Published online Dec 7, 2012. doi: 10.3748/wjg.v18.i45.6690
Revised: August 24, 2012
Accepted: August 25, 2012
Published online: December 7, 2012
Malakoplakia is a rare granulomatous disease probably caused by infection and characterized histologically by Michaelis-Gutmann bodies. We report a more rarely seen case esophageal malakoplakia in a 54-year-old woman. She presented with coughing while eating and drinking. Gastroscopy showed yellow nodules in the esophagus, and endoscopic ultrasonography showed a space-occupying lesion in the substratum of the esophageal mucosa. All findings highly resembled esophageal cancer. Histopathological examination finally indentified this space-occupying lesion as malakoplakia and not cancer. Immunohistochemistry showed that she had human papillomavirus (HPV) infection in the esophagus, which indicates that infection was responsible for the malakoplakia. This is believed to be the first case of malakoplakia in the esophagus, and more importantly, we established that HPV infection was the initiator of esophageal malakoplakia.
- Citation: Yang YL, Xie YC, Li XL, Guo J, Sun T, Tang J. Malakoplakia of the esophagus caused by human papillomavirus infection. World J Gastroenterol 2012; 18(45): 6690-6692
- URL: https://www.wjgnet.com/1007-9327/full/v18/i45/6690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i45.6690
Malakoplakia is a rare granulomatous disease that was first described by Michaelis and Gutmann[1] in 1902, and is characterized by Michaelis-Gutmann bodies with cytoplasmic concentric laminated inclusions[2,3]. Malakoplakia most frequently involves the urinary tract, and less frequently, the gastrointestinal tract[4], and the esophagus is seldom involved. We report a case of esophageal malakoplakia in a 54-year-old woman.
A 54-year-old woman was referred to the gastroenterology department with complaints of coughing while eating and drinking. Her past medical history included chronic atrophic gastritis, duodenitis and rheumatoid arthritis.
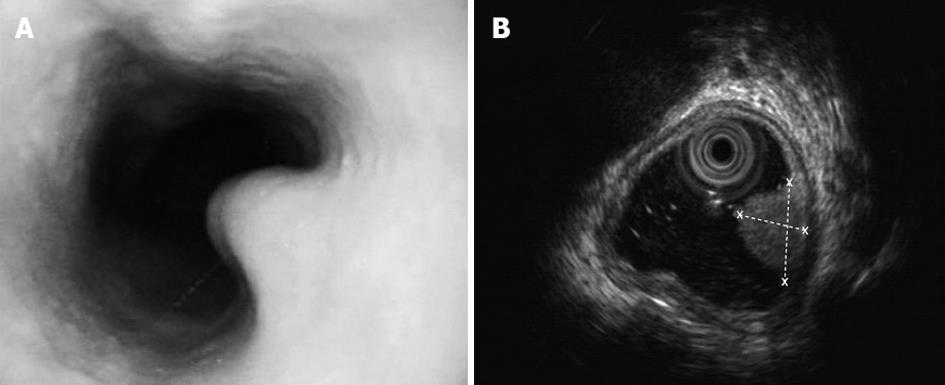
On clinical examination she was pale. Her chest X-ray, electrocardiogram, blood tests and serum α-fetoprotein results were normal. Gastroscopy showed soft yellow nodules in the right wall of the esophagus, which was 23.5-25.0 cm from the cutting tooth (Figure 1A). Endoscopic ultrasonography revealed a space-occupying lesion (8.1 mm × 5.1 mm) in the substratum of the esophageal mucosa (Figure 1B). 14C-Urea breath tests were positive for Helicobacter pylori (H. pylori).
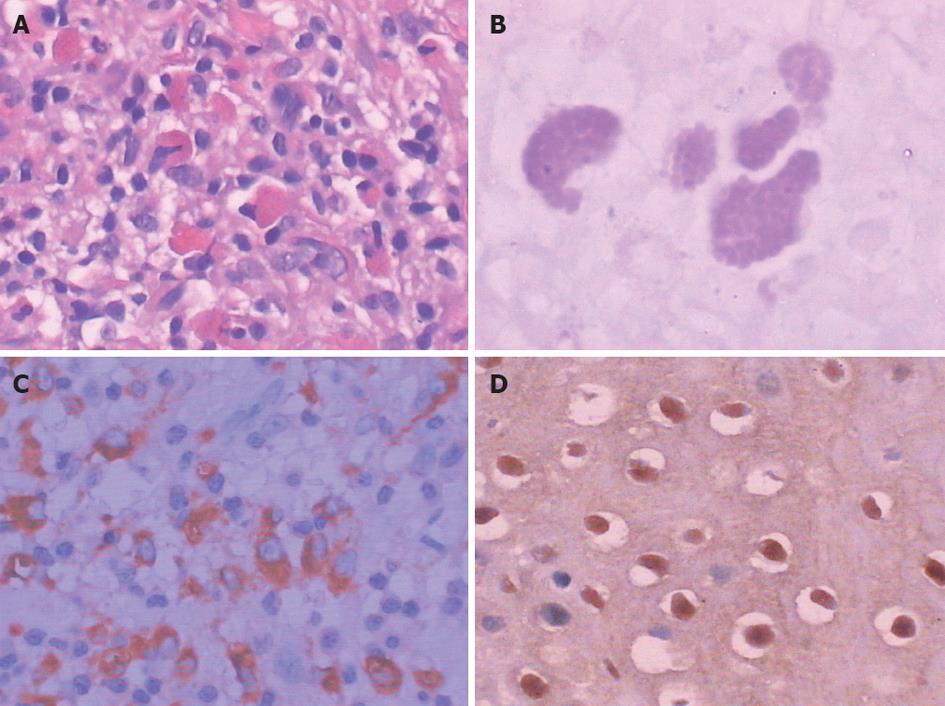
A local specimen of 1 cm × 1 cm was excision under gastroscopy for histopathological examination. Microscopic examination of hematoxylin and eosin stained sections showed a large amount of inflammatory cell infiltration in the substratum of the esophageal mucosa, mainly with lymphoid and histiocytic cells (Figure 2A). The presence of Michaelis-Gutmann bodies, with cytoplasmic concentric laminated inclusions of 5-15 mm confirmed the diagnosis. Follow-up examination supported this diagnosis. The Michaelis-Gutmann bodies were periodic acid-Schiff-positive (Figure 2B), and CD68-positive (Figure 2C) and human papillomavirus (HPV)-positive (Figure 2D) by immunohistochemistry, but negative for Eumycetes by hexamethylene diamine staining and H. pylori by Giemsa staining.
Malakoplakia is a chronic granulomatous inflammatory disease characterized by accumulation of granular basophilic Michaelis-Gutmann bodies. These bodies are generated from histiocytes, which are positive for CD68 antibodies, as well as positive for periodic acid-Schiff stain, and exhibit a targetoid appearance with a dense central core under light microscopy[4].
Malakoplakia has a worldwide distribution and does not have any racial, sex or age predilection[5]. Malakoplakia most commonly affects the urinary tract, as well as the gastrointestinal system, regional lymph nodes, skin, liver, and spleen[6-9].
The mechanism of malakoplakia is not well understood. Three postulates have been suggested. The first considers that microorganisms play a role in the pathogenesis. Escherichia coli infection is often found in the urinary tract[10], Rhodococcus equii in the lungs[11], and H. pylori in the stomach[12]. However, there have been no previous reports of microbial infection in the esophagus. In the present case, HPV infection was identified by immunohistochemistry. We consider that HPV infection plays an important role in development of esophageal malakoplakia. Another possibility is that abnormal immune responses are involved in the pathogenesis[13]. Some immunosuppressive or chronic prolonged illnesses such as organ transplantation, acquired immunodeficiency syndrome, tuberculosis, sarcoidosis, and malignancy[14] can be associated with malakoplakia. The woman in this case report suffered from rheumatoid arthritis whose pathogenesis is considered to be related to an abnormal immune response, which we consider may also have been related to her malakoplakia. The third hypothesis is an abnormal macrophage response caused by defective lysosomal function. This results in macrophages being unable to digest fully the phagocytosed bacteria, accumulation of partially digested bacteria, and generation of Michaelis-Gutmann bodies.
Malakoplakia typical presents as irregular nodules or plaque, but it also exists as widespread mucosal multinodular or polypoid lesions, or large mass lesions under endoscopy. In the present case, it presented as endoscopic nodules.
The clinical appearance of malakoplakia varies from silent nodules to various different presentations according to the organ involved. In the urinary tract it presents with lower tract irritative symptoms such as frequency, dysuria and hematuria[15]. In the gastrointestinal system it can be clinically silent or can cause clinical symptoms such as diarrhea, abdominal pain, hemorrhage, or obstruction[16,17]. In the respiratory system it can appear as silent nodules that mimic bronchogenic carcinoma or tuberculosis[11]. Malakoplakia of the female genital tract usually presents with vaginal bleeding[18]. In the present case, the patient presented with coughing while eating and drinking, which resembled esophageal cancer.
Malakoplakia is generally considered a chronic, self-limiting inflammatory disease that may undergo spontaneous regression[19]. In the present case, despite the patient rejecting further treatment after receiving the pathological report, her symptoms disappeared and her condition did not develop. Follow-up endoscopic examinations 12 mo after resection revealed no changes in the patient’s condition.
There are two therapeutic approaches to malakoplakia. Most cases have been successfully treated with antibiotics, for example, rifampicin, quinolone, and trimethoprim-sulfamethoxazole. The second approach is to attempt to correct the lysosomal defect by a cholinergic agonist, bethanechol chloride. Combination of antibiotic therapy and surgery provides satisfactory results. However, unnecessary radical surgical treatment should be avoided. The best choice depends on each specific patient. Our patient appeared to be cured by resection of the malakoplakia and showed no development during 1-year follow-up.
Peer reviewer: Luigi Bonavina, Professor, Department of Surgery, IRCCS Policlinico San Donato, Piazza Malan, 20097 Milano, Italy
S- Editor Gou SX L- Editor Kerr C E- Editor Li JY
| 1. | Michaelis L, Gutmann C. Uber einschlusse in blasentumoren. Z Klin Med. 1902;47:208-215. [Cited in This Article: ] |
| 2. | Diapera MJ, Lozon CL, Thompson LD. Malacoplakia of the tongue: a case report and clinicopathologic review of 6 cases. Am J Otolaryngol. 2009;30:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Jain M, Arora VK, Singh N, Bhatia A. Malakoplakia of the appendix. An unusual association with eggs of Taenia species. Arch Pathol Lab Med. 2000;124:1828-1829. [PubMed] [Cited in This Article: ] |
| 4. | Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med. 2007;131:297-300. [PubMed] [Cited in This Article: ] |
| 5. | Ben Amna M, Hajri M, Oumaya C, Anis J, Bacha K, Ben Hassine L, Chebil M, Ayed M. Genito-urinary malacoplakia. Report of 10 cases and review of the literature. Ann Urol (Paris). 2002;36:388-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gidwani A, Gidwani S, Khan A, Carson J. Concurrent malakoplakia of cervical lymph nodes and prostatic adenocarcinoma with bony metastasis: case report. Ghana Med J. 2006;40:151-153. [PubMed] [Cited in This Article: ] |
| 7. | Sormes M, Siemann-Harms U, Brandner JM, Moll I. Cutaneous malakoplakia. J Dtsch Dermatol Ges. 2011;9:914-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 8. | Hartman G, Yong SL, Chejfec G. Malakoplakia of liver diagnosed by a needle core biopsy: a case report and review of the literature. Arch Pathol Lab Med. 2002;126:372-374. [PubMed] [Cited in This Article: ] |
| 9. | Greaves WO, Wang LJ. Malakoplakia of the spleen: a case report. Int J Surg Pathol. 2010;18:584-587. [PubMed] [Cited in This Article: ] |
| 10. | Wagner D, Joseph J, Huang J, Xu H. Malakoplakia of the prostate on needle core biopsy: a case report and review of the literature. Int J Surg Pathol. 2007;15:86-89. [PubMed] [Cited in This Article: ] |
| 11. | Gupta K, Thakur S. Pulmonary malakoplakia: a report of two cases. Indian J Pathol Microbiol. 2011;54:133-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Sanusi ID, Tio FO. Gastrointestinal malacoplakia. Report of a case and a review of the literature. Am J Gastroenterol. 1974;62:356-366. [PubMed] [Cited in This Article: ] |
| 13. | Kumar V, Coady MS. Malakoplakia of the neck in an immunosuppressed patient. Plast Reconstr Surg. 2005;116:125e-127e. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | El Jamal SM, Malak SF, Cox RM, Lorsbach RB. Extragenitourinary malakoplakia in a patient with myeloma clinically mimicking extramedullary myelomatous disease. Hum Pathol. 2011;42:602-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Velásquez López JG, Vélez Hoyos A, Uribe Arcila JF. Malakoplakia in urology: six cases report and review of the literature. Actas Urol Esp. 2006;30:610-618. [PubMed] [Cited in This Article: ] |
| 16. | Gustavo LC, Robert ME, Lamps LW, Lagarde SP, Jain D. Isolated gastric malakoplakia: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:e153-e156. [PubMed] [Cited in This Article: ] |
| 17. | Bouguila J, Brahim K, Mokni M, Skandrani K, Harbi A, Essoussi AS, Boughammoura L. Digestive malacoplakia in children: case report. ISRN Gastroenterol. 2011;2011:597350. [PubMed] [Cited in This Article: ] |
| 18. | Rafailidis SF, Ballas KD, Symeonidis N, Pavlidis TE, Emoniotou E, Psarras K, Pantzaki A, Marakis GN, Sakadamis AK. Pelvic malakoplakia simulating recurrence of rectal adenocarcinoma: report of a case. Tech Coloproctol. 2009;13:79-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Garg M, Eley KA, Bond SE, Shah KA, Browning L, Watt-Smith SR. Malakoplakia presenting as an enlarging neck mass: Case presentation and review of the literature. Head Neck. 2010;32:1269-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |