INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies. HCC accounts for more than 660 000 new cases of cancer arising annually all around the world[1,2]. HCC is a highly malignant tumor and the majority of HCC patients are diagnosed at the intermediate or advanced stages of tumor growth. HCC is often accompanied by poor liver function, which makes surgical resection impossible. Liver transplantation is one treatment option for HCC patients. However, the availability of organ donors limits the application of this treatment. Currently, nonsurgical therapy has been accepted as the main treatment for HCC, and gene therapy for the treatment of HCC has begun to attract increasing attention. RNA interference is a powerful method for the knockdown of pathologically relevant genes. Small interfering RNAs (siRNAs) have been widely demonstrated as effective biomedical genetic-therapy applications for many diseases[3,4]. siRNAs induce sequence-specific gene silencing of the target mRNAs to which they are perfectly complementary by directing the RNA-induced silencing complex to mediate site-specific cleavage, thus destroying the targeted mRNA[5]. In view of its powerful gene-silencing properties, RNAi has been proposed as an important option to validate new therapeutic targets and to develop innovative anticancer therapies.
Intratumoral hypoxia is a common finding in human cancers and associated with an increased risk of tumor regeneration, invasion, metastasis and patient mortality[6]. Hypoxia inducible factor-1 (HIF-1) functions as a master regulator of oxygen homeostasis in almost all nucleated mammalian cells and is composed of the constitutively expressed HIF-1β subunit and the highly regulated HIF-1α subunit. The HIF-1α subunit is the functional subunit of HIF-1 and plays a critical role in cellular adaptation to change in oxygen availability[7-9]. HIF-1α is a central transcription factor produced by tumor cells under hypoxic conditions and is a key regulator of several genes that are important in cancer biology. Over-expression of HIF-1α in human tumors is associated with poor prognosis and poor therapeutic outcomes, and HIF-1α has been suggested as a significant target for cancer therapy[10,11].
According to previous studies[12-14], it is possible to silence HIF-1α using siRNA interference technology. Thus, in this study we constructed specific HIF-1α siRNA interference sequences, and transfected these into hypoxic CBRH-7919 rat hepatoma cells to study the effects of HIF-1α silencing on the activation of the phosphatidylinositol 3’-kinase(PI3K)/AKT signaling pathway and the proliferation of hepatoma cells. Consequently, we found that the HIF-1α silencing significantly inhibits the activation of the PI3K/AKT signaling pathway and the proliferation of hypoxic CBRH-7919 rat hepatoma cells.
MATERIALS AND METHODS
Cell lines and cell culture
The CBRH-7919 rat hepatoma cell line was provided by the Central Laboratory of Sun Yat-Sen University (Guangzhou, China). The hepatoma cells were routinely grown in RPMI1640 medium, supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin in a 37 °C incubator with 5% CO2. The cells were passaged every 2-3 d to maintain exponential growth.
Hypoxia model construction
Cells were plated in 60-mm dishes or 6-well plates according to the experimental requirements and were cultured at 37 °C with 5% CO2. In preparation for the experiment, the medium was replaced with a thin layer of fresh medium which contained 10% FCS to decrease the diffusion distance of the ambient gas. Different densities of CoCl2 (100, 150, 200 and 300 μmol/L) were then mixed into the culture dishes, and the samples were processed for 12 and 24 h. A control sample containing 0 μmol/L CoCl2 was also processed.
Design of siRNA and transfection studies
siRNAs targeted against HIF-1α were designed according to the gene coding sequence of rat HIF-1α obtained from GeneBank. The secondary structure of the HIF-1α gene sequence was analyzed using RNA draw software. Three potential target mRNA sequences were selected and siRNA sequences were determined using online design software (http://www.ambion.com/techlib/misc/sirna_finder.htm): 1:P1:5’-AGUGACUGAUUCUGGCAGCTT-3’, P2: 5’-GCUGCCAGAAUCAGUCACUTT-3’; 2:P1: 5’-GGAUGACUUUAAGCAAGAATT-3’, P2: 5’-UUCUUGCUUAAAGUCAUCCTT-3’; 3:P1: 5’-GAAACUCUUCCAAGCAAUUTT-3’, P2: 5’-AAUUGCUUGGAAGAGUUUCTT-3’. Based on the process of siRNA target sequence design, the silencer siRNAs were produced according to the sequence of the selected target mRNA. Then the siRNAs transfection mixture was produced by mixing the siRNA and Lipofectamine2000TM (Invitrogen, Carlsbad, CA, United States). The mixture containing siRNA sequences was then transfected into cultured cells, according to the manufacturer’s protocol. The cells were harvested 24 h after transfection for analyses. A subset of CBRH-7919 cells was treated only with Lipofectamine 2000 TM reagent as a control group.
Real time reverse transcription-polymerase chain reaction
RNA was extracted directly from all samples using TRIzol reagent (Invitrogen) followed by isopropanol precipitation. An RNA polymerase chain reaction (PCR) kit (TaKaRa) was used to obtain template cDNA for real-time PCR (ABI Prism 7500, Perkin Elmer, Foster City, CA, United States) using SYBR Premix Ex Taq (TaKaRa). Specific primer sequences were designed by TaKaRa as follows: HIF-1α: 5’-ACTGCACAGGCCACATTCAT-3’ (sense) and 5’-CGAGGCTGTGTCGACTGAGA-3’ (antisense); vascular endothelial growth factor (VEGF): 5’-AGGCGAGGCAGCTTGAGTTA-3’ (sense) and 5’-CTGTCGACGGTGACGATGGT-3’ (antisense); β-actin: 5’-CCTAGGCACCAGGGTGTGAT-3’ (sense) and 5’-TTGGTGACAATGCCGTGTTC-3’ (antisense). The initial denaturation phase was 3 min at 95 °C followed by 39 cycles of denaturation at 95 °C for 10 s and annealing at 55 °C for 30 s. Relative quantification of PCR products was performed after normalization to β-actin.
Western blotting
Cellular proteins were extracted with RIPA lysis buffer, and protein concentrations were measured by the Bradford method. Protein samples (20 μg/well) were separated by 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis, and electrophoretically transferred to nitrocellulose membranes. The membranes were then blocked for 60 min and subsequently incubated with primary antibodies (1:3000) overnight at 4 °C prior to incubation with anti-mouse IgG conjugated to horseradish peroxidase (1:6000) for 120 min at room temperature. Finally, after developing with enhanced chemiluminescence detection reagents, the protein bands of membranes were visualized by exposure to x-ray film. Protein expression was quantified by densitometry and normalized to β-actin expression. Anti-HIF-1α, anti-Akt/phosphorylated Akt, anti-VEGF, anti-p21, cyclinD1, and anti-β-actin, antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, United States).
Methyl thiazolyl tetrazolium cell proliferation assay
Cells were seeded into 96-well plates (Costar; Corning Inc., Corning, NY, United States) for 9 h. Following exposure to 0.5% O2, the cells were exposed to various concentrations of cisplatin or doxorubicin for 48 h in a CO2 incubator. Methyl thiazolyl tetrazolium (MTT) (10 μL; 5 g/L in PBS) was added to each well followed by incubation for 4 h at 37 °C. Subsequently, the formazan crystals were solubilized with 150 μL of 10% SDS in 0.01 mol/L HCl for 24 h. The absorbances at 500 nm relative to a reference wave length of 490 nm were determined with a microplate reader (Bio-Rad 680, Bio-Rad, United States). The absorbance values were expressed as percentages relative to the untreated controls, and the concentrations resulting in 50% inhibition of cell growth (IC50 values) were calculated.
Bromodeoxyuridine incorporation assay
CBRH-7919 cells were seeded in 96-well plates and treated with various drugs as indicated in each experiment for 48 h. At the end of treatment, bromodeoxyuridine (BrdU) incorporation was assayed by incubating the cells with BrdU for 0.5-1 h using a BrdU Cell Proliferation Assay Kit (Calbiochem, San Diego, CA, United States) according to the manufacturer’s instructions.
Statistical analysis
All experimental data were expressed as mean ± SD. Comparisons between two groups were made using the Student’s t-test. Statistical significance was determined by analysis of variance followed by Fisher’s least significant difference analysis using the SPSS 19.0 software package. The statistical significance level was set at P < 0.05.
RESULTS
Viability of hepatoma cells under hypoxia
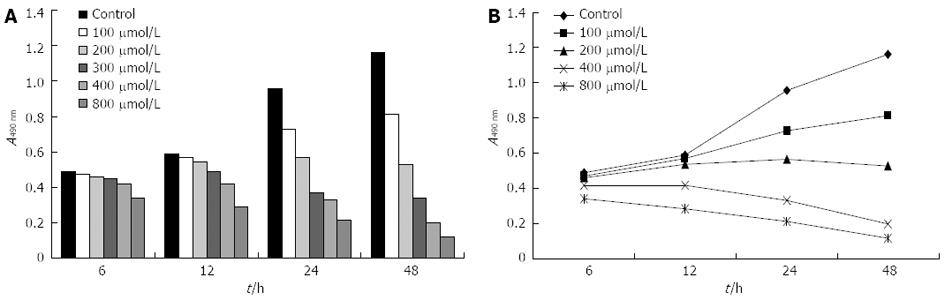
CBRH-7919 hepatoma cells were cultured in a hypoxia model, which was constructed using various concentrations of CoCl2 (Figure 1). The viability of hepatoma cells under hypoxia was observed using MTT, and the results of the analysis indicated that the viability of hepatoma cells was gradually decreased as CoCl2 concentrations increased from 100 μmol/L to 800 μmol/L. The viability of the hepatoma cells reached a minimum at 800 μmol/L CoCl2; however, the viability of the cells was relatively high at CoCl2 concentrations between 100 μmol/L and 200 μmol/L (Figure 1).
Figure 1 Viability of hepatoma cells under hypoxia was measured using methyl thiazolyl tetrazolium cell proliferation assay.
A: Histograms; B: Polygon.
Hypoxia increased the expressions of HIF-1α and VEGF
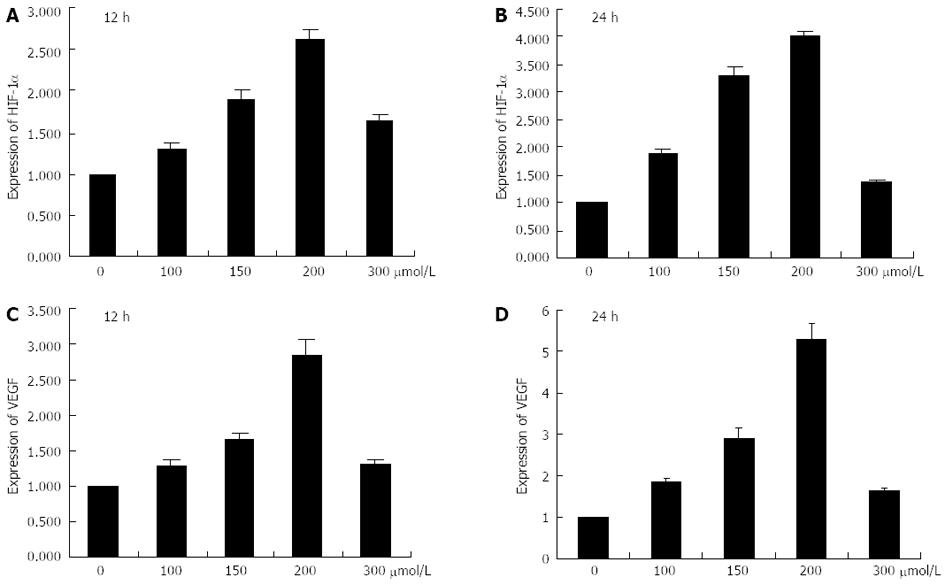
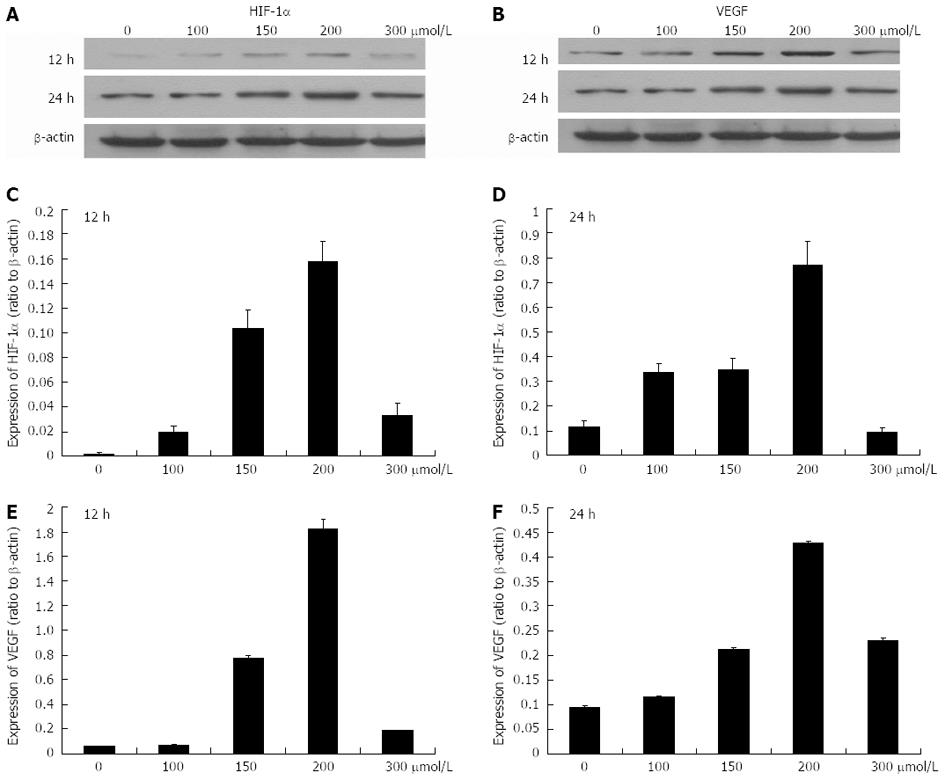
Hepatoma cells were cultured under 0, 100, 150, 200 and 300 μmol/L CoCl2 using processing times of 12 and 24 h, respectively. CoCl2 at 0 μmol/L was the control group. The results of real-time RT-PCR assay indicated that the mRNA levels of HIF-1α and VEGF gradually increased as the concentrations of CoCl2 increased from 0 μmol/L to 200 μmol/L with a 12 h processing time and the expression levels of HIF-1α and VEGF reached a maximum at 200 μmol/L CoCl2 (Figure 2). However, the expressions of HIF-1α and VEGF were declined as the concentration of CoCl2 reached 300 μmol/L. When the processing time was increased to 24 h, the expression levels of HIF-1α and VEGF were higher than those observed at 12 h. Additionally, the protein expression levels of HIF-1α and VEGF demonstrated the same patterns as their respective mRNA expression levels (Figure 3).
Figure 2 mRNA expression levels of hypoxia-inducible factor-1α and vascular endothelial growth factor.
A, B: Histograms illustrating hypoxia-inducible factor-1α (HIF-1α) mRNA expression after exposure to various concentrations of CoCl2 for 12 h and 24 h; C, D: Histograms illustrating vascular endothelial growth factor (VEGF) mRNA expression after exposure to various concentrations of CoCl2 for 12 h and 24 h.
Figure 3 Protein expression levels of hypoxia-inducible factor-1α and vascular endothelial growth factor after exposure to 0-300 μmol/L CoCl2 for 12 and 24 h.
A, B: The Western blotting analysis of protein expression levels of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF); C, D: Histograms illustrating HIF-1α protein expression after exposure to various concentrations of CoCl2 for 12 h and 24 h; E, F: Histograms illustrating VEGF protein expression after exposure to various concentrations of CoCl2 for 12 h and 24 h.
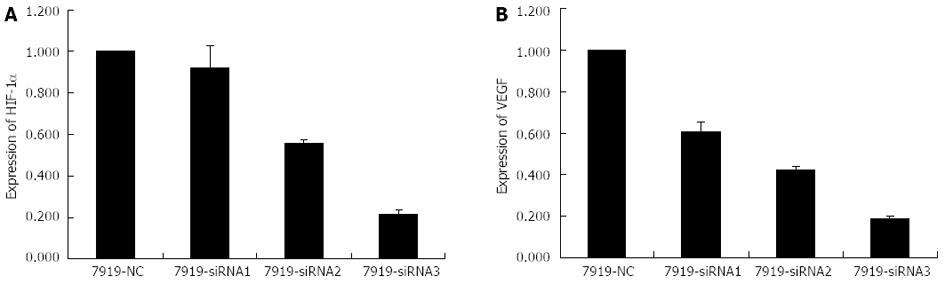
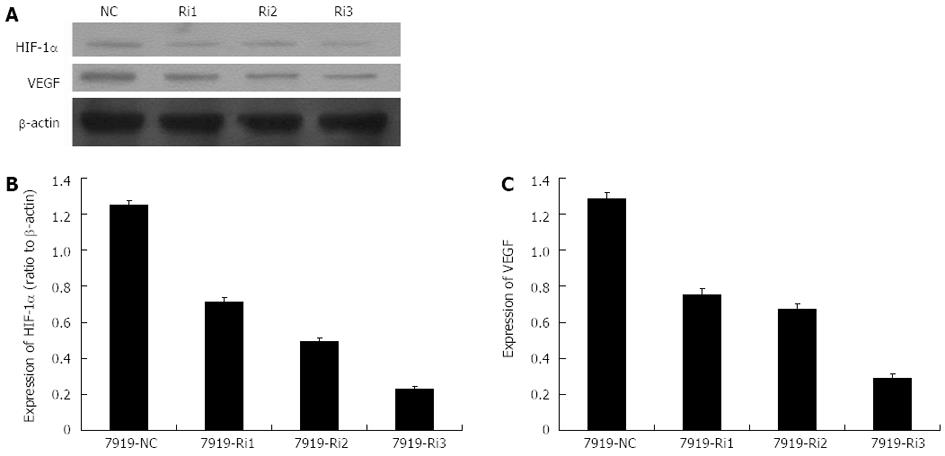
Expressions of HIF-1α and VEGF were inhibited by siRNA interference
Based on the previous phase of our study, the optimal hypoxia-mimicking condition was determined to be the concentration of 150 μmol/L CoCl2 with a 24 h processing time. Thus, CBRH-7919 hepatoma cells were cultured under these conditions prior to the next step of the experiment. The specific siRNA sequences were designed and the hepatoma cells were transfected using three different groups of specific siRNA vectors. The protein and mRNA expression levels of HIF-1α and VEGF were measured using real-time RT-PCR and western blot analysis, respectively. The results indicated that both the mRNA and protein expression levels of HIF-1α and VEGF were significantly decreased after the transfection with specific siRNA sequences with a processing time of 24 h (Figures 4 and 5). However, there were no differences in the mRNA and protein expression levels of HIF-1α and VEGF of hepatoma cells in the control group.
Figure 4 Histograms illustrating hypoxia-inducible factor-1α (A) and vascular endothelial growth factor (B) mRNA expression after small interfering RNAs transfection (24 h processing time).
siRNA: Small interfering RNA.
Figure 5 Protein expression levels of hypoxia-inducible factor-1α and vascular endothelial growth factor after small interfering RNAs transfection (24 h processing time).
A: The Western blotting analysis of protein expression levels of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) after small interfering RNA (siRNA) transfection; B, C: Histograms illustrating HIF-1α (B) and VEGF (C) protein expression after siRNA transfection. NC: Control group without siRNA transfection; Ri1, Ri2, Ri3: Three different specific siRNA sequences.
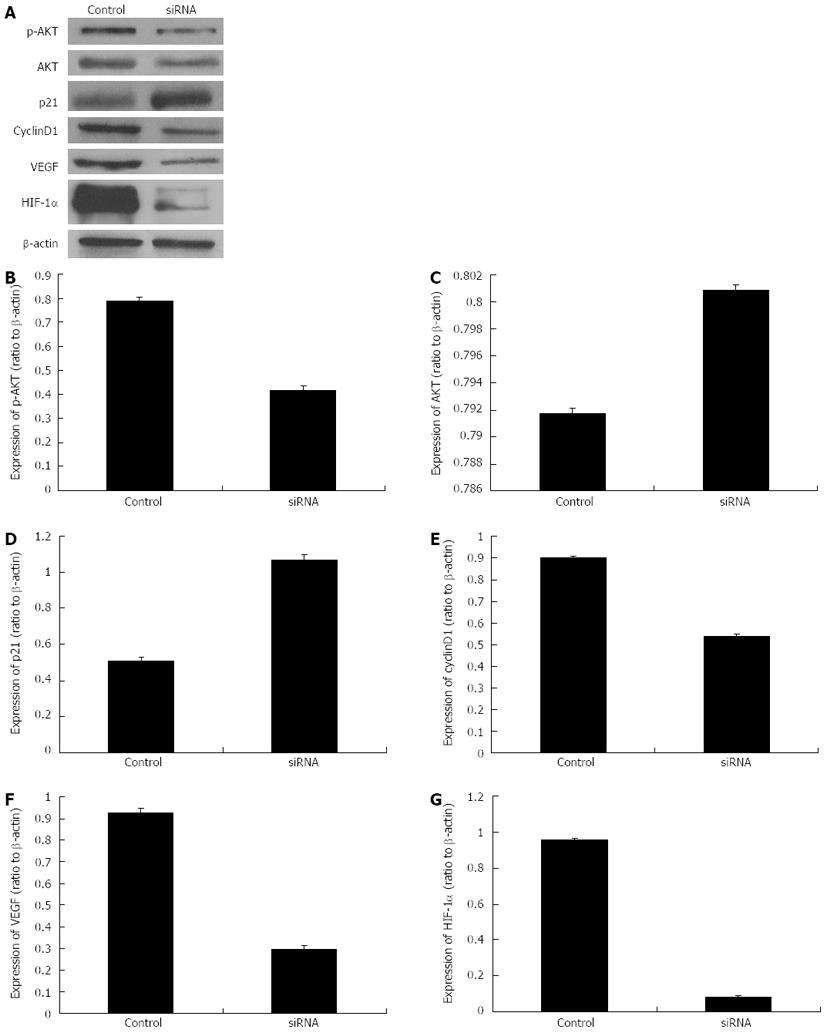
Effects of HIF-1α silencing on the expressions of VEGF, AKT, p-AKT, p21 and cyclinD1
Based on the previous phases of our study, the specific siRNA sequence “Ri3” was selected for the next phase of this study. “Ri3” was transfected into the hepatoma cells cultured with 150 μmol/L CoCl2 with a 24 h processing time, and Western blotting analysis was performed to detect the protein expression levels of HIF-1α, VEGF, AKT, p-AKT, p21 and cyclinD1. The results indicated that the protein expression levels of HIF-1α, VEGF, p-AKT and cyclinD1 were significantly decreased compared with those of the control group; however, the expression of p21 protein was significantly increased and there was no significance difference in the expression of AKT protein (Figure 6).
Figure 6 Protein expression levels of p-AKT, AKT, p21, cyclinD1, vascular endothelial growth factor and hypoxia-inducible factor-1α after the transfection with specific small interfering RNAs (processing time of 24 h).
A: The Western blotting analysis of protein expression levels of p-AKT, AKT, p21, cyclinD1, vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) after specific small interfering RNA (siRNA) transfection; B-G: Histograms illustrating p-AKT (B), AKT (C), p21 (D), cyclinD1(E), VEGF (F) and HIF-1α (G) protein expression after specific siRNA transfection.
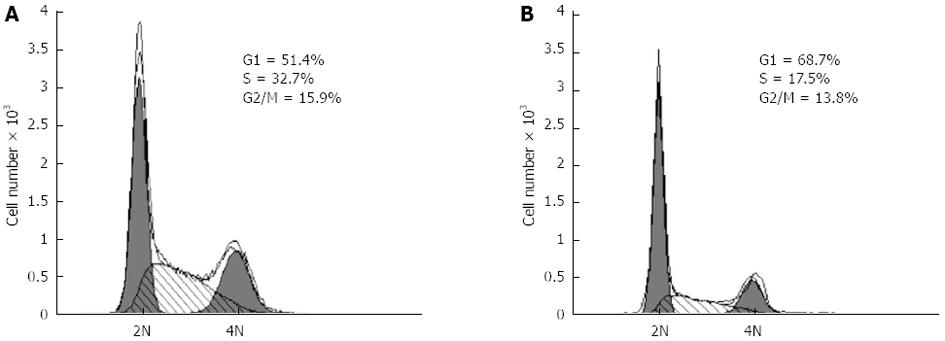
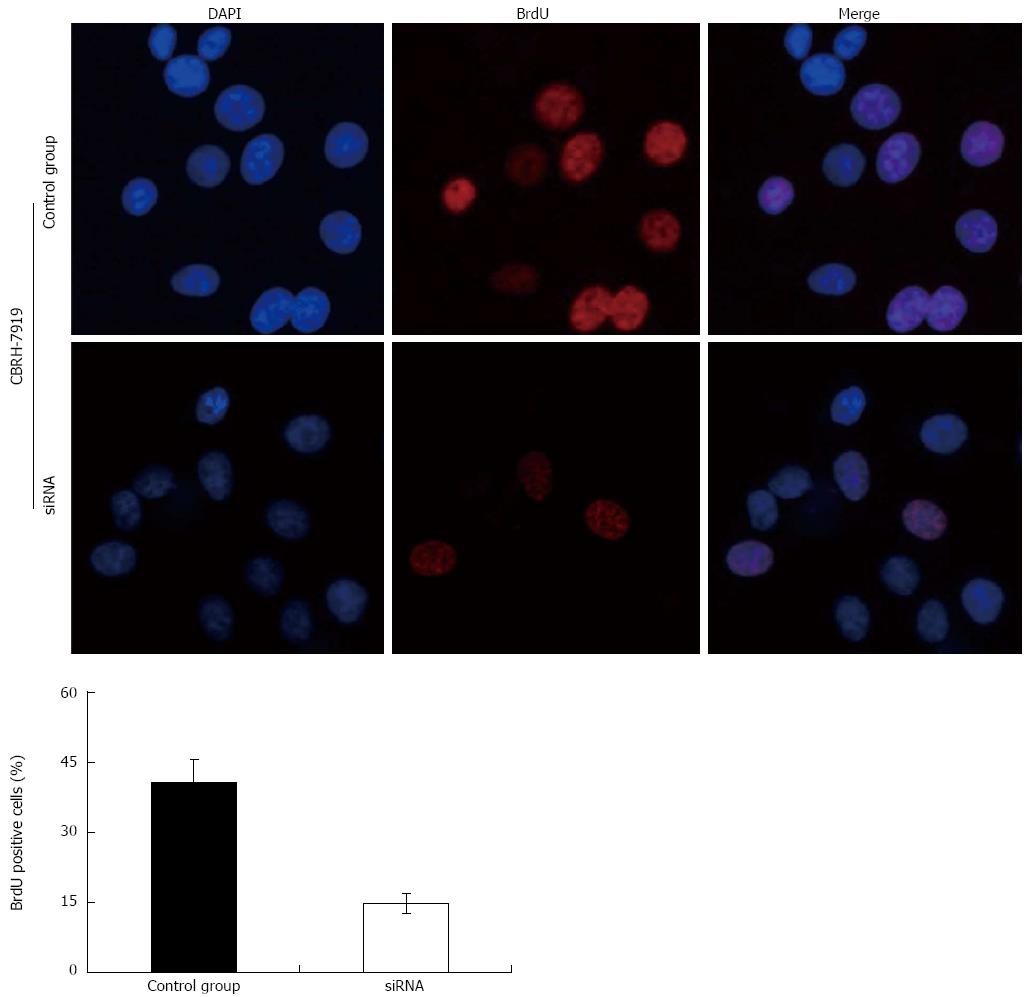
Effects of HIF-1α silencing on the proliferation of hepatoma cells
In consideration of the functions of p21 and cyclinD1 in the cell cycle, the decrease in the expressions of p21 and cyclinD1 caused by HIF-1α silencing suggested that the knockdown of HIF-1α may suppress the proliferation of the hepatoma cells. Thus, we studied the effects of HIF-1α-specific siRNAs on the proliferation of the CBRH-7919 hepatoma cells. HIF-1α-specific siRNAs or controls were transfected using Lipofectamine 2000TM and the proliferation of hepatoma cells was determined using the MTT assay and the BrdU incorporation assay at 24 h after the transfection. The results of the MTT assay and BrdU incorporation assay indicated that the HIF-1α silencing significantly inhibited the proliferation of CBRH-7919 hepatoma cells when compared with the control group at the processing time of 24 h (Figures 7 and 8 ).
Figure 7 Methyl thiazolyl tetrazolium cell proliferation assay indicated that the hypoxia-inducible factor-1α silencing via specific small interfering RNAs significantly inhibited the proliferation of CBRH-7919 hepatoma cells.
A: Contro group; B: Processing time of 24 h.
Figure 8 Proliferation of hepatoma cells detected by bromodeoxyuridine incorporation illustrates that hypoxia-inducible factor-1α silencing via specific small interfering RNAs significantly inhibited the proliferation of CBRH-7919 hepatoma cells.
DAPI: 4',6'-diamidino-2-phenylindole hydrochloride; BrdU: Bromodeoxyuridine.
DISCUSSION
The hypoxia inducible transcription factors mediate the primary transcriptional responses to hypoxic stress in normal and transformed cells[15]. HIF-1α was firstly described by Semenza in 1992 and was shown to play a critical role in mediating O2-dependent transcriptional responses. HIF-1 activity in tumors depends on the availability of the HIF-1α subunit, the expression level of which is increased under hypoxic conditions and is associated with the activation of oncogenes and/or the inactivation of tumor suppressor genes. Over-expression of HIF-1α has been correlated with increased angiogenesis, tumor progression, invasion, metastasis and poor patient prognosis, and this has led to the current interest in HIF-1α as a promising anticancer genetic target for anticancer therapies[16,17].
In this study, rat CBRH-7919 hepatoma cells were cultured in a hypoxic model constructed using CoCl2, and specific HIF-1α siRNA sequences were designed and transfected into hypoxic hepatoma cells. Our study demonstrated that the expressions of HIF-1α, VEGF, p-AKT and cyclinD1 were significantly decreased compared with those of the control group; however, the expression of p21 was significantly increased and there was no significant difference in the expression of AKT. This finding indicates that silencing of HIF-1α using specific siRNA can inhibit the expressions of VEGF, p-AKT and cyclinD1 while up-regulating the expression of p21. The subsequent MTT cell proliferation and BrdU incorporation assays revealed that the proliferation of hepatoma cells was significantly inhibited by HIF-1α silencing.
The PI3K/AKT signaling transduction pathway is one of the most important pathways inside human cancer and plays a critical role in various cellular functions, such as proliferation, adhesion, migration, invasion, metabolism and survival[18-20]. The activation of the PI3K/AKT signaling pathway depends on the expression of p-AKT (phosphorylated AKT). Over-expression of p-AKT is one of the most common indications of human malignancies, such as gastric cancer, liver cancer, colorectal cancer, pancreatic cancer and breast cancer[21,22]. Additionally, p-AKT is believed to play an important role in cancer cell survival and chemotherapy resistance. In our study, although there was no significant difference in the expression of AKT, the expression of p-AKT was significantly inhibited after the specific siRNA sequences were transfected into hepatoma cells. This indicated that the silencing of HIF-1α using specific siRNAs inhibited the activation of the PI3K/AKT signaling transduction pathway. The inhibition of the PI3K/AKT signaling pathway may be associated with inhibition of the activation of the PI3K-independent pathway. Under hypoxia or within tumor cells, the normal degradation of HIF-1α is inhibited and HIF-1α accumulates in the nucleus, which promotes the activation of downstream targeted genes and activates AKT[23-25]. Over-expression of VEGF is also observed in various types of human cancer cells. VEGF promotes the proliferation of vessel endothelial cells, inhibits the apoptosis of vessel endothelial cells, and stimulates the formation of blood vessels. Previous studies reported that VEGF alone can activate the PI3K/AKT pathway[26,27]. Hence, it is indicated that inhibition of the expression of VEGF by HIF-1α silencing may affect the activation of the PI3K/AKT pathway. p21 and cyclinD1 are the downstream targets of the PI3K/AKT signaling transduction pathway and their expressions are also regulated by the pathway[28,29]. Our data revealed that the expression of p21 was significantly increased; however, the expression of cyclinD1 was significantly decreased after HIF-1α was silenced by specific siRNAs. The results of our study provide strong evidence to support our hypothesis that the activation of the PI3K/AKT signaling pathway can be inhibited by HIF-1α silencing.
Considering the functions of p21 and cyclinD1 in cell cycle processing[30-34], HIF-1α silencing may have an effect on PI3K/AKT signaling pathway-dependent proliferation of hepatoma cells as well. In our study, the results of the MTT cell proliferation and BrdU incorporation assays indicated that the transfection of specific siRNAs effectively increased the number of cells in G0/G1 phase, and the numbers of cells in S and G2/M phases were significantly decreased compared with those of the control group. Additionally, the proportion of BrdU-positive cells in the siRNA interference group was smaller than that of the control group. Taken together, these data indicate that HIF-1α silencing by the specific siRNAs can significantly inhibit the activation of the PI3K/AKT signaling transduction pathway and the proliferation of hepatoma cells.
HIF-1α is currently accepted as one of the most important genetic targets for anticancer therapy. However, to our knowledge, there have only been a few studies examining the effects of HIF-1α on the proliferation of hepatoma cells. Our study demonstrated that HIF-1α silencing can inhibit the proliferation of hypoxic rat CBRH-7919 hepatoma cells. However, the correlation between HIF-1α and the PI3K/AKT signaling transduction pathway is still contested[35-38] and further research is urgently needed to provide new evidence. The evidence obtained in our study indicated that HIF-1α can be silenced using specific siRNAs and that the silencing of HIF-1α can significantly inhibit the activation of the PI3K/AKT signal transduction pathway.
Transcatheter arterial chemoembolization (TACE) is the most commonly used palliative treatment for HCC in clinical practice[39-41]. However, the hypoxic microenvironment of tumor tissue after TACE often induces high expression levels of HIF-1α and promotes tumor progression, invasion and metastasis of tumor, providing a poor prognosis for patients with HCC. Thus, the long-term results of TACE are not satisfying. The combination of TACE and siRNA interference for the treatment of HCC can be expected to down-regulate the expression of HIF-1α after TACE. Unfortunately, it is unclear whether the effects of siRNA interference in vivo are the same as (or similar to) that which are observed in vitro. Thus, further studies are required to explore the effects of siRNA interference in vivo.
COMMENTS
Background
The over-expression of hypoxia-inducible factor-1α (HIF-1α) has been demonstrated in multiple types of malignant tumors. HIF-1α contributes to tumor angiogenesis and metastasis and plays a critical role in mediating O2-dependent transcriptional responses. Preliminary tests showed that it is possible to silence HIF-1α using small interfering RNA (siRNA) interference technology.
Research frontiers
Gene therapy is a potential treatment for malignant tumors and some other diseases. siRNAs have been widely demonstrated as effective biomedical genetic-therapy applications for many diseases. In this study, the authors demonstrate that HIF-1α silencing using siRNAs can significantly inhibit the proliferation of hepatoma cells, which could be a potential gene therapy for anti-cancer treatment.
Innovations and breakthroughs
Previous studies reported that HIF-1α contributes to tumor angiogenesis and metastasis and plays a critical role in mediating O2-dependent transcriptional responses. However, the relationship between HIF-1α and the PI3K/AKT signaling pathway, and the effects of HIF-1α on the proliferation of hepatoma cells, have not been unequivocally addressed. The results of this study showed that HIF-1α silencing can significantly inhibit the activation of the PI3K/AKT signaling pathway and the proliferation of hepatoma cells. This study proves that HIF-1α could be an effective and important gene target for anti-cancer treatment.
Applications
By understanding the effect of HIF-1α silencing on the PI3K/AKT signaling pathway and the proliferation of hepatoma cells, an effective gene target has been identified for the gene treatment of hepatocellular carcinoma (HCC), providing an experimental basis for the clinical practice of gene therapies for malignant tumors.
Terminology
HIF-1α has been correlated with increased angiogenesis, tumor progression, invasion, metastasis and poor patient prognosis. In this study, HIF-1α was silenced using siRNA technology. It demonstrated that HIF-1α silencing significantly inhibited the activation of the PI3K/AKT signaling pathway and the proliferation of hepatoma cells, and HIF-1α was proved to be an effective gene target for the gene therapy of HCC.
Peer review
This paper reported that hypoxia induces HIF-1α expression, and knocking-down HIF-1α by siRNA inhibits the PI3K/AKT signaling pathway and the proliferation of hypoxic CBRH-7919 rat hepatoma cells. Those findings indicate HIF-1α could be a potential drug target for cancer therapy.