Published online Mar 28, 2013. doi: 10.3748/wjg.v19.i12.1962
Revised: February 5, 2013
Accepted: February 7, 2013
Published online: March 28, 2013
Processing time: 174 Days and 13.7 Hours
AIM: To investigate whether curcumin could attenuate hepatitis in mice with paracetamol overdose.
METHODS: Male mice were divided into four groups. Group 1 (control, n = 8); was fed with distilled water; Group 2 [N-acetyl-P-aminophenol (APAP), n = 8]; was fed with a single dose of 400 mg/kg APAP dissolved in distilled water; Group 3 [APAP + curcumin (CUR) 200, n = 8], was fed with a single dose of 400 mg/kg APAP and 200 mg/kg CUR; Group 4 (APAP + CUR 600, n = 8), was fed with a single dose of 400 mg/kg APAP and 600 mg/kg CUR. Twenty-four hours later, the liver was removed to examine hepatic glutathione (GSH), hepatic malondialdehyde (MDA), and histopathologically. Then whole blood was withdrawn from heart to determine transaminase (serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase) and inflammatory cytokines [interleukin (IL)-12 and IL-18] levels by enzyme linked immunosorbent assay.
RESULTS: Serum transaminase, hepatic MDA, and inflammatory cytokines increased significantly in the APAP compared with the control group. Curcumin supplementation in APAP + CUR 200 and APAP + CUR 600 groups significantly decreased these parameters compared with the APAP group. The level of GSH decreased significantly in the APAP compared with the control group. Curcumin supplementation in APAP + CUR 200 and APAP + CUR 600 groups significantly increased these parameters compared with the APAP group. The histological appearance of the liver in the control group showed normal. In the APAP-treated group, the liver showed extensive hemorrhagic hepatic necrosis at all zones. Curcumin supplementation in APAP + CUR 200 and APAP + CUR 600 groups, caused the liver histopathology to improve. In the APAP + CUR 200 group, the liver showed focal necrosis and but the normal architecture was well preserved in APAP + CUR 600 group.
CONCLUSION: APAP overdose can cause liver injury. Results indicate that curcumin prevents APAP-induced hepatitis through the improvement of liver histopathology by decreased oxidative stress, reduced liver inflammation, and restoration of GSH.
- Citation: Somanawat K, Thong-Ngam D, Klaikeaw N. Curcumin attenuated paracetamol overdose induced hepatitis. World J Gastroenterol 2013; 19(12): 1962-1967
- URL: https://www.wjgnet.com/1007-9327/full/v19/i12/1962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i12.1962
In therapeutic doses, N-acetyl-P-aminophenol (APAP) or paracetamol is mainly metabolized via glucuronidation and sulfation pathway and in conjugated forms is excreted from the cells. Besides, APAP is partly metabolized by cytochrome P450 (CYP 2E1), to produce toxic metabolites such as N-acetyl-p-benzoquinone imine. These metabolites are produced in the liver and detoxified by reduced glutathione (GSH) and then removed from cells. In APAP overdose, it is mainly metabolized by CYP 2E1 and causes increase of toxic metabolites and GSH depletion. These metabolites interact with biomolecules such as protein, lipid, and nucleic acid via covalent binding, which disrupts hepatocytes function causing hepatic necrosis and liver injury[1-6].
A previous study demonstrated that APAP overdose treatment showed significantly increase in serum transaminase, hepatic malondialdehyde (MDA), and decreased hepatic GSH. Histological examination showed a severe centrilobular hepatic necrosis with fatty changes[7-9].
Curcumin (diferuloymethane), a polyphenol, is an active ingredient of turmeric (Curcuma longa) and is pharmacologically safe for humans and animals. Curcumin has many biological activities, including anti-inflammatory, anti-oxidant, anti-carcinogenic, anti-mutagenic, and anti-diabetic activities[10-13]. The hepatoprotection of curcumin has been widely acknowledged and used in traditional medicines for treatment of inflammatory conditions such as hepatitis[14].
A previous study demonstrated that curcumin treatment showed significantly decrease in serum transaminase, hepatic MDA, increase hepatic GSH, and caused improvement of liver histopathology[7-9].
However, it is still unclear whether curcumin has any effect in APAP-induced hepatotoxicity. Therefore, the present study aims to examine the protective effect of curcumin on hepatitis in mice with APAP overdose.
Male mice (4-5 wk), weighing 25-30 g, were purchased from the National Laboratory Animal Center, Mahidol University (Bangkok, Thailand). They were acclimatized at least 1 wk in a climate-controlled room on a 12-h light-dark cycle and were fed ad libitum. The experimental protocol was approved by the Ethical Committee of Faculty of Medicine, Chulalongkorn University, Thailand.
A single dose of 400 mg/kg of APAP (Tylenol®) was dissolved in distilled water that was freshly prepared for the experiment. A single dose of 200 and 600 mg/kg of curcumin (95% purified curcumin, Cayman Chemical Company, Ann Arbor, MI, United States) were dissolved in corn oil that was freshly prepared for the experiment.
All mice were fasted, with free access to water ad libitum, for 18 h before the experiment. They were randomly divided into four experimental groups.
Group 1 (control, n = 8); mice were fed distilled water orally via an intragastric tube; Group 2 (APAP, n = 8); mice were fed a single dose of 400 mg/kg of APAP orally via an intragastric tube; Group 3 [APAP + curcumin (CUR) 200, n = 8]; mice were fed a single dose of 400 mg/kg of APAP with a single dose of 200 mg/kg of curcumin orally via an intragastric tube; Group 4 (APAP + CUR 600, n = 8); mice were fed a single dose of 400 mg/kg of APAP with a single dose of 600 mg/kg of curcumin orally via an intragastric tube.
Twenty-four hours later, the mice were anesthetized with intraperitoneal injection of thiopental (50 mg/kg body weight). The abdominal wall was incised and liver was removed and washed with cold normal saline (4 °C-8 °C). The liver was chopped into small pieces, frozen in liquid nitrogen, and stored at -80 °C to examine hepatic MDA and hepatic GSH. The hepatic MDA was quantified by thiobarbituric acid reaction as described by Ohkawa et al[15]. The hepatic GSH was quantified by GSH Assay Kit (Cayman Chemical Company, United States). The remaining liver was fixed in 10% formalin solution to examine histologically. Then whole blood was withdrawn from heart. The blood was allowed to coagulate at room temperature (2 h) and centrifuged for 20 min at 3000 ×g to obtain serum. The serum was collected to examine transaminase (serum glutamic oxaloacetic transaminase, serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase, SGPT) and inflammatory cytokines interleukin (IL)-12 (R and D Systems, Inc., United States) and IL-18 (Medical and Biological Laboratory Co., Ltd, Japan) by enzyme-linked immunosorbent assay method.
Samples of the liver were excised and transferred to formalin and later processed by routine techniques prior to embedding in paraffin. Sections were cut at the thickness of 5 μm and stained with hematoxylin and eosin (HE). An experienced pathologist blinded to the experiment evaluated all samples. All histopathological changes were observed under light microscope. Hepatic necroinflammation score in each section was graded according to the criteria described by Brunt et al[16] from 0 to 3 as follow; Score 0 = No hepatocyte injury/inflammation; Score 1 = Sparse or mild focal zone 3 hepatocyte injury/inflammation; Score 2 = Noticeable zone 3 hepatocyte injury/inflammation; Score 3 = Severe zone 3 hepatocyte injury/inflammation.
The data were expressed as mean ± SD. For comparison among all groups of animals, one-way analysis of variance (one-way ANOVA) and Tukey PostHoc comparisons were employed. P-value at less than 0.05 was considered statistically significant. The data were analyzed using the SPSS software version 17.0 for windows.
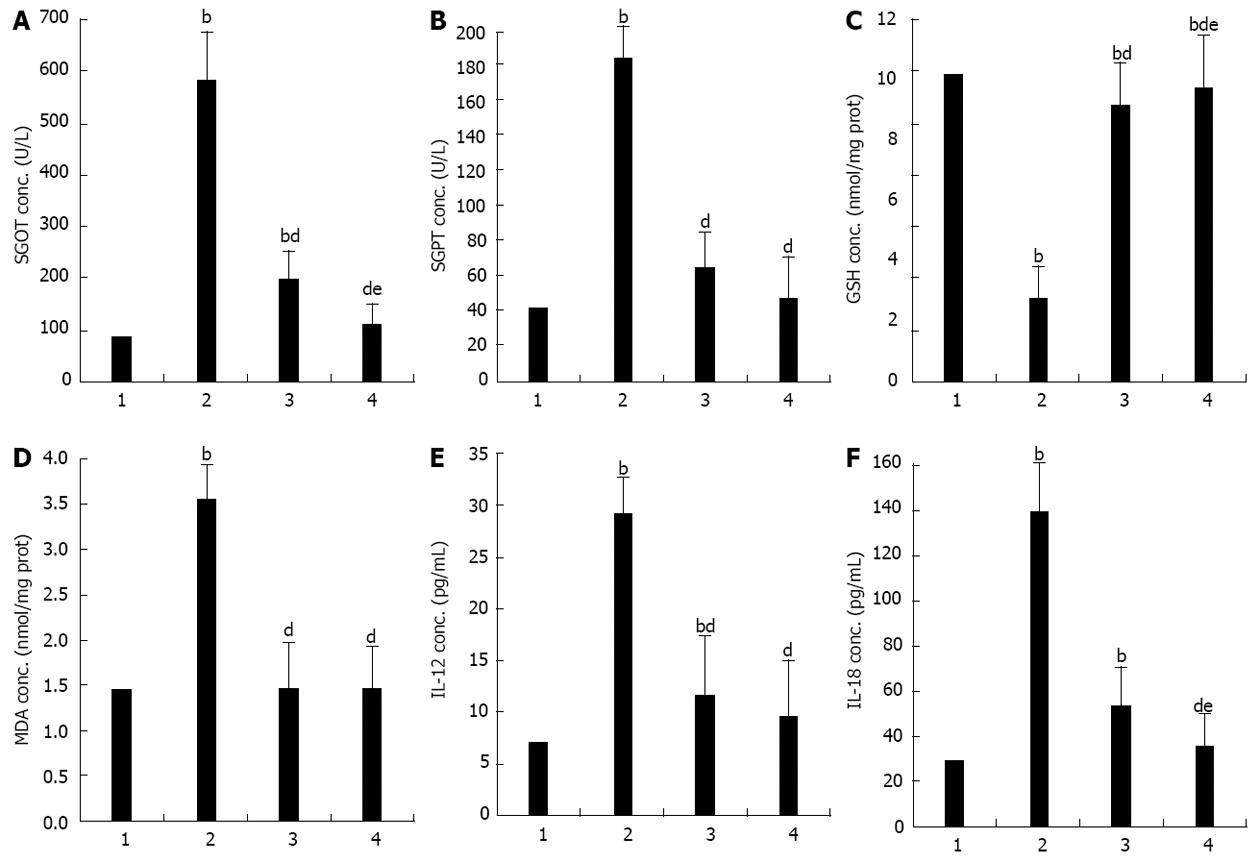
Serum SGOT increased significantly in the APAP when compared to the control group (SGOT, 583.25 ± 118.30 U/L vs 86.13 ± 6.90 U/L, P < 0.001). These were significantly lower in the APAP + CUR 200 and APAP + CUR 600 groups than that in the APAP group (197.38 ± 14.39 U/L vs 538.25 ± 118.30 U/L and 111.38 ± 8.33 U/L vs 583.25 ± 118.30 U/L, P < 0.001, respectively). There was statistically significant difference in serum SGOT in APAP + CUR 200 and APAP + CUR 600 groups (197.38 ± 14.39 U/L vs 111.38 ± 8.33 U/L, P < 0.05) (Figure 1A).
Serum SGPT increased significantly in the APAP group when compared to the control group (186.00 ± 43.73 U/L vs 42.63 ± 6.95 U/L, P < 0.001). They were significantly lower in the APAP + CUR 200 and APAP + CUR 600 groups than in the APAP group (65.25 ± 3.11 U/L vs 186.00 ± 43.73 U/L and 47.50 ± 4.72 U/L vs 186.00 ± 43.73 U/L, P < 0.001, respectively). There was no statistically significant difference in serum SGPT in APAP + CUR 200 and APAP + CUR 600 groups (65.25 ± 3.11 U/L vs 47.50 ± 4.72 U/L, P > 0.05) (Figure 1B).
Hepatic GSH were significantly lower in the APAP group compared to the control group (2.75 ± 0.16 nmol/mg protein vs 10.17 ± 0.11 nmol/mg protein, P < 0.001). Hepatic GSH in APAP + CUR 200 and APAP + CUR 600 groups were significantly higher than in the APAP group (9.16 ± 0.49 nmol/mg protein vs 2.75 ± 0.16 nmol/mg protein and 9.72 ± 0.22 nmol/mg protein vs 2.75 ± 0.16 nmol/mg protein, P < 0.001, respectively). There was statistically significant difference in hepatic GSH in APAP + CUR 200 and APAP + CUR 600 groups (9.16 ± 0.49 nmol/mg protein vs 9.72 ± 0.22 nmol/mg protein, P < 0.05) (Figure 1C).
Hepatic MDA was elevated significantly in the APAP group when compared to the control group (3.55 ± 0.05 nmol/mg protein vs 1.45 ± 0.01 nmol/mg protein, P < 0.001). Hepatic MDA in APAP + CUR 200 and APAP + CUR 600 groups were significantly lower than in the APAP group (1.47 ± 0.01 nmol/mg protein vs 3.55 ± 0.05 nmol/mg protein and 1.46 ± 0.01 nmol/mg protein vs 3.55 ± 0.05 nmol/mg protein, P < 0.001, respectively). There was no statistically significant difference in hepatic MDA in APAP + CUR 200 and APAP + CUR 600 groups (1.47 ± 0.01 nmol/mg protein vs 1.46 ± 0.01 nmol/mg protein, P > 0.05) (Figure 1D).
The level of serum IL-12 increased significantly in the APAP group compared with the control group (29.16 ± 3.34 pg/mL vs 7.08 ± 1.40 pg/mL, P < 0.001). Serum IL-12 significantly decreased in the APAP + CUR 200 and APAP + CUR 600 groups compared with the APAP group (11.60 ± 1.68 pg/mL vs 29.16 ± 3.34 pg/mL and 9.63 ± 1.38 pg/mL vs 29.16 ± 3.34 pg/mL, P < 0.001, respectively). There was no statistically significant difference in serum IL-12 in APAP + CUR 200 and APAP + CUR 600 groups (11.60 ± 1.68 pg/mL vs 9.63 ± 1.38 pg/mL, P > 0.05) (Figure 1E).
The level of serum IL-18 increased significantly in the APAP group compared with the control group (139.52 ± 15.59 pg/mL vs 29.17 ± 3.72 pg/mL, P < 0.001). Serum IL-18 significantly decreased in the APAP + CUR 200 and APAP + CUR 600 groups compared with the APAP group (53.48 ± 18.19 pg/mL vs 139.52 ± 15.59 pg/mL and 35.21 ± 2.18 pg/mL vs 139.52 ± 15.59 pg/mL, P < 0.001, respectively). There was statistically significant difference in serum IL-18 in APAP + CUR 200 and APAP + CUR 600 groups (53.48 ± 18.19 pg/mL vs 35.21 ± 2.18 pg/mL, P < 0.05) (Figure 1F).
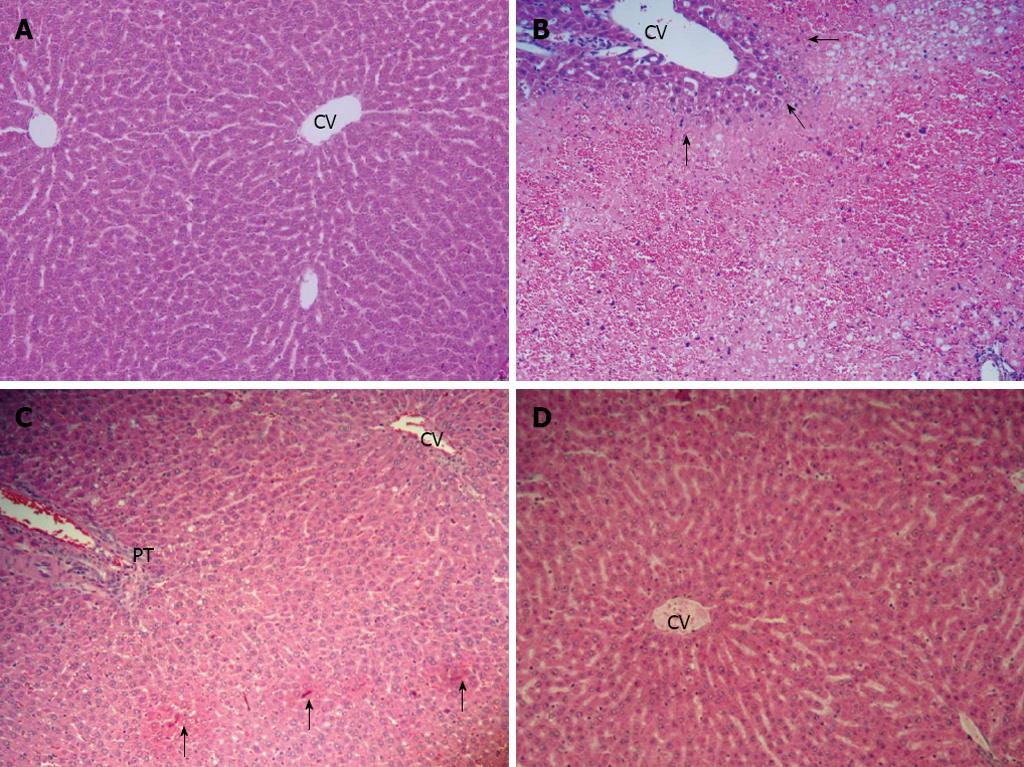
The histological appearance of the liver in the control group showed normal (Figure 2A). In the APAP group, the liver showed extensive hemorrhagic hepatic necrosis of all zones (Figure 2B). In APAP + CUR 200 and APAP + CUR 600 groups, the liver histopathology improved. The APAP + CUR 200 group showed focal necrosis (Figure 2C), whereas the majority of hepatic lobules were preserved as normal architecture with limited hepatic change in the APAP + CUR 600 groups (Figure 2D). The summary of hepatic necroinflammation score in the control and experimental groups are shown in Table 1.
| Group | n | Hepatic necroinflammation scores | |||
| 0 | 1 | 2 | 3 | ||
| Control | 8 | 8 | - | - | - |
| APAP | 8 | - | 1 | 1 | 6 |
| APAP + CUR 200 | 8 | 5 | 2 | 1 | - |
| APAP + CUR 600 | 8 | 5 | 3 | - | - |
This study demonstrated that treatment of APAP overdose induced hepatitis in mice and could be attenuated by treatment with curcumin. This result corresponds to previous observations studied in mice and rat models[7-9].
Chemoprevention is promising as a preventive approach for hepatitis. Curcumin (diferuloylmethane), a polyphenol compound, is an active ingredient of turmeric (Curcuma longa)[10]. The phenolic and methoxy groups on the benzene rings of curcumin are important structural features that contribute to its anti-oxidant properties and its ability to reduce the amount of free radicals[17-20]. Curcumin shows beneficial effects in inflammatory conditions including hepatitis[14].
To assess lipid peroxidation we used MDA as a marker[21] and reduced GSH as indicator of hepatoprotectivity for cells. In this study the oxidative damage caused by APAP overdose was significantly attenuated by administrating curcumin. It was conceivable that curcumin could protect against free radical mediated oxidative stress by scavenging for free radicals that limits lipid peroxidation involved in membrane damage. In this study the GSH depletion caused by APAP overdose was significantly attenuated by administrating curcumin. The protective action of curcumin can be explained by GSH inducer through induction of glutathione reductase enzyme system.
Serum transaminase, SGOT and SGPT, are often used as markers as their increase indicates liver damage[22,23]. There is a significant increase of both SGOT and SGPT in APAP overdose. We demonstrated that this increase was reduced by the administration of curcumin.
It has been reported that IL-12 is produced by dendritic cells, monocytes, Langerhans cells, neutrophils, and keratinocytes[24]. IL-18 is produced by activated macrophages, keratinocytes, intestinal epithelial cells, oeteoblasts, adrenal cortex cells, and the murine diencephalon. IL-18 is synthesized as a precursor 24 kilodalton molecule without a signal peptide and must be cleaved to produce an active molecule. IL-1 converting enzyme (or caspase-1) cleaves pro-IL-18, producing the mature, bioactive peptide that is readily released from cells[25-29]. Importantly, both of these cytokines are produced from Kupffer cells or hepatic macrophages in the liver. It may be possible that inflammation is due to hepatocytes releasing cytokines into the blood stream. Furthermore, IL-12, in combination with IL-18, causes inflammation via the activity of interferon-gamma, which is produced from T-lymphocyte and NK cells.
Curcumin is modulates the inflammatory response by down-regulating the activity of COX-2, inducible NO synthase, tumor necrosis factor-α, IL-1, IL-2, IL-6 and IL-8[30]. This shows that curcumin is an anti-inflammatory substance. These cytokines are required for the expression of many cells linked with the inflammatory response. However, there are no experiments which study the effect of curcumin in preventing hepatitis resulting from APAP overdose and influence the levels of cytokines IL-12 and IL-18. Therefore, we studied the effect of curcumin on decreasing hepatitis resulting from APAP overdose. We showed that curcumin could prevent hepatitis resulting from APAP overdose and cause decrease in IL-12 and IL-18 levels. It may be possible that curcumin can inhibit caspase-1 enzyme, which is cleaved pro-IL-18 into bioactive peptide or active-IL-18[26]. This could explain that another pathway may also reduce liver inflammation that is indirectly mediated by oxidative stress inhibition.
In the present study, APAP overdose caused extensively hepatic necrosis. In curcumin supplementation, liver histopathology improved and showed only a focal hepatic necrosis of lobules.
In conclusion, APAP overdose can cause liver injury. Our results show curcumin could attenuate APAP-induced liver injury by decrease oxidative stress, reduce liver inflammation, restore hepatic GSH, and improve liver histopathology. In addition, curcumin at the dose of 600 mg/kg tends to be more potent than 200 mg/kg in preventing the effects of APAP hepatotoxicity. Hence, curcumin might be a novel therapeutic strategy against hepatitis caused by APAP overdose.
N-acetyl-P-aminophenol (APAP) overdose induced liver damage is one of the most widespread drug-induced side-effects. Although the exact mechanism of APAP remains largely unknown, it appears to involve two pathways: direct hepatotoxicity and adverse immune reactions. This impairment of liver functions can culminate in cell death, leading to a variety of pathological conditions, including acute hepatitis. Curcumin has been shown to possess a wide spectrum of biological actions. These include anti-inflammatory and anti-oxidant activities. Authors postulated that curcumin, acting through the oxidative stress inhibition, could reduce the production of inflammatory cytokines; thus resulting in the attenuation of liver injury in APAP-induced hepatitis in mice.
Curcumin (diferuloymethane) is the main yellow bioactive component of turmeric (Curcuma longa). It has been shown to possess a wide spectrum of biological actions by the inhibition of oxidative stress and reduction of inflammatory cytokines. APAP can cause liver injury through the increase in oxidative stress and release of inflammatory cytokines leading to liver injury. The hallmark of this study was that we showed an attenuation of liver damage and decrease in oxidative stress, reduced liver inflammation, restoration of hepatic glutathione (GSH), and improved liver histopathology after curcumin administration in APAP-treated animals.
The previous study showed that curcumin is an anti-inflammatory and anti-oxidant agent in an in vivo study. However, it is unclear whether curcumin has any effect in APAP-induced hepatitis in vivo. Therefore, in this study, authors examined the protective effects of curcumin in APAP-induced liver damage in mice and authors found that curcumin could attenuate APAP-induced liver injury through the decrease in oxidative stress, reduce liver inflammation, restoration of hepatic GSH, and improve liver histopathology.
Curcumin might be a novel therapeutic strategy against hepatitis caused by APAP overdose.
This is an interesting study of the effects of curcumin on APAP-induced hepatitis in mice. This study showed the effects of curcumin in attenuation of APAP-induced hepatitis reflected in attenuated levels of hepatic malondialdehyde, transaminase, inflammatory cytokines, hepatic GSH, and improved liver histopathology.
P- Reviewer Rege RV S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Hart SG, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD. Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 1994;126:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 464] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | McCuskey RS, Bethea NW, Wong J, McCuskey MK, Abril ER, Wang X, Ito Y, DeLeve LD. Ethanol binging exacerbates sinusoidal endothelial and parenchymal injury elicited by acetaminophen. J Hepatol. 2005;42:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Mladenović D, Radosavljević T, Ninković M, Vucević D, Jesić-Vukićević R, Todorović V. Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem Toxicol. 2009;47:866-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ishibe T, Kimura A, Ishida Y, Takayasu T, Hayashi T, Tsuneyama K, Matsushima K, Sakata I, Mukaida N, Kondo T. Reduced acetaminophen-induced liver injury in mice by genetic disruption of IL-1 receptor antagonist. Lab Invest. 2009;89:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Somanawat K, Thong-Ngam D, Klaikeaw N. Effects of curcumin attenuated hepatitis in mice with paracetamol overdose. Thai J Gastroenterol. 2012;13:43-49. |
| 8. | Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol. 2010;628:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010;48:3246-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1090] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 11. | Murathanum R, Thong-Ngam D, Klaikaew N. Curcumin Prevents Indomethacin-induced Acute Gastric Mucosal Damage in Rats. Thai J Gastroenterol. 2008;9:118-123. |
| 12. | Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol. 2010;16:4039-4046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 13. | Cerný D, Lekić N, Váňová K, Muchová L, Hořínek A, Kmoníčková E, Zídek Z, Kameníková L, Farghali H. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: relationship to HO-1/CO antioxidant system. Fitoterapia. 2011;82:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol. 2009;2009:981963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18815] [Article Influence: 409.0] [Reference Citation Analysis (0)] |
| 16. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2887] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 17. | Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169-171. [PubMed] |
| 18. | Sreejayan MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 559] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 19. | Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 250] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206:533-540. [PubMed] |
| 21. | Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1862] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 22. | Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;369-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 612] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 23. | Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 959] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1022] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 26. | Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Thong-Ngam D, Tangkijvanich P, Lerknimitr R, Mahachai V, Theamboonlers A, Poovorawan Y. Diagnostic role of serum interleukin-18 in gastric cancer patients. World J Gastroenterol. 2006;12:4473-4477. [PubMed] |
| 28. | Tangkijvanich P, Thong-Ngam D, Mahachai V, Theamboonlers A, Poovorawan Y. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol. 2007;13:4345-4349. [PubMed] |
| 29. | Finotto S, Siebler J, Hausding M, Schipp M, Wirtz S, Klein S, Protschka M, Doganci A, Lehr HA, Trautwein C. Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut. 2004;53:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141-153. [PubMed] |