Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7384
Revised: October 29, 2012
Accepted: November 6, 2012
Published online: December 28, 2012
Processing time: 199 Days and 21.8 Hours
AIM: To investigate the effectiveness of 5-flurouracil-based neoadjuvant chemotherapy (NAC) for gastroesophageal and gastric cancer by meta-analysis.
METHODS: MEDLINE and manual searches were performed to identify all published randomized controlled trials (RCTs) investigating the efficacy of the flurouracil-based NAC for gastroesophageal and gastric cancer, and RCTs of NAC for advanced gastroesophageal and gastric cancer vs no therapy before surgery. Studies that included patients with metastases at enrollment were excluded. Primary endpoint was the odds ratio (OR) for improving overall survival rate of patients with gastroesophageal and gastric cancer. Secondary endpoints were the OR of efficiency for down-staging tumor and increasing R0 resection in patients with gastroesophageal and gastric cancer. Safety analyses were also performed. The OR was the principal measurement of effect, which was calculated as the treatment group (NAC plus surgery) vs control group (surgery alone) and was presented as a point estimate with 95% confidence intervals (CI). All calculations and statistical tests were performed using RevMan 5.1 software.
RESULTS: Seven RCTs were included for the analysis. A total of 1249 patients with advanced gastroesophageal and gastric cancer enrolled in the seven trials were divided into treatment group (n = 620) and control group (n = 629). The quality scores of the RCTs were assessed according to the method of Jadad. The RCT quality scores ranged from 2 to 7 (5-point scale), with a mean of 3.75. The median follow-up time in these studies was over 3 years. The meta-analysis showed that NAC improved the overall survival rate (OR 1.40, 95%CI 1.11-1.76; P = 0.005), which was statistically significant. The 3-year progression-free survival rate was significantly higher in treatment group than in control group (37.7% vs 27.3%) (OR 1.62, 95%CI 1.21-2.15; P = 0.001). The tumor down-stage rate was higher in treatment group than in control group (55.76% vs 41.38%) (OR 1.77, 95%CI 1.27-2.49; P = 0.0009) and the R0 resection rate of the gastroesophageal and gastric cancer was higher in treatment group than in control group (75.11% vs 68.56%) (OR 1.38, 95%CI 1.03-1.85; P = 0.03), with significant differences. No obvious safety concerns about mortality and complications were raised in these trials. There were no statistically significant differences in perioperative mortality (5.08% vs 4.86%) (OR 1.05, 95%CI 0.57-1.94; P = 0.87 fixed-effect model) and in the complication rate between the two groups (13.25% vs 9.66%) (OR 1.40, 95%CI 0.91-2.14; P = 0.12 fixed-effect model). Trials showed that patients from Western countries favored NAC compared with those from Asian countries (OR 1.40, 95%CI 1.07-1.83). Monotherapy was inferior to multiple chemotherapy (OR 1.40, 95%CI 1.07-1.83). Intravenous administration of NAC was more advantageous than oral route (OR 1.41, 95%CI 1.09-1.81).
CONCLUSION: Flurouracil-based NAC can safely improve overall survival rate of patients with gastroesophageal/gastric cancer. Additionally, NAC can down the tumor stage and improve R0 resection.
- Citation: Ge L, Wang HJ, Yin D, Lei C, Zhu JF, Cai XH, Zhang GQ. Effectiveness of 5-flurouracil-based neoadjuvant chemotherapy in locally-advanced gastric/gastroesophageal cancer: A meta-analysis. World J Gastroenterol 2012; 18(48): 7384-7393
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7384
Gastric and esophageal cancers are among the leading causes of cancer-related death worldwide[1,2]. In spite of a declining incidence of the distal stomach cancer in the Western countries over the past decades, the incidence of adenocarcinoma of the lower esophagus and the gastroesophageal junction has dramatically increased in the world[3]. Early-stage gastric and gastroesophageal cancers are curable with surgical treatment alone, with a 5-year overall survival rate of 90%. However, the majority of gastric and gastroesophageal cancer patients are diagnosed with advanced diseases (stages III or IV)[4]. The advanced gastric and gastroesophageal cancer without distant metastasis is still a potentially curable disease, but the prognosis is poorer than the early-stage diseases. Treatment of advanced gastroesophageal cancer is still a challenge for gastrointestinal surgeons. Localized tumors, limited to the submucosa, can be best treated surgically, with a long-term survival of 70%-95%, but the prognosis of locally advanced tumors is poor due to a high unresectability rate at presentation, and a much higher relapse rate after radical surgery[5,6], thus demanding further studies regarding neoadjuvant treatment.
Theoretically, the administration of the neoadjuvant chemotherapy (NAC) appears to have several potential benefits for gastroesophageal and gastric cancers: to reduce the tumor volume, to improve the R0 resection rate, to act on micrometastases and to evaluate tumor chemosensitivity to cytotoxic medications. Randomized trials and meta-analyses have demonstrated a benefit with neoadjuvant or perioperative chemotherapy in gastric and gastroesophageal cancers. However, the optimal approach in individual patients is not clear and remains controversial[7,8]. The aim of the current meta-analysis under such circumstances was to evaluate the effectiveness of NAC in treatment of gastric and gastroesophageal cancers and explore the optimal strategy for chemotherapy delivery.
MEDLINE and manual searches were carried out to identify all published RCTs that compared the flurouracil-based NAC plus surgery with surgery alone for advanced gastric and gastroesophageal cancers. The search was done on PudMed using three sets of terms: “esophagogastric junction/gastroesophageal/gastric”; “carcinoma/cancer”; and “neoadjuvant chemotherapy/preoperative chemotherapy”. A limit was set on the randomized controlled trials (RCTs) and the terms were set to title/abstract.
The following inclusion criteria were used: (1) RCTs that compared the flurouracil-based NAC plus surgery with no treatment before surgery for gastric and gastroesophageal cancers; (2) blindness of the trial was not required; (3) patients with pathologically diagnosed esophagogastric junction or gastric adenocarcinoma, without prior treatment before entering the trial, but with a history of potentially curative surgery; and (4) studies which were considered updated.
(1) Studies on preoperative radiotherapy or immunotherapy; and (2) studies with the control group receiving chemotherapy were excluded.
The data of each RCT were collected by two reviewers (Zhang GQ and Wang HJ) independently. The results were consistent.
Methodological quality of trials was evaluated using the modified Jadad quality scores[9], which include secure method of randomization, allocation concealment, double-blinding, and information on withdrawals, and losses to follow-up. Based on these criteria, the studies were divided into high-quality group (score ≥ 3) and low-quality group (score ≤ 2). Two reviewers independently assessed the eligibility of each trial.
The following data were extracted from each study and recorded using a predesigned form: authors, year of publication, patient population, country of investigators, sample size (total, eligible, and per arm), chemotherapy regimen, cycles of chemotherapy, follow-up period, curative effect (survival rate, rate of macroscopic radical resection and cancer stage at pathological examination), and adverse events. Two reviewers did the extraction independently.
Data were obtained directly from included articles or calculated by percentage in each article. The meta-analysis was performed using Review Manager 5.1 software (provided by Cochrane Collaboration). Outcomes assessed by this meta-analysis included the overall survival, three-year progression-free survival rate, tumor down-staging rate, R0 resection rate, safety analysis and subgroup analysis. Overall survival was defined as the time between the treatment randomization and the date of the last follow-up or of the patient’s death. Patients who were lost to follow-up were considered as dead. Locoregional recurrence was measured either from the date of treatment randomization to the occurrence of the event or to the date of last follow-up. Heterogeneity between the trials was assessed to determine which model would be used in the meta-analysis. A sensitivity analysis was performed by changing the meta-analysis model. An odds ratio (OR) was the principal measurement of effect. It was calculated as the treatment group vs the control group.
All statistical analysis were performed spontaneously using Review Manager 5.1 software[10]. Heterogeneity between the trials was assessed using Chi-square test. The OR was presented with a 95%CI. I2 statistics was used for the degree of heterogeneity evaluation, and P < 0.05 was considered statistically significant.
Seven RCTs were identified, and the quality scores of the RCTs were assessed according to the method of Jadad. The details are shown in Table 1. The RCT quality scores ranged from 2 to 7 (5-point scale), with a mean of 3.75 (Tables 2 and 3).
| No. | Authors and year of publication | Country | Patients (n) | Treatment group | Control group | |||
| Treatment | Control | Pre-op | Post-op | Pre-op | Post-op | |||
| 1 | Schuhmacher et al[15], 2010 | Germany | 72 | 72 | 5-FU + DDP | None | None | None |
| 2 | Boige et al[11], 2011 | France | 113 | 111 | FP | FP | None | None |
| 3 | Cunningham et al[12], 2006 | United Kingdom | 250 | 253 | ECF | ECF | None | None |
| 4 | Hartgrink et al[13], 2004 | Holland | 27 | 29 | FAMTX | None | None | |
| 5 | Zhang et al[17], 2004 | China | 37 | 54 | IV (no details) | None | None | |
| 6 | Kobayashi et al[14], 2000 | Japan | 91 | 80 | 5-FU (oral) | CT | None | None |
| 7 | Wang et al[16], 2000 | China | 30 | 30 | 5-FU (oral) | None | None | |
| No. | Authors | Titles | Jadad score |
| 1 | Schuhmacher et al[15] | Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organization for Research and Treatment of Cancer randomized trial 40954 | 4 |
| 2 | Boige et al[11] | Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial | 4 |
| 3 | Cunningham et al[12] | Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer | 7 |
| 4 | Hartgrink et al[13] | Neoadjuvant chemotherapy for operable gastric cancer: long-term results of the Dutch randomized FAMTX trial | 5 |
| 5 | Zhang et al[17] | Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer | 2 |
| 6 | Kobayashi et al[14] | Long-term outcome of preoperative chemotherapy with 5'-deoxy-5-fluorouridine (5'-DFUR) for gastric cancer | 3 |
| 7 | Wang et al[16] | A favorable impact of preoperative FPLC chemotherapy on patients with gastric cardia cancer | 3 |
| Authors and year of publication | Randomization | Allocation concealment | Blinding | Withdrawal and dropout | Jadad score |
| Schuhmacher et al[15], 2010 | Without details | Without details | Without details | Well reported | 4 |
| Boige et al[11], 2011 | Without details | Without details | Without details | Well reported | 4 |
| Cunningham et al[12], 2006 | Well reported | Envelope | Double-blind | Well reported | 7 |
| Hartgrink et al[13], 2004 | Well reported | Envelope | No | Well reported | 5 |
| Zhang et al[17], 2004 | Without details | None | No | Well reported | 2 |
| Kobayashi et al[14], 2000 | Well reported | Envelope | No | Well reported | 3 |
| Wang et al[16], 2000 | Without details | Without details | No | Well reported | 3 |
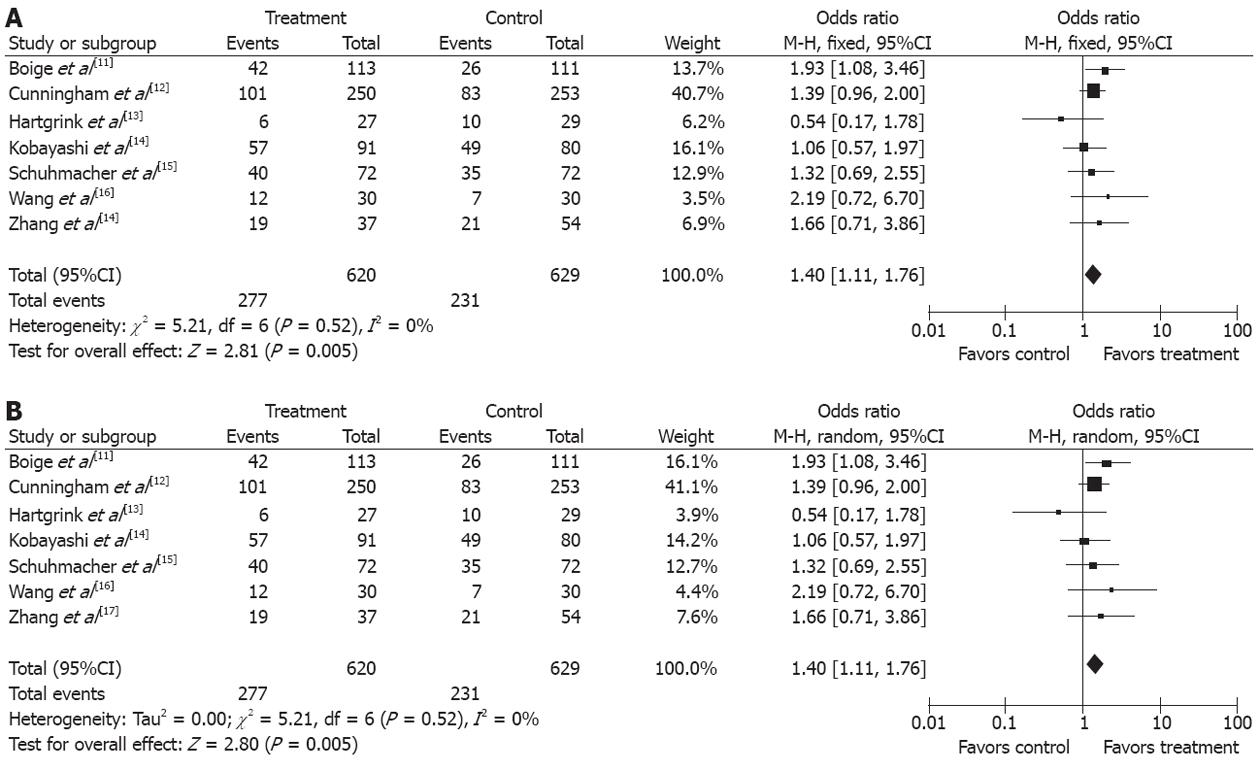
There was no significant heterogeneity between the trials (P = 0.52), and the fixed effects model was used. The data in the seven studies[11-17] were available for the analysis of overall survival. There were 620 patients in the treatment group and 629 patients in the control group in this meta-analysis. The median follow-up time was over three years. The OR, expressed as treatment group vs control group, was 1.40 (95%CI 1.11-1.76; P = 0.005). The difference of the overall survival between the treatment group and the control group was statistically significant. The overall survival was increased by 7.96% in the treatment group compared with the control group (Figure 1A). The number needed to treat (NNT) was 12. A sensitivity analysis was performed by changing the effect model into the random effect model. The results showed that the confidence interval of the odds ratio did not lie across the non-effect line, and the difference of the overall survival was statistically significant between the treatment group and the control group when the random effect model was used. The fixed effect model and the random effect model were used respectively to evaluate the sensitivity of the results of the analysis (Figures 1B and 2).
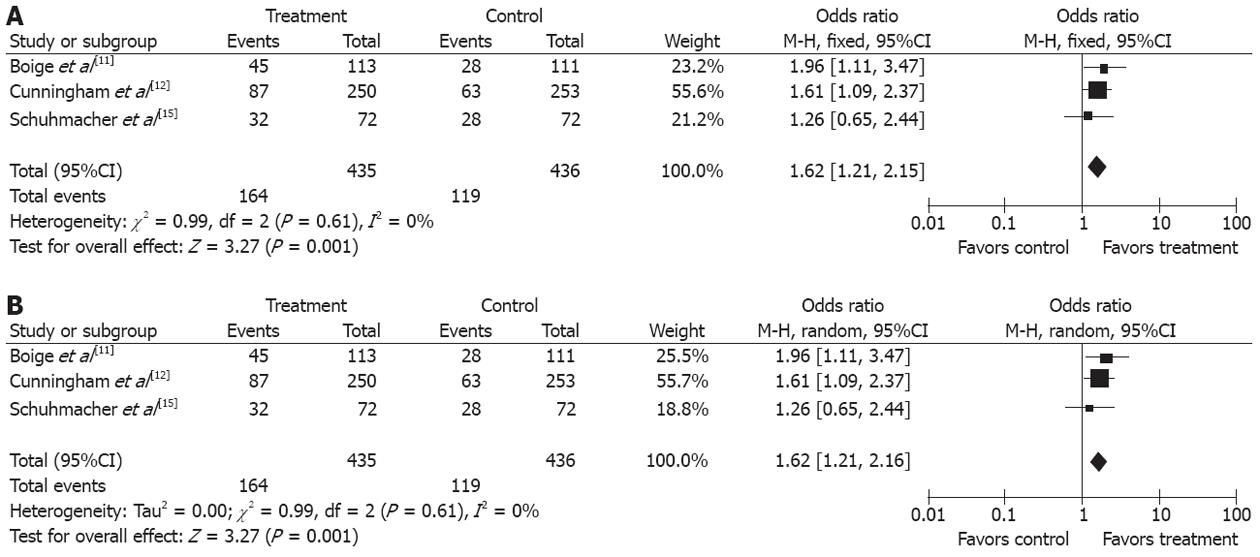
Three studies[11,12,15] compared the 3-year progression-free survival (PFS) rates between the two groups. The 3-year PFS rate was higher in treatment group than in control group (37.7% vs 27.3%), (OR 1.62, 95%CI 1.21, 2.15; P = 0.001, fixed-effect model) and NNT was 10 (Figure 3A and B).
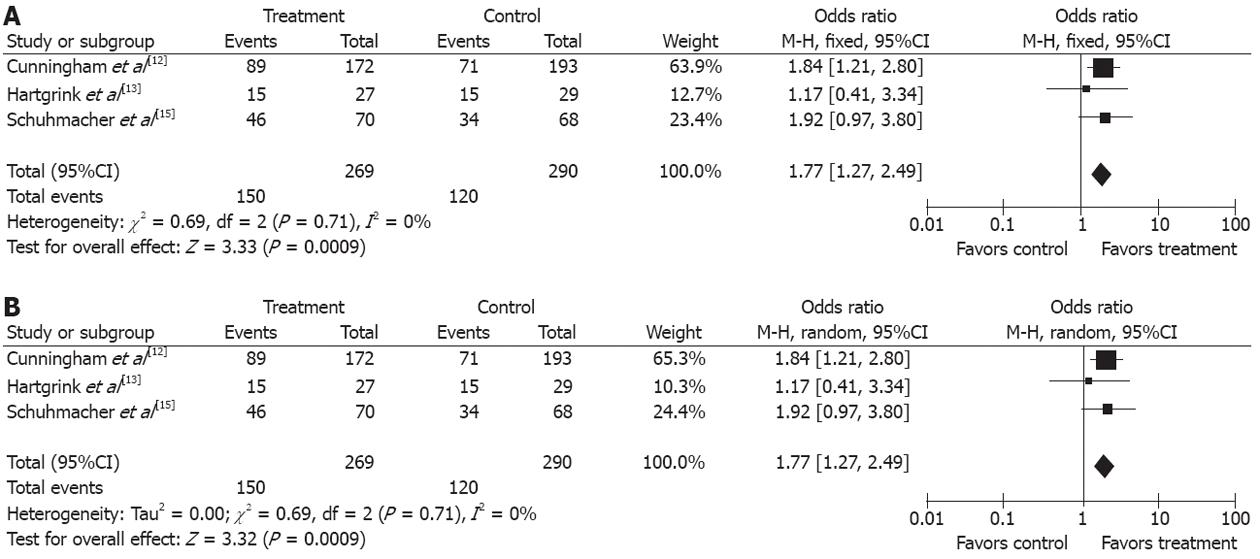
Three studies[12,13,15] describing the pathological staging of gastroesophageal and gastric cancers after resection (269 in treatment group and 290 in control group) were included in the analysis. The rate of pT1-2 was higher in treatment group than in control group (55.76% vs 41.37%) (OR 1.77, 95%CI 1.27, 2.49; P = 0.0009, fixed-effect model) and the NNT was 7 (Figure 4A and B).
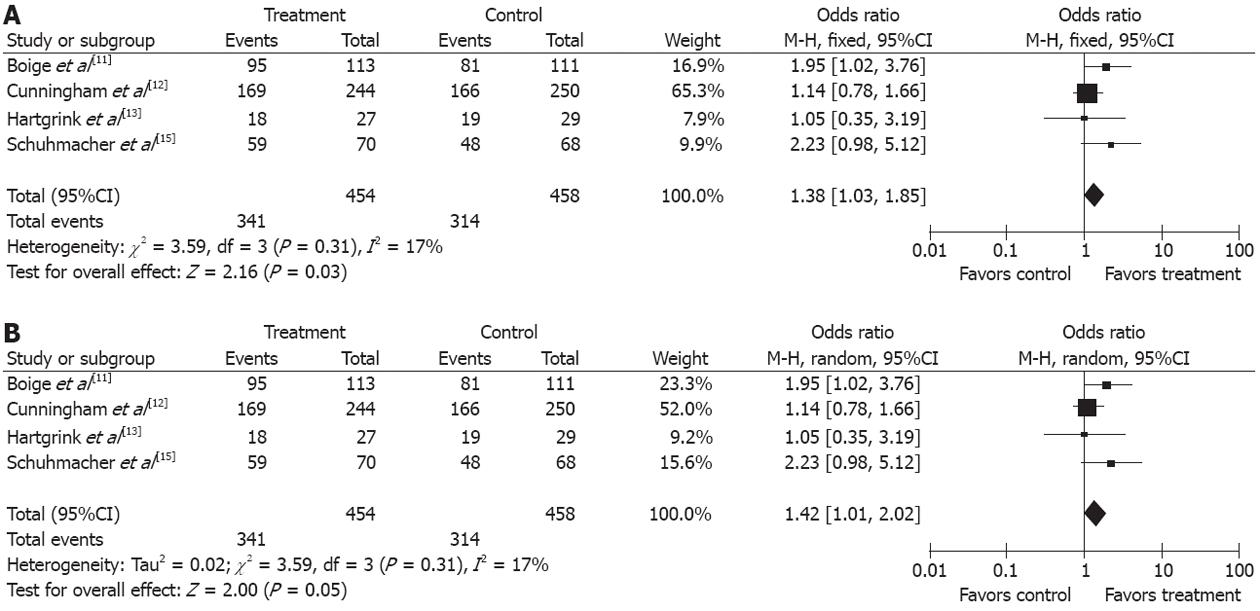
The resection rate of gastroesophageal and gastric cancers was reported in four trials[11,12,13,15]. Since no obvious heterogeneity was observed in these studies (P = 0.31, I2 = 17%), the fixed-effect model was used. The R0 resection rate of the gastroesophageal and gastric cancers was higher in treatment group than in control group (OR 1.38, 95%CI 1.03-1.85, P = 0.03, fixed-effect model) and the NNT was 15 (Figure 5A and B).
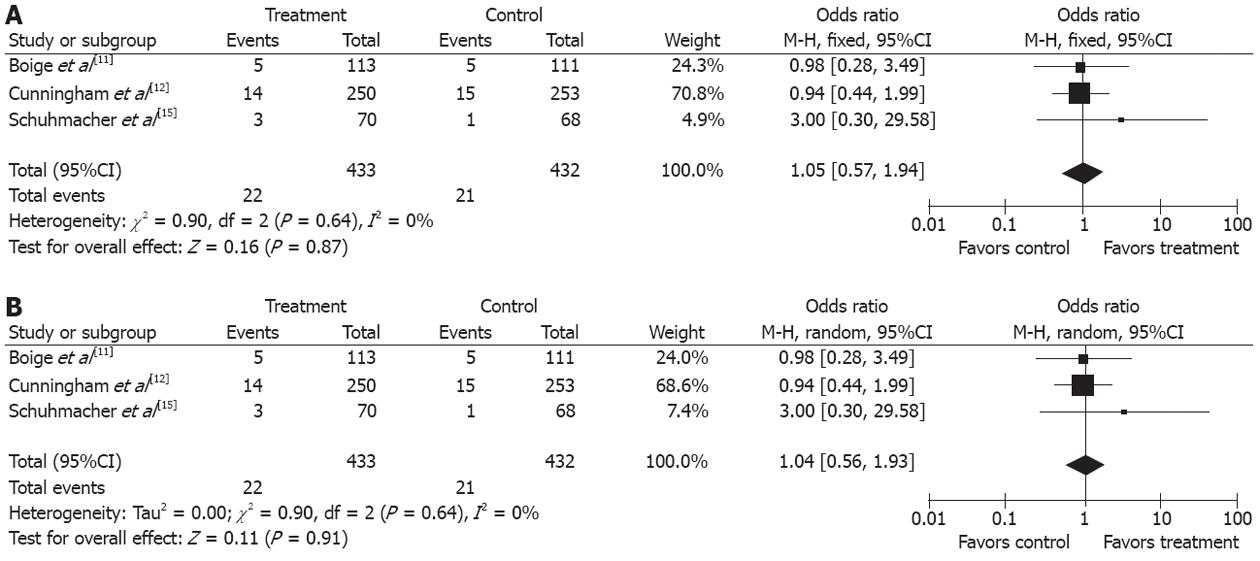
Safety analysis included both chemotherapy-induced adverse effects (grade 3/4, defined according to the Common Toxicity Criteria of the National Cancer Institute, version 2.0) and postoperative complication and mortality. Two studies reported[11,12] grade 3/4 adverse effects of NAC, including gastrointestinal side effect in 18.1 % (60/332) and leukopenia in 9.9% (33/332). Three studies[11,12,15] reported perioperative mortality with no statistically significant difference (P = 0.87) between the two groups (5.08% vs 4.86%), (OR 1.05, 95%CI 0.57-1.94, fixed-effect model) (Figures 6 and 7).
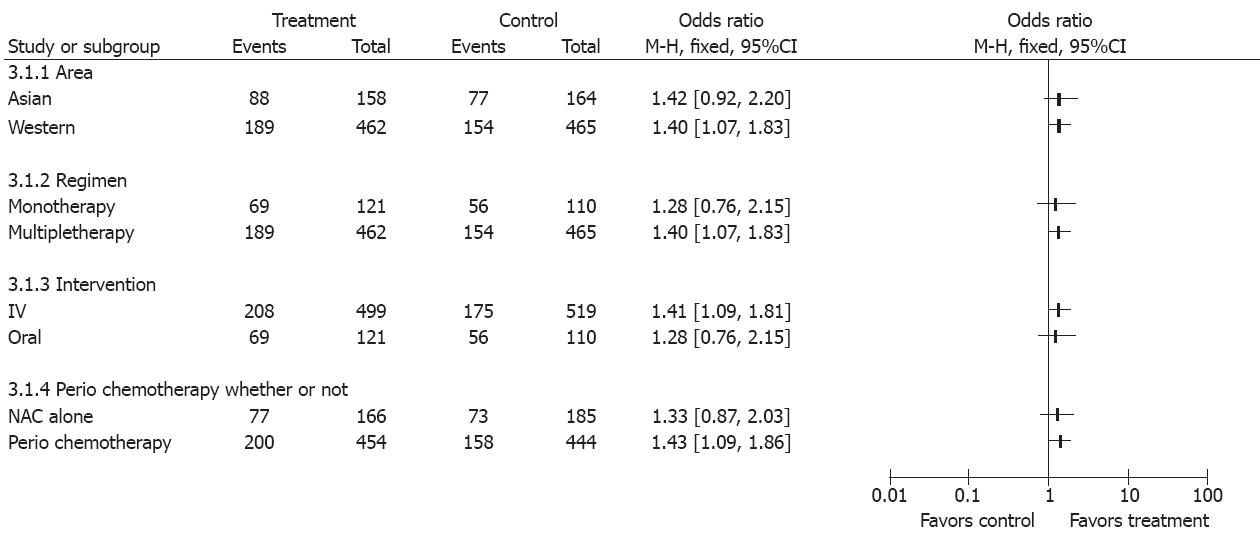
Factors that might influence the results in the two groups were studied (Figure 8). When the overall survival rate was set as the end point, gastroesophageal and gastric cancer patients were benefited more from perioperative chemotherapy than from NAC alone (OR 1.40, 95%CI 1.11-1.76, NNT = 12). Trials showed that patients from Western countries favored NAC compared with those from Asian countries (OR 1.40, 95%CI 1.07-1.83). Monotherapy was inferior to multiple chemotherapy (OR 1.40, 95%CI 1.07-1.83). Intravenous administration of NAC was more advantageous than oral route (OR 1.41, 95%CI 1.09-1.81) (Figure 7).
Gastric cancer remains to be an important health issue worldwide. Over the past two decades, the incidence of distal gastric cancer has been decreased, but the incidence of proximal and esophagogastric junction cancers has been increased significantly[18]. The treatment of the gastroesophageal and gastric cancer is dependent on the TMN staging of the tumor. The advanced gastroesophageal and gastric cancers (stage III-IVB) without evidence of distant metastasis are potentially curable, but these tumors usually present with a more advanced stage and are associated with a worse prognosis[19], and a combination therapy, including surgery, chemotherapy and radiotherapy, is often needed. Chemotherapy is an adjuvant treatment modality in the form of adjuvant chemotherapy, NAC and concomitant chemoradiotherapy[20]. NAC has several advantages: (1) it is well tolerated; (2) it can better control the micrometastasis[21]; and (3) it might downstage the tumor to the greatest extent and increase the probability of R0 resection so as to facilitate the surgery[22,23], thus improving the survival rate of the patients with gastroesophageal and gastric cancer. However, it might delay the curative treatment if the tumor does not respond to the NAC, which is also costly and may be detrimental to the patients. Theoretically, NAC can increase the survival rate and improve the quality of life of the patients. However, the meta-analysis by Him[8] failed to show the benefit of NAC in patient survival. Li ’s study[7] demonstrated a minor but significant benefit in patient survival. The result coincided with our studies, which shows that 5-flurouracil-based NAC has a benefit on the overall survival of gastroesophageal and gastric cancer patients. Our meta-analysis demonstrated the feasibility of NAC for locally gastroesophageal and gastric cancer patients. NAC could downstage the tumor (NNT = 7) and increase the R0 rate of gastric cancer (NNT = 15). To examine the role of NAC alone in improving the overall survival rate of gastroesophageal and gastric cancer patients who did not receive postadjuvant chemotherapy, data from four trials[13,15-17] were further analyzed, showing that NAC had no effect on the overall survival rate of gastroesophageal and gastric cancer patients (OR 1.34, 95%CI 0.87-2.06). These may contribute to the factors that the included studies had a very small sample size with different follow-up time, different cycles of treatment as well as different tumor locations, which could be too underpowered to demonstrate any positive results. Alternatively, this might be attributed to the fact that currently used preoperative chemotherapeutic agents may not be effective enough to eradicate micrometastases. No clear conclusion could be reached as yet about the effect of NAC alone on the overall survival rate of gastroesophageal and gastric cancer patients. Fortunately, the perio chemotherapy shows some effect in the overall survival rate of gastroesophageal and gastric cancer patients (OR 1.43, 95%CI 1.09-1.86). It has been found that 5-flurouracil-based NAC benefits the overall survival of gastroesophageal and gastric cancer patients.
The regimen of the NAC might affect the outcome of the treatment. Therefore, the regimen factors concerning NAC for gastroesophageal and gastric cancer should be taken into account. Several regimens have been used in the NAC of gastroesophageal and gastric cancer. Our current meta-analysis showed that flurouracil-based combination regimen and intravenous route of NAC had a high efficiency for gastroesophageal and gastric cancer patients. The effective response rate will help down-stage tumors to the greatest extent and increase the probability of R0 resection, thus improving the survival rate of the patients.
Subgroup analysis also showed that the outcome of NAC for gastroesophageal and gastric cancer was better in trials from Western countries than in those from Asian countries and in multi-agent regimens as well. The increasingly higher incidence of the gastroesophageal cancer in Western countries compared with Asian countries[12] and the different constituent of tumor stage may influence the final results.
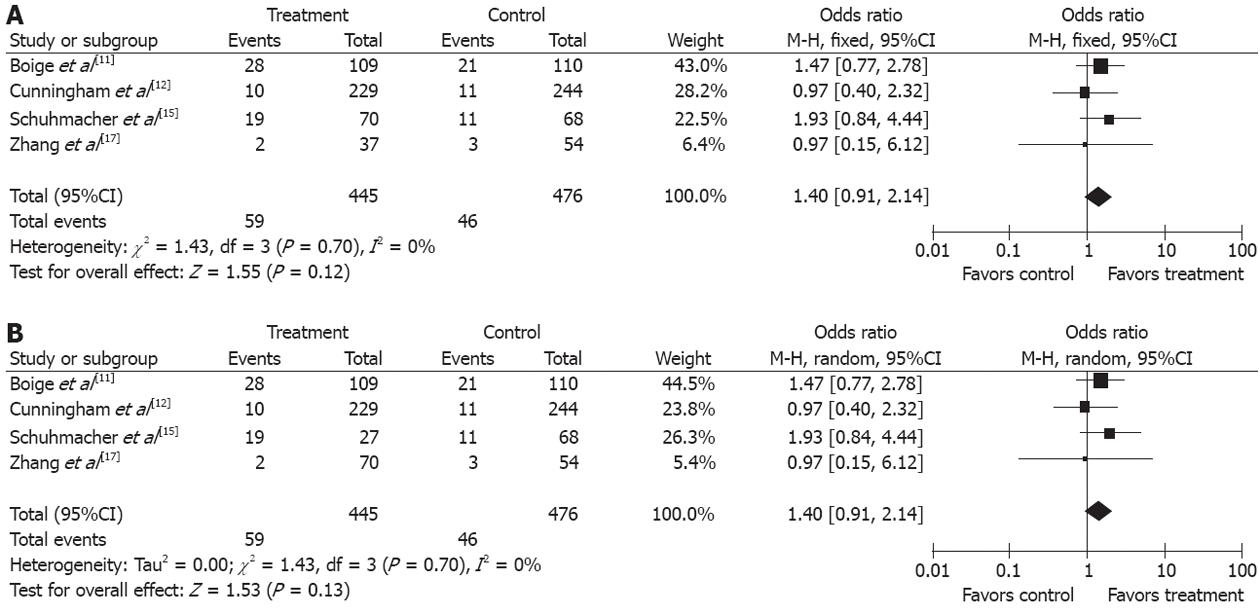
Another major concern in our meta-analysis is the efficiency and safety of NAC in the studies included. Mortality directly links with treatment failure, which mainly comprises locoregional recurrence, secondary primary malignancy, and distant metastasis. In this meta-analysis, the outcomes of 3-year PFS were analyzed. The difference of 3-year PFS was statistically significant between the treatment group and the control group, which suggests that NAC with a 5-flurouracil-based regimen is effective in the locoregional control of gastroesophageal and gastric cancer when a locoregional treatment is administered. Our meta-analysis showed that gastric cancer patients could well tolerate NAC. Grade 3/4 gastrointestinal and leukopenia adverse events of NAC occurred in 32% (112/343) of gastroesophageal and gastric cancer patients. The rate of complications in the treatment group was not obviously higher than in the control group, indicating that NAC is a safe modality for gastric cancer (OR 1.05, 95%CI 0.75-1.48). The efficiency and the safety were assessed in treatment group, but disease progression during NAC is another potential concern in patients with a loss of opportunity for surgery. In this meta-analysis, three trials[11,12,15] showed a disease progression rate of 10.6% (46/435). The R-0 resection rate was higher in treatment group than in control group (OR 1.57, 95%CI 1.21-2.02, NNT = 11), indicating that disease progression after NAC is not a major concern for its resection. The results were consistent with the study showing that lack of response to NAC may delay curative surgery, and chemotherapy-induced toxicity may lead to increased surgical complications[24,25].
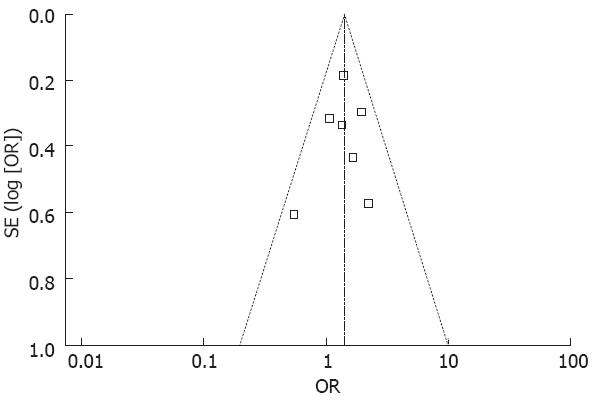
In addition, funnel plot observation did not indicate obvious publication bias in the two subgroups (Figure 2). The sensitivity analysis showed similar results by excluding the trials with Jadad score less than 2. In this meta-analysis, the primary endpoint about the overall survival rate with a fixed model OR of 1.40 (95%CI 1.11-1.76) vs a random model OR of 1.40 (95%CI 1.11-1.76) (Figure 1A and B), indicated that the trial quality was good, which was not influenced by the factors, such as selection bias and other trials.
NAC has been proven effective against some cancers, such as breast cancer[26], head and neck squamous cell carcinoma[27]. However, it is not recommended as a standard regimen for gastroesophageal and gastric cancer, primarily because of the significantly different strategies used around the world. However, adjuvant chemoradiotherapy is a standard treatment in the USA, perioperative chemotherapy is the first choice in Europe, and surgery and adjuvant chemotherapy are recommended in Japan, where D2 surgery is effective and safe[28-30]. Some studies have demonstrated that NAC and D2 surgery can effectively improve the overall survival[31] , whereas a recent study showed no benefits[32]. Whether NAC benefits D2 dissection lacks strong evidence. Fortunately, our meta-analysis provided the up-to-date evidence for the positive effect of NAC in gastroesophageal and gastric cancers, but further studies are required to determine its best regimen and response-based neoadjuvant concept.
In conclusion, our study provides information on the efficacy of NAC with 5-flurouracil-based regimen in gastroesophageal and gastric cancer patients. This regimen was regarded as the most effective one before the emergence of Taxanes. The incorporation of Taxanes into the 5-FU/cisplatin (FP) regimen makes up the Taxol/5-FU/cisplatin (TPF) regimen, which is a promising treatment strategy for gastroesophageal and gastric cancers[33]. A number of trials have been registered to examine the role of NAC in treatment of advanced gastric cancer, such as S-1 plus cisplatin or S-1 and cisplatin plus Taxanes[34,35]. However, its effectiveness is yet to be confirmed by the meta-analysis. With all these clinical and scientific efforts, these treatment strategies will definitely continue to further improve the outcome of gastroesophageal and gastric cancer patients.
Although the prevalence of distal gastric cancer has declined, the world-wide incidence of gastroesophageal junctional adenocarcinoma is increasing. For patients who present with gastric and gastroesophageal cancer, surgery remains the cornerstone of treatment, which can potentially improve the long-term survival. However, the prognosis of the patients remains poor. Chemotherapy has been proven to be effective for advanced gastric cancer. Neoadjuvant chemotherapy (NAC) plays a role in improving the prognosis of the patients with advanced gastric cancer and gastroesophageal junctional adenocarcinoma, but its value remains controversial because of lack of well-powered trials.
Meta-analysis was used to evaluate the effectiveness of NAC for advanced gastric/gastroesophageal cancer in this study.
The meta-analysis provided the up-to-date evidence for the positive effect of NAC in locally advanced gastric and gastroesophageal cancer. NAC improved the R0 resection rate (95%CI: 1.03-1.85), tumor down-staging (95%CI: 1.27-2.49) and survival rate (95%CI: 1.11-1.76) for the 1249 patients enrolled in seven trials. Surgery was safe after preoperative 5-flurouracil-based chemotherapy while no obvious morality and complication concerns were raised in these trials. These findings suggest that NAC can improve the survival rate of patients with advanced gastric and gastroesophageal cancer.
Based on the studies on the efficacy and feasibility of the preoperative chemotherapy with 5-flurouracil-based regimen and with the increasing acceptance of the concept of NAC, additional studies on new regimens and well-designed powerful trials are highly encouraged in patients with locally advanced gastric and gastroesophageal cancer. More experiments are expected to focus on individualized treatment under the guidance of molecular marker for the NAC.
D2 surgery: the extent of lymphadenectomy (D1-3), in which complete dissection of up to the second group nodes was defined as D2 according to the rules of the Japanese Research Society for Gastric Cancer; TNM classification of malignant tumors: “pT” indicates the pathological stage after surgery and “cTNM” indicates the clinical pretreatment tumor stage; Taxanes: they represent one of the more effective targets in current cancer therapy, which block cell cycle progression through centrosomal impairment, induction of abnormal spindles and suppression of spindle microtubule dynamics. Paclitaxel (Taxol) is the prototype of the taxane family of anti-tumor drugs, which has been approved for treatment of metastatic gastric cancer; Number needed to treat: It is often used to describe how many patients would need to be treated to prevent one event. It is determined from the absolute difference between one treatment and another.
This is an excellent analysis. The authors submit a meta-analysis of the available randomized controlled trials investigating the utility of neoadjuvant therapy for gastric and gastroesophageal cancer. Overall, the paper is very strong, the data appropriately analyzed and the conclusions reasonable. The supposition the neoadjuvant therapy for gastroesophageal cancers remaining controversial is not entirely the case. Most centers across the world have embraced this approach based on the strong data supporting efficacy.
Peer reviewer: Oscar Joe Hines, MD, FACS, Professor, Director, Surgery Residency Program, Department of Surgery, UCLA School of Medicine, 10833 Le Conte Ave, Los Angeles, CA 90095-6904, United States
S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] |
| 2. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2644] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 3. | Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 390] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, Sasako M, Kunii Y, Motohashi H, Yamamoto S. Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Briasoulis E, Liakakos T, Dova L, Fatouros M, Tsekeris P, Roukos DH, Kappas AM. Selecting a specific pre- or postoperative adjuvant therapy for individual patients with operable gastric cancer. Expert Rev Anticancer Ther. 2006;6:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16:5621-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | He LF, Yang KH, Tian JH, Bai ZG. [Meta analysis of clinical effectiveness of neoadjuvant chemotherapy for gastric cancer]. Ai Zheng. 2008;27:407-412. [PubMed] |
| 9. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Jiang H, Sun MW, Hefright B, Chen W, Lu CD, Zeng J. Efficacy of hypocaloric parenteral nutrition for surgical patients: a systematic review and meta-analysis. Clin Nutr. 2011;30:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1491] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 12. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4582] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 13. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Kobayashi T, Kimura T. [Long-term outcome of preoperative chemotherapy with 5'-deoxy-5-fluorouridine (5'-DFUR) for gastric cancer]. Gan To Kagaku Ryoho. 2000;27:1521-1526. [PubMed] |
| 15. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 16. | Wang XL, Wu GX, Zhang MD, Guo M, Zhang H, Sun XF. A favorable impact of preoperative FPLC chemotherapy on patients with gastric cardia cancer. Oncol Rep. 2000;7:241-244. [PubMed] |
| 17. | Zhang CW, Zou SC, Shi D, Zhao DJ. Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer. World J Gastroenterol. 2004;10:3070-3072. [PubMed] |
| 18. | Pera M. Epidemiology of esophageal cancer, especially adenocarcinoma of the esophagus and esophagogastric junction. Recent Results Cancer Res. 2000;155:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kim DY, Joo JK, Park YK, Ryu SY, Kim YJ, Kim SK. Predictors of long-term survival in node-positive gastric carcinoma patients with curative resection. Langenbecks Arch Surg. 2007;392:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Menges M, Hoehler T. Current strategies in systemic treatment of gastric cancer and cancer of the gastroesophageal junction. J Cancer Res Clin Oncol. 2009;135:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Bölke E, Peiper M, Knoefel WT, Baldus SE, Schauer M, Matuschek C, Gerber PA, Hoff NP, Budach W, Gattermann N. [Multimodal therapy in locally advanced gastric cancer]. Dtsch Med Wochenschr. 2011;136:2205-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Zhou DL, Zheng CZ, Li JH, Yin K, Ke CW, Liu HT, Hu XG, Chen DL, Zhang DY. [Application of neoadjuvant chemotherapy in laparoscopic gastrectomy for advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Zazhi. 2009;12:126-129. [PubMed] |
| 23. | Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;183:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Misra S, Pedroso FE, Dipasco PJ, Solomon NL, Gennis E, Franceschi D, Ardalan B, Koniaris LG. Does neoadjuvant chemotherapy improve outcomes for patients with gastric cancer? J Surg Res. 2012;178:623-631. [PubMed] |
| 25. | Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR, Schwaiger M. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-3065. [PubMed] |
| 26. | Untch M, von Minckwitz G. Recent advances in systemic therapy: advances in neoadjuvant (primary) systemic therapy with cytotoxic agents. Breast Cancer Res. 2009;11:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Su YX, Zheng JW, Zheng GS, Liao GQ, Zhang ZY. Neoadjuvant chemotherapy of cisplatin and fluorouracil regimen in head and neck squamous cell carcinoma: a meta-analysis. Chin Med J (Engl). 2008;121:1939-1944. [PubMed] |
| 28. | Ott K, Lordick F, Blank S, Büchler M. Gastric cancer: surgery in 2011. Langenbecks Arch Surg. 2011;396:743-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2431] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 30. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Schwarz RE, Smith DD. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2005;23:5404-5405; author reply 5405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Wu AW, Ji JF. Neoadjuvant chemotherapy for locally advanced gastric cancer: With or without radiation. World J Gastrointest Surg. 2012;4:27-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868-874. [PubMed] |
| 34. | Inoue K, Nakane Y, Kogire M, Fujitani K, Kimura Y, Imamura H, Tamura S, Okano S, Kwon AH, Kurokawa Y. Phase II trial of preoperative S-1 plus cisplatin followed by surgery for initially unresectable locally advanced gastric cancer. Eur J Surg Oncol. 2012;38:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Fushida S, Nashimoto A, Fukushima N, Kawachi Y, Fujimura T, Kuwabara S, Musha N. Phase II trial of preoperative chemotherapy with docetaxel, cisplatin and S-1 for T4 locally advanced gastric cancer. Jpn J Clin Oncol. 2012;42:131-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |