Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6996
Revised: May 31, 2012
Accepted: June 8, 2012
Published online: December 21, 2012
AIM: To evaluate the efficacy and safety of tenofovir disoproxil fumarate (TDF) for chronic hepatitis B (CHB) patients after multiple failures.
METHODS: A total of 29 CHB patients who had a suboptimal response or developed resistance to two or more previous nucleoside/nucleotide analogue (NA) treatments were included. Study subjects were treated with TDF alone (n = 13) or in combination with lamivudine (LAM, n = 12) or entecavir (ETV, n = 4) for ≥ 6 mo. Complete virologic response (CVR) was defined as an achievement of serum hepatitis B virus (HBV) DNA level ≤ 60 IU/mL by real-time polymerase chain reaction method during treatment. Safety assessment was based on serum creatinine and phosphorus level. Eleven patients had histories of LAM and adefovir dipivoxil (ADV) treatment and 18 patients were exposed to LAM, ADV, and ETV. Twenty-seven patients (93.1%) were hepatitis B e antigen (HBeAg) positive and the mean value of the baseline serum HBV DNA level was 5.5 log IU/mL ± 1.7 log IU/mL. The median treatment duration was 16 mo (range 7 to 29 mo).
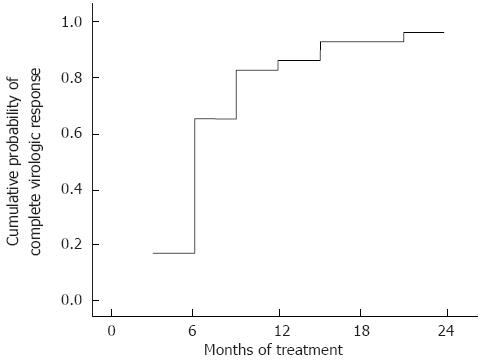
RESULTS: All the patients had been treated with LAM and developed genotypic and phenotypic resistance to it. Resistance to ADV was present in 7 patients and 10 subjects had a resistance to ETV. One patient had a resistance to both ADV and ETV. The cumulative probabilities of CVR at 12 and 24 mo of TDF containing treatment regimen calculated by the Kaplan Meier method were 86.2% and 96.6%, respectively. Although one patient failed to achieve CVR, serum HBV DNA level decreased by 3.9 log IU/mL from the baseline and the last serum HBV DNA level during treatment was 85 IU/mL, achieving near CVR. No patients in this study showed viral breakthrough or primary non-response during the follow-up period. The cumulative probability of HBeAg clearance in the 27 HBeAg positive patients was 7.4%, 12%, and 27% at 6, 12, and 18 mo of treatment, respectively. Treatment efficacy of TDF containing regimen was not statistically different according to the presence of specific HBV mutations. History of prior exposure to specific antiviral agents did not make a difference to treatment outcome. Treatment efficacy of TDF was not affected by combination therapy with LAM or ETV. No patient developed renal toxicity and no cases of hypophosphatemia associated with TDF therapy were observed. There were no other adverse events related to TDF therapy observed in the study subjects.
CONCLUSION: TDF can be an effective and safe rescue therapy in CHB patients after multiple NA therapy failures.
- Citation: Kim YJ, Sinn DH, Gwak GY, Choi MS, Koh KC, Paik SW, Yoo BC, Lee JH. Tenofovir rescue therapy for chronic hepatitis B patients after multiple treatment failures. World J Gastroenterol 2012; 18(47): 6996-7002
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6996
Nucleoside/nucleotide analogues (NAs), which inhibit reverse transcription of hepatitis B virus (HBV) polymerase, are an important class of drugs that changed the treatment paradigm and prognosis of chronic hepatitis B (CHB). Oral NA therapy has advantages over interferon therapy due to its potent antiviral effects, good tolerance, lower side-effect profile, and convenience[1,2]. However, the development of antiviral resistance to the drug is the major limitation of NA therapy and frequently leads to treatment failure. The development of drug resistance begins with mutations in the polymerase gene, followed by viral breakthrough, biochemical breakthrough, clinical deterioration, and even progressive liver failure. Favorable effects obtained by NA therapy are lost in patients who developed antiviral resistance. Today, an increasing number of patients experience multiple NA treatment failures, especially when they are sequentially treated with drugs that have similar characteristics[3]. To achieve sustained suppression of HBV replication and remission of liver disease, successful management of CHB patients who developed treatment failure due to antiviral resistance or incomplete inhibition of viral replication is critical.
Tenofovir disoproxil fumarate (TDF) is an oral NA with the most potent activity against HBV and a high genetic barrier that has been approved in the United States and Europe for the treatment of CHB since 2009. In treatment-naive patients, viral resistance to TDF was not detected after up to 144 wk of therapy, and persistent viremia through week 144 was only 0.8%, mostly due to poor compliance[4]. TDF has also shown efficacy in NA experienced patients with various results. TDF monotherapy in patients with prior failure or resistance to two or more NAs showed a 79% viral suppression rate at 23 mo of treatment[5]. In another study, TDF rescue therapy following lamivudine (LAM) and adefovir dipivoxil (ADV) treatment failure achieved a viral suppression rate of only 46% at 48 wk and 64% at 96 wk of treatment[6]. However, experience with TDF in Asian countries, including Korea, is limited because this drug has not yet been approved for the treatment of CHB in this region of the world. A single report of six South Korean patients with prior LAM and ADV treatment failure stated that complete virologic suppression was achieved in all patients at 12 mo with TDF plus LAM therapy[7].
Many CHB patients in Korea have undergone sequential treatment with LAM, ADV, and/or entecavir (ETV) at 1 mg to manage antiviral resistance or insufficient suppression of HBV DNA and they have begun to emerge as an important and difficult issue for clinicians[8]. However, the efficacy of TDF treatment in such patients with previous treatment failures using multiple NAs, including ETV, is not well known. Multiple treatment failures of NAs in CHB patients are not limited to Korea; it is considered to be a global problem. In this study, we evaluated the efficacy and safety of a TDF containing treatment regimen in CHB patients after the failure of multiple NA therapies.
CHB patients with failures of two or more previous NA therapies were treated with TDF containing regimens for at least 6 mo. The TDF containing regimens included TDF monotherapy (300 mg/d), or combination therapy with LAM (100 mg/d) or ETV (1 mg/d). Failures of previous NA therapies included suboptimal viral suppression (serum HBV DNA level > 2000 IU/mL despite continued therapy for more than 1 year) or the development of resistance. Exclusion criteria were coinfection with human immunodeficiency virus or hepatitis C virus, and history of underlying renal problems. Since TDF is not approved for use with CHB patients in Korea, informed consent was obtained from all subjects. The study protocol was approved by the Institutional Review Board of Samsung Medical Center (IRB file number: 2011-06-027) and was conducted in accordance with the principles of the Declaration of Helsinki.
Serum HBV DNA, hepatitis B e antigen (HBeAg), anti-hepatitis B e antibody, alanine aminotransferase, creatinine, and phosphorus levels were recorded every 3 mo. All data were collected from medical records and analyzed retrospectively.
The mean reduction of HBV DNA levels was assessed during treatment. Complete virologic response (CVR) was defined as a decrease of serum HBV DNA ≤ 60 IU/mL. The primary non-response was defined as a decrease in serum HBV DNA of less than 2 log IU/mL at 24 wk of therapy. Viral breakthrough (BT) was defined as an increase of HBV DNA > 1 log IU/mL from nadir during TDF treatment. HBeAg clearance included HBeAg loss or seroconversion to anti-HBe.
The safety assessment was based on the development of renal toxicity or hypophosphatemia. Renal toxicity was defined when the estimated glomerular filtration rate (eGFR) decreased by more than 20% from baseline. Calculation of eGFR was performed with this formula: eGFR = (140-age) × weight (kg) (× 0.85 if female)/(72 × serum creatinine). Investigation of other adverse events was performed by review of medical records.
Serum HBV DNA level was determined by real-time polymerase chain reaction (PCR) assay, using the COBAS TaqMan HBV quantitative test (Roche Molecular System Inc., Branchburg, NJ, United States), which has a lower limit of detection at 9 IU/mL. To identify mutations associated with resistance in the gene encoding HBV polymerase, PCR amplification and direct sequencing was performed in a single reference laboratory as previously described[9].
To describe continuous variables with normal distributions, the mean ± SD was used. Continuous variables without normal distributions were expressed as the median with range. Cumulative probability of CVR during the treatment period was calculated using the Kaplan-Meier method and comparison between groups was performed with a log-rank test. P values less than 0.05 were considered statistically significant. All data were analyzed using SPSS (version 15.0; Chicago, IL, United States).
A total of 29 patients were included in the study. Detailed demographics of the patients are presented in Table 1. The median age was 56 years (range 22 to 63 years) and 72.4% were male. Twenty-seven patients were HBeAg positive (93.1%) and the mean baseline serum HBV DNA level was 5.5 log IU/mL ± 1.7 log IU/mL. Eleven patients (37.9%) had been exposed to LAM and ADV, and 18 (62.1%) subjects had been additionally exposed to ETV. TDF treatment alone was used in 13 subjects (44.8%), with LAM in 12 (41.4%), or with ETV in 4 (13.8%). The median treatment duration of the TDF containing regimen was 16 mo (range 7 to 29 mo).
| Characteristics | |
| Age, yr, median (range) | 56 (22-63) |
| Male, n (%) | 21 (72.4) |
| HBeAg positive, n (%) | 27 (93.1) |
| Serum HBV DNA, log IU/mL, mean ± SD | 5.5 ± 1.72 |
| ALT, U/L, median (range) | 47 (12-763) |
| Prior exposed NAs | |
| LAM + ADV, n (%) | 11 (37.9) |
| LAM + ADV + ETV, n (%) | 18 (62.1) |
| Treatment duration or prior NAs, mo, median (range) | 66 (28-125) |
| Treatment regimen | |
| TDF/TDF+ LAM/TDF + ETV, n (%) | 13 (44.8)/12 (41.4)/4 (13.8) |
| Treatment duration of TDF, mo, median (range) | 16 (7-29) |
| Serum creatinine, mg/dL, median (range) | 0.93 (0.6-1.16) |
| Serum phosphorus, mEq/dL, median (range) | 3.3 (2.8-4.4) |
More detailed information about prior NA therapy history and genotypic resistance profiles is described in Table 2. All patients had been treated with LAM at first, and then developed a resistance to it. In LAM and ADV experienced patients, ADV was used as a sequential or add-on therapy in an attempt to suppress LAM resistant strains; however, the patients developed viral BT with or without genotypic resistance to ADV, or showed suboptimal viral response. In LAM, ADV, and ETV experienced patients, patients were moved to an 1 mg ETV regimen, due to the failure of sequential or add-on ADV therapy. 10 out of these 18 patients were confirmed to have genotypic resistance to ETV (Table 2).
| Treatment history | LAM resistance | ADV resistance | ETV resistance | Outcome | n |
| LAM→ADV | rtM204V/I ± rtL180M ± rtV173L | None | None | No VR | 1 |
| LAM→LAM + ADV | rtA181V/T | Viral BT | 1 | ||
| None | No VR | 6 | |||
| LAM→ADV→LAM + ADV | rtN236T,rtN238A | Viral BT | 1 | ||
| rtA181V/T | No VR | 2 | |||
| LAM→ADV→ETV | rtM204V/I ± rtL180M | None | rtT184L + rtI169T | Viral BT | 1 |
| rtT184S | 2 | ||||
| rtS202G | 3 | ||||
| rtS202G + rtV207I | 1 | ||||
| rtS202G + rtT184A | 1 | ||||
| None | 5 | ||||
| rtA181V/T | None | No VR | 2 | ||
| None | 1 | ||||
| LAM→LAM+ADV→ETV | rtN238H | rtS202G | Viral BT | 1 | |
| None | rtS202G + rtV207I | 1 |
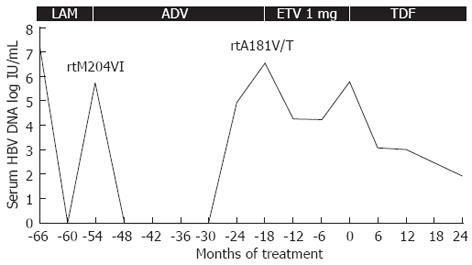
The cumulative probability of CVR during the treatment period is presented as a Kaplan-Meier curve (Figure 1). The probability of CVR was 86.2% at 12 mo and 96.6% at 24 mo of treatment. In this study, only one patient did not achieve CVR with TDF treatment. The detailed clinical course of this patient is given in Figure 2. This patient underwent sequential therapy with LAM, ADV, and ETV at 1 mg: however, the development of resistance and suboptimal viral suppression led to treatment failure. TDF monotherapy was introduced and maintained for 24 mo. Even though the patient did not reach CVR, the patient’s serum HBV DNA decreased to 85 IU/mL with a reduction of 3.9 log IU/mL, therefore becoming near CVR. No patient in this study developed primary non-response or viral BT during follow-up.
To define whether there is any difference in the rates of CVR according to prior exposures to antiviral agents, genotypic resistance profile, or TDF monotherapy vs combination therapy with LAM or ETV, the CVR rates were compared according to these variables using a log-rank test. There were no significant differences between patients with prior exposure to LAM and ADV vs LAM, ADV, and ETV (P = 0.93). Genotypic resistance to ADV (rtA181V/T or rtN236T) and resistance to ETV did not affect CVR rates (P = 0.99 and 0.14, respectively). TDF monotherapy, or in combination with other NAs, was not related to the achievement of CVR (P = 0.19).
Cumulative probability of HBeAg clearance was calculated with the Kaplan-Meier method. The rate of HBeAg clearance in the 27 HBeAg positive patients was 7.4%, 12%, and 27% at 6, 12, and 18 mo of treatment, respectively.
No patient developed renal toxicity, defined as a decrease of eGFR more than 20% from baseline. No cases of hypophosphatemia associated with TDF therapy were observed. There were no other adverse events related to TDF therapy observed in the subjects.
The management of CHB has improved markedly over the last decade, primarily due to the availability of oral NA therapy. LAM was the first NA approved for CHB in 1998 and has been used extensively for treatment since that time. However, resistance to LAM occurs frequently and is observed in up to 80% of patients treated for 5 years[10]. When sequential ADV monotherapy was introduced to these patients, ADV resistance was found in up to 21% of the patients after 1 to 2 years[11-13]. Sequential therapy with ETV, a newer generation NA, has been attempted to counteract LAM resistance. Despite good treatment efficacy and low levels of ETV resistance in treatment naive patients, rescue therapy in LAM resistance patients revealed that the treatment was less effective and had a higher rate of resistance[14,15]. Sequential therapies with multiple NAs in this manner can promote selection for multidrug-resistant strains of HBV and frequently leads to viral BT or inadequate viral response[3]. As a result, increased numbers of CHB patients with NA (mostly LAM and ADV, or ETV) treatment failures are becoming a global problem.
TDF is an oral NA that has been used for the treatment of human immunodeficiency virus infection and is approved for the treatment of CHB in the United States and Europe[16]. Owing to its potent antiviral activity and high genetic barrier to the development of resistance for up to 3 years as determined in phase 4 clinical trials[4,17], TDF is now recommended as a first-line therapy for HBV infected patients in recently published guidelines[1,18].
The efficacy of TDF in the treatment of prior NA refractory HBV infection has also been evaluated. TDF showed an excellent antiviral activity on LAM resistant virus independently of the resistance mutation profile in vitro[19]. In patients with LAM resistant CHB, treatment with TDF was well tolerated without significant adverse events such as renal toxicity. Treatment resulted in a median decline of 4.5 log copies/mL in HBV DNA levels after median treatment duration of 12 mo[20]. TDF alone, or combined with LAM, exerted a greater viral reduction than ADV for LAM-resistant HBV infection without developing phenotypic resistance, and showed a high antiviral efficacy in patients with LAM resistance and an inadequate response during therapy with ADV[21-23]. TDF plus LAM therapy improved the Child-Pugh score in decompensated liver cirrhosis patients with multiple NA treatment failures[7].
This current study included 29 patients with a prior history of treatment failure with two or more NAs. Treatment with a TDF containing regimen in these subjects resulted in 86.2% of CVR at 12 mo and 96.6% at 24 mo of treatment. A distinctive feature of our study is that we included 18 patients who had failed treatment with ETV at 1 mg in addition to LAM and ADV. A retrospective multicenter study conducted by van Bömmel et al[5] included only 3 patients who had failed ETV therapy; two who had been only ETV experienced and one who had been treated with sequential LAM and ETV at 1 mg. ETV has an extremely high anti-HBV suppressive effect and very low chance of resistance emergence at only 1.2% after 5 years in treatment-naive subjects[24]. In this study, among the 18 patients who failed multiple NA treatments including LAM, ADV, and ETV, 17 patients achieved CVR and one patient showed a viral reduction of 3.9 log IU/mL, nearly reaching CVR. Genotypic resistance to EVT did not affect the probability of CVR. Based on these results, it can be suggested that the strong antiviral activity of TDF is also valid in ETV failed CHB patients.
Patterson et al[6] reported 64% of CVR rate at 96 wk of TDF rescue therapy in CHB patients following failures of both LAM and ADV treatment. 21 of the 60 patients had baseline ADV resistance (14 patients with the rtA181T/V mutation and 7 patients with the rtN236T mutation), and the authors concluded that the viral response was independent of mutations conferring ADV resistance. However, in another study, the presence of ADV resistance was considered to decrease the efficacy of TDF. During the observation period, the probability of achieving HBV DNA levels below 400 copies/mL was 52% for patients with ADV resistant variants and 100% for those without[5]. In the current study, 6 patients had genotypic resistance to ADV. Although one patient with the rtA181V/T mutant strain did not reach CVR, resistance to ADV did not influence CVR during the treatment period. The effect of ADV resistance on the antiviral efficacy of TDF cannot be concluded from the results of this study because of the small sample size, and is an area that should be explored in further research. However, considering that in vitro cross resistance of ADV and TDF has been described previously[25-27], the possibility of altered response to TDF in CHB patients with genotypic resistance to ADV should be considered.
The ultimate goal of CHB therapy is to prevent cirrhosis, hepatic failure, hepatocellular carcinoma, and death. This goal can be achieved by the complete and sustained suppression of HBV replication. Therefore, the primary aim of treatment in chronic HBV infection is to suppress HBV replication to an undetectable level[1,18,28], leading to decreased infectivity and pathogenicity of the virus and resulting in reduced hepatic necroinflammation[29]. Viral BT resulting from resistance or insufficient suppression of HBV DNA due to the use of a less potent drug can result in failure to completely suppress HBV DNA. After the availability of ETV and TDF, NAs with potent antiviral activity and a high genetic barrier, insufficient viral suppression or resistance in treatment naive patients is not common. However, in the era when these drugs with a high genetic barrier were not available, the majority of patients started therapy with LAM and subsequently received ADV and/or ETV to manage LAM resistance. Many of these patients developed resistance to ADV or ETV and remained viremic despite prolonged treatment[11-15,22]. Multiple failures of NA therapies are a growing and global problem, especially in some parts of Asia, where the use of LAM remains common due to the high prevalence of CHB and generics are available at a low cost[30]. In the present study, we have demonstrated the high efficacy of a TDF containing regimen in patients with sequential resistance to multiple NAs, and that TDF containing regimens are effective and safe rescue therapies for CHB patients after multiple failures with NAs.
Treatment efficacy, evaluated as CVR, did not differ in patients treated with TDF alone or in patients treated with combination therapy with LAM or ETV. Generally, combination therapy of drugs that are not in the same cross-resistance group is recommended for the management of resistant strains[1,3,18]. However, since TDF monotherapy without other NAs was also effective without viral BT after approximately 2 years of follow-up[5], it is questionable as to whether combination therapy with this strong antiviral agent is really necessary when considering the cost and potential adverse effects of combination therapy. As such, further studies are necessary to explore these results.
This study has some limitations. The number of subjects included was not large enough (n = 29) and the follow-up period was relatively short (median 16 mo). Nevertheless, the patients in this study represent the most difficult-to-treat population in the management of CHB. Further studies with larger sample sizes are necessary to remedy these shortcomings and to elucidate the long-term outcome of TDF treatment. Although the genotype of HBV could be a factor affecting the treatment efficacy of antiviral agents, we did not perform an analysis of the genotype. However, previous studies have documented that almost all HBV infected patients in Korea have genotype C; constituting 98%-100% of HBV infected patients[31-33]. Therefore these results could be applicable to our study patients, and can represent the genotype C HBV infected patients.
In conclusion, a TDF containing treatment regimen suppressed HBV DNA in CHB patients with multiple treatment failures of NA therapy, regardless of genotypic resistance or treatment regimen. TDF can be an effective and safe rescue therapy for these patients.
The excellent treatment efficacy of tenofovir disoproxil fumarate (TDF) in treatment naive chronic hepatitis B (CHB) has been documented in various clinical studies. However, data on its role in CHB patients with prior treatment failure to other oral anti-viral agent is insufficient.
TDF has shown efficacy in nucleoside/nucleotide analogue (NA) experienced patients with various results outside of Asia. A single report including six South Korean patients with prior treatment failure to lamivudine (LAM) and adefovir stated that a complete virologic suppression was achieved in all patients at 12 mo with a TDF containing regimen, and this has been the only study of its type conducted in Asian country.
Many CHB patients in Asian countries develop sequential antiviral resistance to oral anti-viral agents or develop insufficient suppression of hepatitis B virus (HBV) DNA, which has begun to emerge as an important and difficult issue for clinicians. In this study, the authors documented the efficacy and safety of a TDF rescue therapy in Asian CHB patients after failure of multiple NA therapies. The efficacy was not affected by genotypic resistance or prior exposure to a specific agent.
After documenting the efficacy and safety of TDF regimen in CHB patients with multiple NA treatment failure, the authors suggest the possibility of using TDF as a rescue therapy for CHB patients, even whose with prior entecavir therapy failure.
The authors evaluated the efficacy and safety of a TDF treatment for CHB patients after failures of multiple NA therapies, and showed that TDF successfully suppressed HBV DNA levels in these patients. The study is very important, as the authors stated in the manuscript, in some countries where LAM treatment is still common due to financial problems.
Peer reviewer: Masahiro Arai, MD, PhD, Department of Gastroenterology, Toshiba General Hospital, 6-3-22 Higashi-ooi, Shinagawa-ku, Tokyo 140-8522, Japan
S- Editor Lv S L- Editor Rutherford A E- Editor Zhang DN
| 1. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology. 2009;49:S112-S121. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Halaris AE, Belendiuk KT, Freedman DX. Antidepressant drugs affect dopamine uptake. Biochem Pharmacol. 1975;24:1896-1897. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Snow-Lampart A, Chappell B, Curtis M, Zhu Y, Myrick F, Schawalder J, Kitrinos K, Svarovskaia ES, Miller MD, Sorbel J. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology. 2011;53:763-773. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73-80. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247-254. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Choe WH, Kwon SY, Kim BK, Ko SY, Yeon JE, Byun KS, Kim GH, Lee CH. Tenofovir plus lamivudine as rescue therapy for adefovir-resistant chronic hepatitis B in hepatitis B e antigen-positive patients with liver cirrhosis. Liver Int. 2008;28:814-820. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15 Suppl 6:S13-S24. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Shin SR, Yoo BC, Choi MS, Lee DH, Song SM, Lee JH, Koh KC, Paik SW. A comparison of 48-week treatment efficacy between clevudine and entecavir in treatment-naïve patients with chronic hepatitis B. Hepatol Int. 2011;5:664-670. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385-1391. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, Seo YS, Chung HJ, Moon MS, Kim SO. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488-1495. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307-313. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Rescue therapy for lamivudine-resistant chronic hepatitis B: comparison between entecavir 1.0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. J Gastroenterol Hepatol. 2010;25:1374-1380. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Ryu HJ, Lee JM, Ahn SH, Kim do Y, Lee MH, Han KH, Chon CY, Park JY. Efficacy of adefovir add-on lamivudine rescue therapy compared with switching to entecavir monotherapy in patients with lamivudine-resistant chronic hepatitis B. J Med Virol. 2010;82:1835-1842. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Froese A, Sehon AH. Kinetics of antibody--hapten reactions. Contemp Top Mol Immunol. 1975;4:23-54. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132-143. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Lada O, Benhamou Y, Cahour A, Katlama C, Poynard T, Thibault V. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther. 2004;9:353-363. [PubMed] [Cited in This Article: ] |
| 20. | Kuo A, Dienstag JL, Chung RT. Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin Gastroenterol Hepatol. 2004;2:266-272. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, Wiedenmann B, Berg T. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421-1425. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Hann HW, Chae HB, Dunn SR. Tenofovir (TDF) has stronger antiviral effect than adefovir (ADV) against lamivudine (LAM)-resistant hepatitis B virus (HBV). Hepatol Int. 2008;2:244-249. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | van Bömmel F, Zöllner B, Sarrazin C, Spengler U, Hüppe D, Möller B, Feucht HH, Wiedenmann B, Berg T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318-325. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. J Gastroenterol Hepatol. 2011;26 Suppl 1:138-143. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Fabre LF, McLendon DM, Harris RT. Preference studies of triazolam with standard hypnotics in out-patients with insomnia. J Int Med Res. 1976;4:247-254. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Qi X, Xiong S, Yang H, Miller M, Delaney WE. In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355-362. [PubMed] [Cited in This Article: ] |
| 27. | Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174-S184. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Zoulim F. Hepatitis B virus resistance to antiviral drugs: where are we going? Liver Int. 2011;31 Suppl 1:111-116. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: an endemic area of hepatitis B virus infection. Intervirology. 2005;48:133-137. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Cho JH, Yoon KH, Lee KE, Park DS, Lee YJ, Moon HB, Lee KR, Choi CS, Cho EY, Kim HC. [Distribution of hepatitis B virus genotypes in Korea]. Korean J Hepatol. 2009;15:140-147. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Lee JM, Park JY, Kim do Y, Nguyen T, Hong SP, Kim SO, Chon CY, Han KH, Ahn SH. Long-term adefovir dipivoxil monotherapy for up to 5 years in lamivudine-resistant chronic hepatitis B. Antivir Ther. 2010;15:235-241. [PubMed] [DOI] [Cited in This Article: ] |