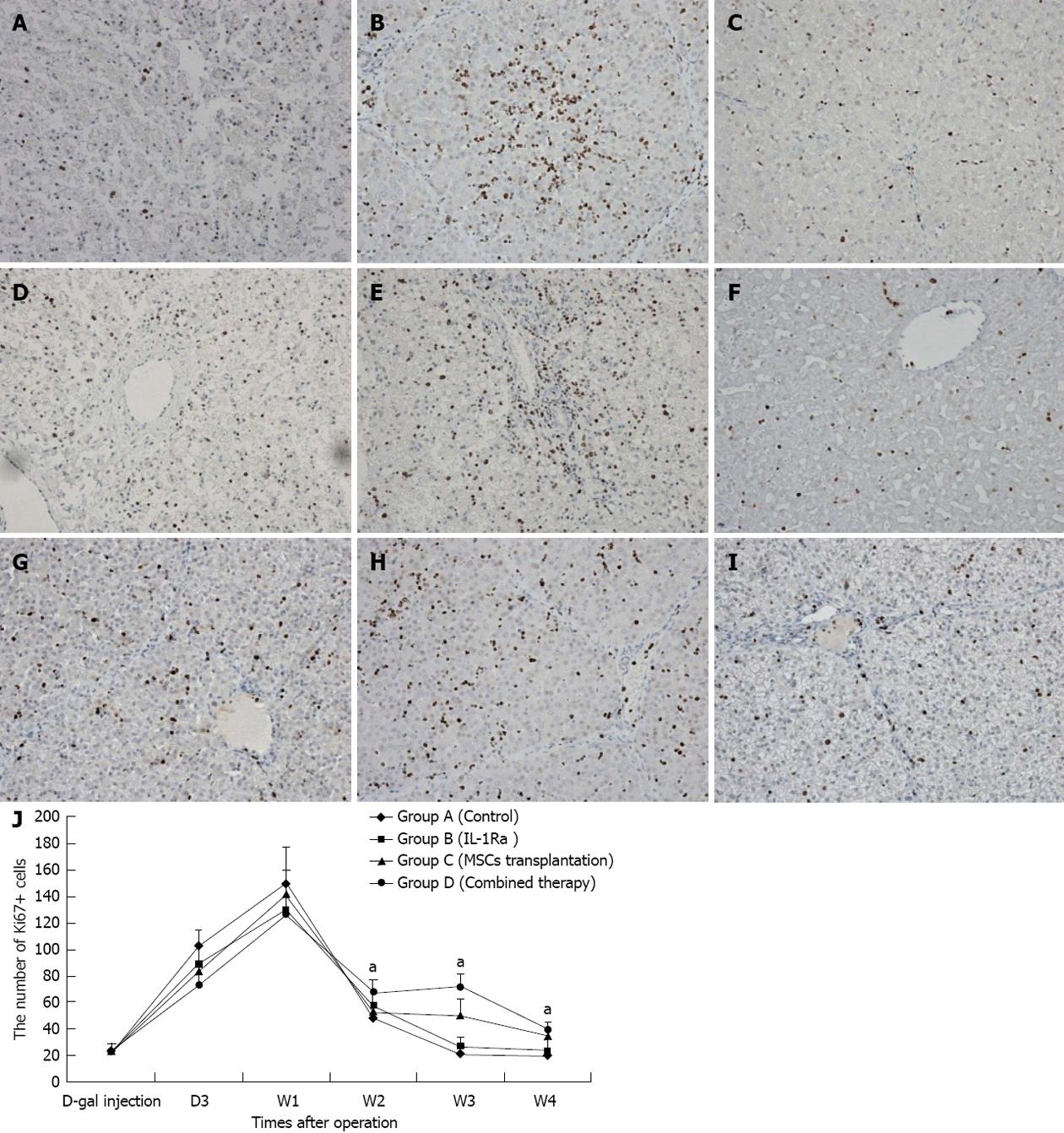
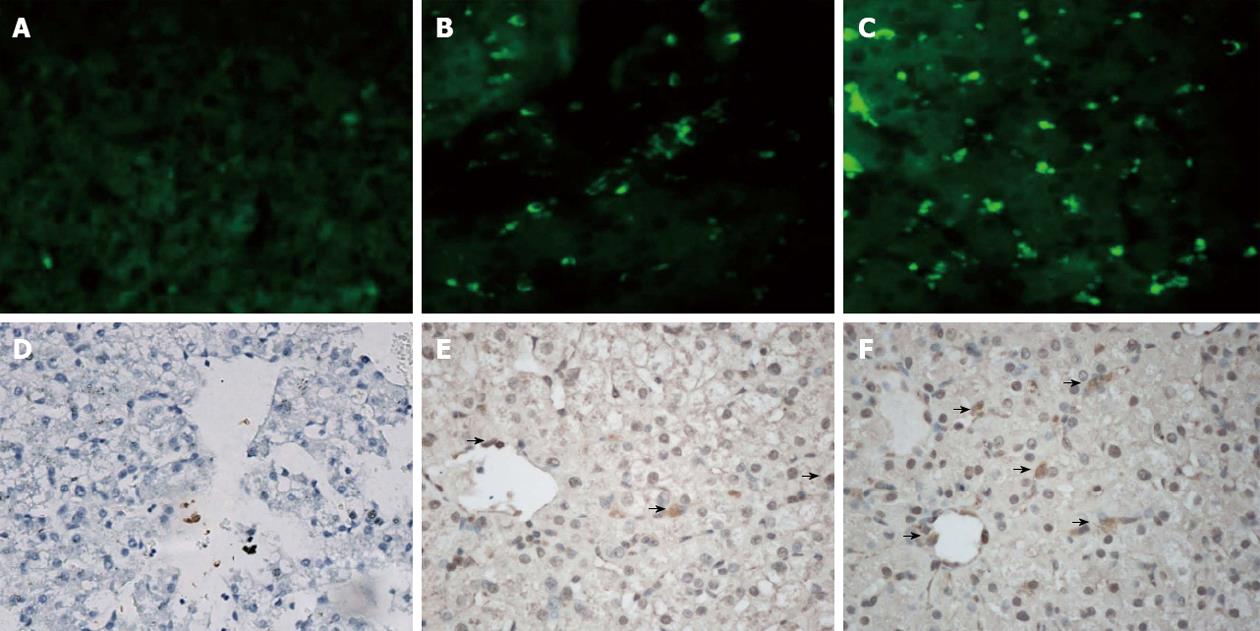
D-gal-induced liver damage was observed by HE staining in all groups, however, less inflammatory cell infiltration and relatively complete lobular architecture were found in Group D, and liver damage was worst in Group A (Figure 3). The number of Ki-67+ cells increased quickly after D-gal induction and reached a peak within 1 wk in all groups, and then a sharp decline was observed in Group A to a normal proliferation level at the end of 3 wk after operation, however, the Ki-67 positive cell index in Group D was maintained at a high level (P < 0.05); the performance of Group B and Group C were better than Group A, but there were no significant differences between the groups (Figure 4). Fluorescent microscopy revealed that there were more GFP-MSCs in Group D than in Group B at 1 wk after treatment, and most of these were distributed in the hepatic lobule along the central vein. Similar results were obtained by immunohistochemistry using anti-GFP (Figure 5).
ALF is a severe liver disease with large quantities of liver cell necrosis; liver transplantation is the only effective treatment, but has a number of difficulties. MSCs can be easily obtained from bone marrow and have multilineage potential. Petersen et al[13] and Schwartz et al[5] showed that MSCs possess the potential ability for hepatocyte differentiation in vitro and in vivo. Sakaida et al[14] confirmed that MSCs were involved in both liver repair and reconstruction. In their research, MSCs transplantation was used to reduce CCl4-induced liver fibrosis in mice, and their findings showed that MSCs transplantation was an ideal candidate treatment for liver disease. What will happen if MSCs transplantation is used for the treatment of ALF? di Bonzo et al[15] xenografted human MSCs into acute liver injured NOD/SCID mice and CCl4-induced liver injury in mice and demonstrated that the number of original human MSCs in acute liver injured mice was less than in chronically injured livers, and the number of hepatocytes undergoing differentiation was even less. In our study, there was no obvious improvement in liver function in the MSCs transplantation group (Group C), few GFP-MSCs were observed on fluorescent microscopy, and little differentiation was seen. Therefore, we concluded that MSCs transplantation for the treatment of ALF is largely limited by its low implantation and differentiation rate in acute liver injured patients.
In recent years, experimental studies have demonstrated that microcirculatory dysfunction and an inflammatory environment are determinants of ALF, and proinflammatory mediators such as IL-1, IL-2 and TNF-α are the key players[9,12,16-18]. Vodovotz also proved that the levels of these cytokines in ALF patients were significantly higher than in normal and chronic hepatitis patients; IL-1 may be a main driver of late inflammation which leads to further injury[3,9,19]. Given this, we presume that the inflammatory environment in ALF patients may be largely related to the low efficiency of MSCs transplantation, that is the inflammatory environment caused by proinflammatory mediators leads to the low survival and differentiation rate of transplanted MSCs. Our pre-experiment results proved this assumption: animals with lower levels of inflammatory cytokines had a higher MSCs implantation rate and differentiation rate. Therefore, we realized that reducing the inflammation level in the acutely injured liver may be a way of improving the efficacy of MSCs transplantation in ALF patients. IL-1Ra is a natural IL-1 antagonist, and can block the inflammatory process by competitively binding to the IL-1 receptor with an avidity equal to that of IL-1, but fails to stimulate downstream signals, thereby reducing the inflammation level[8,20]. An imbalance between IL-1 and IL-1Ra can be observed in a variety of inflammatory diseases including ALF[3,8,12,20]. IL-1Ra is significantly associated with the level of liver inflammation and is an independent marker unaffected by obesity, alcohol consumption, and insulin resistance[21], and can inhibit the process of hepatocellular apoptosis in mice with acetaminophen-induced ALF significantly improving their survival rate[22]. Therefore, will MSCs transplantation become an efficient treatment for ALF when it is combined with IL-1Ra used to relieve liver inflammation?
In this research, the combined therapy of IL-1Ra with MSCs transplantation was administered for acute liver injury, and the results obtained were very promising. Increased levels of proinflammatory cytokines such as IL-1, IL-2 and TNF-α were seen in all animals injected with D-gal, a slight improvement was observed when MSCs were transplanted in Group B animals, and better results were achieved in Group C and Group D animals following IL-1Ra injection. Thus, exogenous IL-1Ra had an enormous effect in reducing some proinflammatory mediators, and improving the inflammatory environment. This effect may last for at least a month as the animals in Group C and Group D showed a continuous reduction in these proinflammatory mediators compared with the other two groups in later experiments, which was thought to be mainly related to the damaged inflammatory cycle caused by IL-1Ra in the very early phase. Improved liver inflammation was then observed following MSCs transplantation. As shown in the results section, Group D treated with combination therapy had the highest GFP-MSCs implantation rate and the best liver function. In addition, the trend in proliferation level in the four groups was different, although the level in all groups peaked at a similar time point after surgery, Group D had a higher proliferation level than the other groups at the end of 2 wk of combination therapy and this difference was maintained for at least 2 wk; which was thought to be caused by both IL-1Ra and MSCs transplantation. IL-1Ra improved the liver inflammatory environment then increased liver cell proliferation rate and MSCs transplantation efficiency, and high MSCs transplantation efficiency may directly lead to a higher hepatocyte differentiation rate, proliferation level and better liver function.
Thus, IL-1Ra can improve liver inflammation and then enhance the effect of MSCs transplantation. Combination therapy with IL-1Ra and MSCs transplantation can promote the restoration and reconstruction of acute liver injury in swine, and is a promising future treatment for patients with ALF.
Cell transplantation is an effective therapy for acute liver failure; however, the activity and function of transplanted cells are largely limited by the inflammatory environment of acute liver failure (ALF) liver. Interleukin-1 (IL-1) is primarily a proinflammatory cytokine and IL-1 receptor antagonist (IL-1Ra) is the most effective antagonist. The combination therapy with IL-1Ra and mesenchymal stem cell (MSC) transplantation for the treatment of ALF is an interesting way and responded well.
The essence of ALF is severe inflammation leading to cell necrosis in a large number of liver cells. Microcirculatory dysfunction and an inflammatory environment are determinants of ALF, and proinflammatory mediators such as IL-1, IL-2 and tumor necrosis factor α are the primary players. The key to the treatment of ALF is the reduction of liver cell necrosis and the stimulation of liver cell regeneration.
Recent reports have highlighted the effect of IL-1Ra injection on ALF models. In this study, the authors investigated the effect of bone marrow MSCs transplantation combined with IL-1Ra injection on ALF swine. Based on the results of the study, the authors concluded that MSCs transplantation is somewhat useful for ALF swine and that the combined therapy of IL-1Ra with MSCs transplantation is a promising treatment for ALF.
According to this article, it may represent a future strategy for therapeutic intervention in the treatment of patients with ALF.
IL-1Ra is the interleukin-1 receptor antagonist, which can bind to the IL-1 receptor and blocks IL-1 action through competitive inhibition, but will not initiate the IL-1 signaling cascade due to its IL-1-like structure which will not induce a signal at all.
The authors evaluated the effect of bone marrow mesenchymal stem cell transplantationcombined with IL-1Ra injection on ALF swine. Group D (combine therapy of MSC transplantation + IL-1Ra) showed significantly improvement of biochemical assay, serum inflammation level and histological results compared with other groups (P < 0.05), labeled MSCs of Group D were more than Group B, and the long-term cell proliferation rate was the best in all groups too. They concluded that MSC transplantation is somewhat useful for ALF swine, IL-1Ra plays an important role in alleviating inflammatory condition, and the combined therapy is a promising treatment for ALF. These results are interesting and important in the therapy for ALF.
P- Reviewers Yamakawa M, Iizuka M, Sumi S, Sakata N S- Editor Huang XZ L- Editor A E- Editor Li JY
| 1. | Foston TP, Carpentar D. Acute liver failure. Crit Care Nurs Clin North Am. 2010;22:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Nguyen NT, Vierling JM. Acute liver failure. Curr Opin Organ Transplant. 2011;16:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Vodovotz Y, Constantine G, Faeder J, Mi Q, Rubin J, Bartels J, Sarkar J, Squires RH, Okonkwo DO, Gerlach J. Translational systems approaches to the biology of inflammation and healing. Immunopharmacol Immunotoxicol. 2010;32:181-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Laleman W, Wilmer A, Evenepoel P, Verslype C, Fevery J, Nevens F. Review article: non-biological liver support in liver failure. Aliment Pharmacol Ther. 2006;23:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Pournasr B, Mohamadnejad M, Bagheri M, Aghdami N, Shahsavani M, Malekzadeh R, Baharvand H. In vitro differentiation of human bone marrow mesenchymal stem cells into hepatocyte-like cells. Arch Iran Med. 2011;14:244-249. [PubMed] |
| 7. | Cho KA, Lim GW, Joo SY, Woo SY, Seoh JY, Cho SJ, Han HS, Ryu KH. Transplantation of bone marrow cells reduces CCl4 -induced liver fibrosis in mice. Liver Int. 2011;31:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 2010;43:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277-5286. [PubMed] |
| 11. | Orelio C, Haak E, Peeters M, Dzierzak E. Interleukin-1-mediated hematopoietic cell regulation in the aorta-gonad-mesonephros region of the mouse embryo. Blood. 2008;112:4895-4904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1668] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 14. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2011;91:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Yazdi AS, Drexler SK, Tschopp J. The role of the inflammasome in nonmyeloid cells. J Clin Immunol. 2010;30:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Kim YS, Park HJ, Hong MH, Kang PM, Morgan JP, Jeong MH, Cho JG, Park JC, Ahn Y. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front Biosci. 2009;14:2845-2856. [PubMed] |
| 19. | Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, Sébire G. Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine. 2008;43:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Arend WP. Cytokine imbalance in the pathogenesis of rheumatoid arthritis: the role of interleukin-1 receptor antagonist. Semin Arthritis Rheum. 2001;30:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Pihlajamäki J, Kuulasmaa T, Kaminska D, Simonen M, Kärjä V, Grönlund S, Käkelä P, Pääkkönen M, Kainulainen S, Punnonen K. Serum interleukin 1 receptor antagonist as an independent marker of non-alcoholic steatohepatitis in humans. J Hepatol. 2012;56:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Hu J, Yan D, Gao J, Xu C, Yuan Y, Zhu R, Xiang D, Weng S, Han W, Zang G. rhIL-1Ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab Invest. 2010;90:1737-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |