Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1639
Revised: January 31, 2013
Accepted: February 5, 2013
Published online: March 14, 2013
Processing time: 188 Days and 18.9 Hours
AIM: To evaluate long-term outcomes and prognostic factors for esophageal squamous cell carcinoma (SCC) treated with three dimensional conformal radiotherapy (3D-CRT).
METHODS: Between January 2005 and December 2006, 153 patients (120 males, 33 females) with pathologically confirmed esophageal SCC and treated with 3D-CRT in Cancer Hospital of Shantou University were included in this retrospective analysis. Median age was 60 years (range: 37-84 years). The proportion of tumor location was as follows: upper thorax (including the cervical region), 73 (48%); middle thorax, 73 (48%); lower thorax, 7 (5%), respectively. The median radiation dose was 64 Gy (range: 50-74 Gy). Fifty four cases (35%) received cisplatin-based concurrent chemotherapy. Univariate and multivariate analysis were performed to determine the association between the correlative factors and prognosis.
RESULTS: The five-year overall survival rate was 26.3%, with a median follow-up of 49 mo (range: 3-66 mo) for patients who were still alive. On univariate analysis, lesion location, lesion length by barium esophagogram, computed tomography imaging characteristics including Y diameter (anterior-posterior, AP, extent of tumor), gross tumor volume of primary lesion (GTV-E), volume of positive lymph nodes (GTV-LN), and the total target volume (GTV-T = GTV-E + GTV-LN) were prognostic for overall survival. By multivariate analysis, only the Y diameter [hazard ratio (HR) 2.219, 95%CI 1.141-4.316, P = 0.019] and the GTV-T (HR 1.372, 95%CI 1.044-1.803, P = 0.023) were independent prognostic factors for survival.
CONCLUSION: The overall survival of esophageal carcinoma patients undergoing 3D-CRT was promising. The best predictors for survival were GTV-T and Y diameter.
- Citation: Chen CZ, Chen JZ, Li DR, Lin ZX, Zhou MZ, Li DS, Chen ZJ. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World J Gastroenterol 2013; 19(10): 1639-1644
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1639.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1639
In the Radiation Therapy and Oncology Group (RTOG) 85-01 trial, patients with locally advanced, unresectable esophageal carcinoma (EC) were randomized to receive either chemoradiation or radiation alone. Those patients receiving chemoradiotherapy had a 26% 5-year survival rate compared with 0% in those treated with radiotherapy alone[1]. Since the results of this study were reported, concurrent chemoradiotherapy has become a widely accepted standard treatment for patients with EC who are treated with non-surgical methods. However, not all the patients are good candidates for chemoradiotherapy due to the presence of multiple co-morbid medical conditions. The outcomes and factors that predict overall survival in patients treated with radiotherapy alone should also be considered in the management of esophageal cancer. The development of three-dimensional conformal radiotherapy (3D-CRT) and other advanced radiotherapy techniques has allowed clinicians to treat patients with increased accuracy and normal tissue sparing capabilities. These advantages may allow for the safe delivery of higher radiation doses compared with historic radiotherapy approaches[2-5].
To our knowledge, this study was the largest single institution experience to report the long-term survival for esophageal squamous cell carcinoma (SCC) in patients treated with 3D-CRT with or without chemotherapy in English.
Patients with non-metastatic and pathologically confirmed SCC who received definitive 3D-CRT, with or without chemotherapy, in the Department of Radiation Oncology, Cancer Hospital of Shantou University Medical College (SUMC) between Jan 2005 and December 2006 were enrolled in the study. Patients were treated with definitive chemoradiotherapy because the disease was not amenable to resection, patients had multiple medical co-morbidities that would preclude surgery, or the patient declined surgery. Patients were treated with radiotherapy alone if other co-morbid medical conditions precluded them from receiving concurrent chemotherapy or the patient declined chemotherapy.
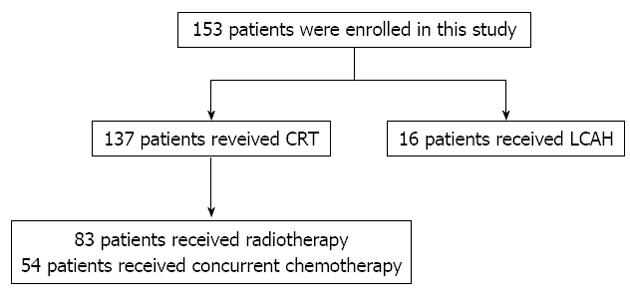
The gross tumor volume (GTV) was identified using both diagnostic and radiotherapy planning computed tomography (CT) images and barium swallow examination. The clinical target volume (CTV) was then expanded from the GTV with a margin of 1.0 cm laterally and a 2 cm margin in the superior and inferior dimensions. An additional 0.5 cm margin expansion around the CTV was included for the planning target volume. For treatment planning purposes, lung V20 30%-35% and spinal cord maximum dose < 45 Gy were used as dose constraints. All 153 patients were treated using 3D-CRT to a median dose 64 Gy (range: 50-74 Gy) in 25 to 37 fractions. Of these, 137 patients received conventionally fractionated radiotherapy at 2 Gy per fraction, five times a week throughout the treatment course, while 16 patients received an accelerated course of treatment in which the first 40 Gy was conventionally fractionated and the latter course was treated with accelerated hyperfractionated radiotherapy consisting of 24 Gy given 1.5 Gy bid, to a total dose of 64 Gy. All fractions were delivered at least 6 h apart. Fifty four patients (35.3%) received cisplatin-based concurrent chemotherapy. The treatment allocation was showed in a patient flow chart (Figure 1).
General clinical features included gender, age, lesion location, late course accelerated hyperfractionation, chemotherapy and radiation dose. Imaging parameters included the lesion length, as defined by esophageal barium swallow; and lesion characteristics on CT imaging, such as the largest diameter, X diameter (maximum lateral extent of tumor) and Y diameter (anterior-posterior, AP, extent of tumor); esophageal wall thickness (maximum thickness of esophageal wall if the esophageal lumen is visible or half of the largest diameter with invisible esophageal lumen); lesion length; invasion to adjacent structures (including thoracic aorta, bronchus, trachea, pericardia); supraclavicular lymph nodes metastasis; number of positive lymph nodes; volume of the primary lesion (GTV-E); the volume of positive lymph nodes (GTV-LN); and the total gross tumor volume (GTV-T = GTV-E + GTV-LN).
Survival curves were generated using the Kaplan-Meier method with mortality data being estimated from first day of radiotherapy until date of death or date of last follow-up. The log-rank test was used to compare the survival curves. A univariate analysis was performed to identify factors for the multivariable model. A multivariate Cox-regression analysis was then performed incorporating significant univariate factors to determine the relevant prognostic factors. Results were reported using hazard ratios (HRs) and 95%CIs. All analyses were performed with SPSS (version 19.0; Chicago, IL). All reported P values were 2-sided with a value < 0.05 considered statistically significant.
Between January 2005 and December 2006, there were 637 patients with EC that were prospectively registered in the Department of Radiation Oncology, Cancer Hospital of SUMC. One hundred and fifty three of these patients met the enrollment criteria. There were 120 males and 33 females, with a median age of 60 years (range: 37-84 years). The number of patients with primary tumor location in upper (including neck), middle and lower thorax was 73 (48%), 73 (48%) and 7 (5%), respectively. Of these patients, 57.5% had invasion to adjacent structures and 64.1% were found with positive regional lymph nodes metastases. Table 1 summarized the radiographic tumor characteristic of 153 patients.
| Characteristic | mean ± SD | Range |
| Length of lesion in barium esophagogram (cm) | 5.8 ± 2.3 | 1.6-12.8 |
| Largest diameter (cm) | 3.4 ± 1.0 | 1.5-6.0 |
| X dimension (cm) | 3.2 ± 1.0 | 1.2-5.9 |
| Y dimension (cm) | 2.5 ± 0.6 | 1.2-4.4 |
| Esophageal wall thickness (cm) | 1.5 ± 0.6 | 0.5-4.2 |
| Length of lesion in CT (cm) | 6.6 ± 2.2 | 2.4-16.0 |
| GTV-E (cm3) | 31.8 ± 20.7 | 3.4-114.3 |
| GTV-LN (cm3) | 4.3 ± 10.6 | 0-78.8 |
| GTV-T (cm3) | 36.1 ± 23.8 | 3.4-124.0 |
For the 153 patients included in this study, the median follow up time was 49 mo (range: 3-66 mo) for the 47 living patients. The 1-, 3- and 5-year overall survival (OS) rates were 72.5%, 34.7% and 26.3%, respectively. The 1-, 3- and 5-year OS rates for the 99 patients that were treated with radiotherapy alone were 69.9%, 34.3% and 26.5%, respectively, and for the 54 patients treated with chemoradiotherapy, the 1-, 3-, and 5-year OS rates were 79.2%, 35.8% and 25.5%, respectively.
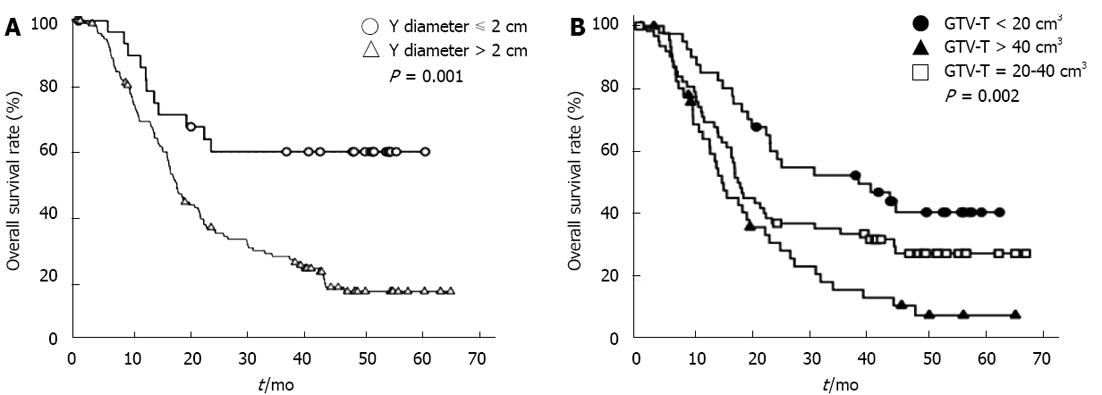
We performed an analysis to identify factors that predicted for OS for patients with EC. The results of univariate analysis and multivariate analysis are shown in Table 2. By univariate analysis, lesion location (P = 0.011); lesion length by barium esophagogram (P = 0.003); lesion characteristics by CT imaging such as the largest diameter (P = 0.002), X diameter (P = 0.006) and Y diameter of lesion (P = 0.001); thickness of esophageal wall (P = 0.016); length of lesion (P = 0.024); invasion to adjacent structure (P = 0.007); invasion of bronchus (P = 0.046); number of metastatic lymph nodes (P = 0.008); GTV-LN (P = 0.010); GTV-E (P = 0.007); and GTV-T (P = 0.002) were significant prognostic factors. By multivariate analysis, only the Y diameter (HR 2.219, 95%CI 1.141-4.316, P = 0.019) and GTV-T (HR 1.372, 95%CI 1.044-1.803, P = 0.023) were independent prognostic factors for survival. The survival curves with different Y diameters and GTV-T are shown in Figure 2.
| Feature | Cases | Median survival (mo) | 5-yr OS | χ2value | P value | |
| Gender | Male | 120 | 19.2 | 24.2% | 0.896 | 0.344 |
| Female | 33 | 24.3 | 34.6% | |||
| Age (yr) | ≤ 60 | 77 | 22.1 | 29.5% | 1.159 | 0.282 |
| > 60 | 76 | 20 | 23.2% | |||
| Lesion location | Neck, upper thorax | 73 | 23.4 | 38.8% | 6.497 | 0.011 |
| Middle, lower thorax | 80 | 18.5 | 13.5% | |||
| LCAH | No | 137 | 19.2 | 26.7% | 0.257 | 0.612 |
| Yes | 16 | 30.4 | 25% | |||
| RT dose (Gy) | ≤ 64 | 86 | 19.4 | 23.8% | 0.429 | 0.512 |
| > 64 | 67 | 22.4 | 29.7% | |||
| Chemotherapy | No | 99 | 18.4 | 26.5% | 0.438 | 0.508 |
| Yes | 54 | 23.4 | 25.5% | |||
| The length of lesion in barium esophagogram (cm) | ≤ 3 | 12 | - | 80.8% | 13.627 | 0.003 |
| > 3, ≤ 5 | 57 | 22.4 | 23.8% | |||
| > 5, ≤ 7 | 52 | 16.8 | 20.9% | |||
| > 7 | 32 | 17.8 | 21.7% | |||
| Largest diameter (cm) | ≤ 2 | 11 | - | 72.7% | 12.729 | 0.002 |
| > 2, ≤ 3 | 43 | 23.4 | 33.7% | |||
| > 3 | 99 | 17.3 | 17.3% | |||
| Y diameter (cm) | ≤ 2 | 29 | - | 60.3% | 11.574 | 0.001 |
| > 2 | 124 | 18.4 | 18.3% | |||
| X diameter (cm) | ≤ 2 | 16 | - | 62.5% | 15.319 | 0.006 |
| > 2, ≤ 3 | 49 | 22.1 | 31.7% | |||
| > 3, ≤ 4 | 59 | 18.1 | 23% | |||
| > 4 | 29 | 17.8 | 0% | |||
| Thickness of esophageal wall (cm) | ≤ 1 | 32 | 34.8 | 46.2% | 8.224 | 0.016 |
| > 1, ≤ 2 | 104 | 18.4 | 22.0% | |||
| > 2 | 17 | 17.2 | 12.8% | |||
| Length of lesion (cm) | < 4 | 10 | - | 57.1% | 7.462 | 0.024 |
| ≥ 4, ≤ 9 | 126 | 20 | 26% | |||
| > 9 | 17 | 13.9 | 9.2% | |||
| GTV-E (cm3) | < 18 | 40 | 37.8 | 42.9% | 9.934 | 0.007 |
| ≥ 18, ≤ 48 | 82 | 18.4 | 24.6% | |||
| > 48 | 31 | 14.5 | 8.6% | |||
| Invasion to adjacent structure | No | 65 | 24.3 | 38.1% | 7.289 | 0.007 |
| Yes | 88 | 17.7 | 17.8% | |||
| Invasion to trachea | No | 96 | 22.5 | 27.5% | 0.865 | 0.352 |
| Yes | 57 | 17.8 | 24.5% | |||
| Invasion to bronchus | No | 131 | 22.1 | 29.9% | 3.992 | 0.046 |
| Yes | 22 | 15.8 | 5.6% | |||
| Invasion to aorta | No | 128 | 22.1 | 29% | 1.469 | 0.226 |
| Yes | 25 | 17.8 | 13.9% | |||
| Invasion to pericardia | No | 145 | 20 | 27.9% | 1.559 | 0.212 |
| Yes | 8 | 16.9 | 0% | |||
| SLNM1 | Yes | 25 | 15.9 | 21.1% | 1.317 | 0.251 |
| No | 107 | 22.4 | 23.6% | |||
| Number of positive lymph nodes2 | 0 | 55 | 30.4 | 38.4% | 9.709 | 0.008 |
| 1-2 | 85 | 18.4 | 21.2% | |||
| > 2 | 13 | 13.8 | 7.7% | |||
| GTV-LN (cm3) | 0 | 55 | 30.4 | 38.4% | 9.229 | 0.010 |
| 0-3 | 59 | 19.2 | 22.8% | |||
| > 3 | 39 | 15.9 | 14% | |||
| GTV-T (cm3) | < 20 | 41 | 39.8 | 41.5% | 12.891 | 0.002 |
| 20-40 | 64 | 18.1 | 28.6% | |||
| > 40 | 48 | 15.4 | 9.2% |
EC is an uncommon malignancy in the United States and adenocarcinoma accounts for nearly 60%[6,7]. In China, EC is endemic and the majority of cases are SCC. We retrospectively analyzed 153 cases of esophageal SCC that were treated with 3D-CRT during 2005 to 2006. The radiation was delivered by 3D-CRT in this patient cohort, which should be contrasted with the 2D conventional radiotherapy technique reported in RTOG 85-01 trial. The 1- 3- and 5-year OS rates were 72.5%, 34.7% and 26.3% respectively, which compares favorably to the results of RTOG 85-01. For the 99 patients who received 3D-CRT alone, the median survival and the 5-year OS rates were 18.4 mo and 26.5%, respectively, which was similar to previous reports from patients receiving chemoradiotherpy[8-12].
Although the RTOG 85-01 trial reported no 5 year survivors in those patients receiving radiation alone, in this current retrospective review, a 5-year OS rate of 26.5% in those patients receiving 3D-CRT alone was observed. We hypothesize that the improvement in outcome may be a function of increased dose conformity and improved tumor targeting. These advances in 3D-CRT likely lead to decreased treatment related toxicities and reduced the potential for “marginal misses”[2-4]. Furthermore, 35.3% of patients received chemotherapy in this study, but no significant difference in survival was found between patients with or without chemotherapy (χ2 = 0.438, P = 0.508). However, due to the limitations of this retrospective study, it is difficult to define the precise role of chemotherapy in EC treated with 3D-CRT.
Tumor length was replaced by depth of esophageal wall invasion as a staging factor in the 1987 version of the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system based on the EC registration database of Japan between 1969 and 1980, which demonstrated that depth of tumor invasion was a better predictor for 10-year survival of EC patients than the superficial extent of tumor[13,14]. In the newly published edition of the AJCC TNM staging system, histological grading and tumor location, as well as depth of esophageal wall invasion, are regarded as prognostic factors for both adenocarcinoma and SCC[15,16]. Nevertheless, several previously published results proposed that tumor length was still an independent staging factor for EC other than previously mentioned prognostic factors[17-20]. However, most, if not all of these results were based on surgery patients, which may be quite different from the outcome of non-surgical cases. Meanwhile, there were few English publications about the prognosis of EC treated with non-surgical approaches.
Many potential prognostic tumor factors achieving significance in univariate analysis were identified in our study. However, only the AP dimension (Y diameter) and GTV-T maintained significance for OS on multivariate analysis. Deep anterior-posterior invasion or large tumor size indicated poor prognosis.
Wang et al[21] reported a Cox model analysis on 100 cases of EC treated with 3D-CRT which also showed similar results as our study, indicating that the GTV-T was an independent predictor for OS. Generally, larger GTV-T means heavier tumor load, increasing numbers of radioresistant hypoxic tumor cells and clonogenic cells and greater limitation of relevant organs at risk that lead to poor survival, which has been regarded as an important predictive factor for cancer in other sites such as lung, breast and head and neck.
Tumor cells tend to invade through the mucosa of the esophagus or spread longitudinally as it is a hollow tube structure. Therefore, deeper invasion through a transverse direction generally means more advanced status. Indeed, the AJCC staging system for EC classifies T staging based upon the depth of tumor invasion[15,16]. However, transverse diameters are more reasonable predictors of the invasion of tumor especially for non-surgical patients due to the imprecise measurement of thickness of esophageal wall in CT images. Y diameter was the only independent prognostic factor in our study. Concerning the adjacent structures, trachea, bronchus and pericardia are anterior and aorta and vertebrae are posterior to the esophagus. The esophageal tumor tends to grow in other directions than anterior or posterior due to the limitation of these adjacent structures. We found Y diameter was much smaller than the other two diameters (2.5 cm vs 3.2 cm or 3.4 cm). This may explain why the Y diameter becomes the most sensitive predictor to the invasion of esophageal cancer, the bigger Y diameter, the lower OS.
In addition to tumor invasion, the AJCC TNM staging system also incorporates the number of metastatic lymph nodes as an independent staging factor for EC[15,16]. Several prior studies have confirmed the prognostic significance of lymph nodes[22-25]. In these studies, lymph node metastases were determined pathologically while in the current study of patients treated by nonsurgical approaches, the lymph node status was determined by CT imaging. Clinical staging is never as accurate as surgical staging which likely explains why lymph node involvement in our study lost significance on multivariate analysis.
In conclusion, 3D-CRT with or without chemotherapy should be considered as a definitive treatment option for patients with inoperable esophageal SCC. Big anterior-posterior tumor dimension (Y diameter) or large tumor size predicted for worse survival which may provide additional prognostic information to the non-surgical staging system and clinical decision making for esophageal SCC.
Esophageal cancer is the eighth most common cancer worldwide. The incidence of this disease in the United States is relatively low and adenocarcinoma accounts for nearly 60%, while in China, esophageal cancer is endemic and the majority of cases are squamous cell cancer (SCC). Radiation therapy is the most commonly used treatment method for patients with this disease under the following conditions: (1) unresectable disease; (2) resectable disease, but with medical co-morbidities that would preclude surgery; (3) patients declined surgery. Since the report of the results of Radiation Therapy and Oncology Group (RTOG) 85-01 trial in 1999, concurrent chemoradiotherapy has become a widely accepted standard treatment for patients with esophageal carcinoma (EC) who are treated with non-surgical methods.
In the RTOG 85-01 trial, patients with locally advanced, unresectable EC were randomized to receive either chemoradiation or radiation alone. Those patients receiving chemoradiotherapy had a 26% 5-year survival rate compared with 0% in those treated with radiotherapy alone. However, not all patients are good candidates for chemoradiotherapy due to the presence of multiple co-morbid medical conditions. The outcomes and factors that predict overall survival in patients treated with radiotherapy alone should also be considered in the management of esophageal cancer. Furthermore, the technique used in this report was a conventional method, whereas the development of three-dimensional conformal radiotherapy (3D-CRT) and other advanced radiotherapy techniques has allowed clinicians to treat patients with increased accuracy and normal tissue sparing capabilities. Therefore, it is meaningful to explore the outcomes and predict outcome factors of patients with the advent of modern radiation techniques.
Patients with esophageal cancer in the previous reports were mostly treated with conventional radiation techniques. This study was the largest single institution experience to report the long-term survival for SCC in patients treated with 3D-CRT with or without chemotherapy in English. The tumor node metastasis (TNM) staging system has long been used to predict the outcomes of patients with esophageal cancer after treatment. However, the current TNM staging category of esophageal cancer is wholly based on pathological findings from surgery. Hence it is inapplicable in patients treated with a non-surgical method, especially radiation therapy. Factors other than pathological findings are desperately needed to help predict outcomes or make clinical decisions. In this study, we enrolled general clinical features (including gender, age, lesion location, late course accelerated hyperfractionation, chemotherapy, radiation dose) and imaging parameters (including the lesion length as defined by esophageal barium swallow and lesion characteristics on computed tomography imaging), (in total 22 factors) in the prediction analysis. These factors could be attained easily through general clinical examinations without surgical approaches.
The study results suggest that 3D-CRT with or without chemotherapy should be considered as a definitive treatment option for patients with inoperable esophageal SCC. Big anterior-posterior tumor dimension (Y diameter) or large tumor size predict for worse survival which may provide additional prognostic information to the non-surgical staging system and clinical decision making for esophageal SCC.
3D-CRT is a type of modern radiation technique, in which the profile of each radiation beam is shaped to fit the profile of the target from a beam’s eye view using multileaf collimator (or lead block) and a variable number of beams. This technique has allowed clinicians to treat patients with increased accuracy and normal tissue sparing capabilities and deliver a higher dose of radiation to the tumor than conventional techniques would allow. Since the start of this century, 3D-CRT has been gradually implemented in the radiation treatment of esophageal cancer.
This is a good study in which authors evaluate long-term outcomes and prognostic factors for esophageal SCC treated with 3D-CRT. The results are interesting and suggest that the overall survival of EC patients undergoing 3D-CRT is promising.
P- Reviewer Koizumi W S- Editor Gou SX L- Editor O’Neill M E- Editor Xiong L
| 1. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 2. | Chen ZJ, Chen CZ, Li DS, Zhang PC, Li DR. Comparison of CT simulation with conventional simulation in the treatment planning of esophageal carcinoma. Zhonghua Fangshe Zhongliu Xue Zazhi. 2001;10:85-87. |
| 3. | Guzel Z, Bedford JL, Childs PJ, Nahum AE, Webb S, Oldham M, Tait D. A comparison of conventional and conformal radiotherapy of the oesophagus: work in progress. Br J Radiol. 1998;71:1076-1082. [PubMed] |
| 4. | Wu VW, Sham JS, Kwong DL. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. Br J Radiol. 2004;77:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Komaki R, Liao Z, Forster K, Lee HK, Stevens CW, Cox JD. Target definition and contouring in carcinoma of the lung and esophagus. Rays. 2003;28:225-236. [PubMed] |
| 6. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 877] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 9. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1081] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 10. | Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 926] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 11. | Crosby TD, Brewster AE, Borley A, Perschky L, Kehagioglou P, Court J, Maughan TS. Definitive chemoradiation in patients with inoperable oesophageal carcinoma. Br J Cancer. 2004;90:70-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Gwynne S, Hurt C, Evans M, Holden C, Vout L, Crosby T. Definitive chemoradiation for oesophageal cancer--a standard of care in patients with non-metastatic oesophageal cancer. Clin Oncol (R Coll Radiol). 2011;23:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Iizuka T, Isono K, Kakegawa T, Watanabe H. Parameters linked to ten-year survival in Japan of resected esophageal carcinoma. Japanese Committee for Registration of Esophageal Carcinoma Cases. Chest. 1989;96:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988;61:2310-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th ed. Chicago, IL: Springer 2010; . |
| 16. | Rice TW, Rusch VW, Blackstone EH. 2009 AJCC/UICC staging of esophageal cancer. General thoracic surgery. 7th ed. Vol 2. Amsterdam, Netherlands: Wolters Kluwer 2009; 2013-2015. |
| 17. | Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Bolton WD, Hofstetter WL, Francis AM, Correa AM, Ajani JA, Bhutani MS, Erasmus J, Komaki R, Maru DM, Mehran RJ. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2009;138:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Wang L, Han C, Zhang X, Wang J, Ma GX, Xiao AQ. Multivariate analysis of three dimensional conformal radiotherapy for esophageal carcinoma. Zhonghua Zhongliu Fangzhi Zazhi. 2009;16:58-61. |
| 22. | Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, Mazumdar M. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994;81:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 196] [Article Influence: 6.3] [Reference Citation Analysis (0)] |