Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6494
Revised: August 17, 2012
Accepted: August 26, 2012
Published online: November 28, 2012
Processing time: 148 Days and 4.5 Hours
We report a case of an 84-year-old male patient with primary small intestinal angiosarcoma. The patient initially presented with anemia and melena. Consecutive endoscopy revealed no signs of upper or lower active gastrointestinal bleeding. The patient had been diagnosed 3 years previously with an aortic dilation, which was treated with a stent. Computed tomography suggested an aorto-intestinal fistula as the cause of the intestinal bleeding, leading to operative stent explantation and aortic replacement. However, an aorto-intestinal fistula was not found, and the intestinal bleeding did not arrest postoperatively. The constant need for blood transfusions made an exploratory laparotomy imperative, which showed multiple bleeding sites, predominately in the jejunal wall. A distal loop jejunostomy was conducted to contain the small intestinal bleeding and a segmental resection for histological evaluation was performed. The histological analysis revealed a less-differentiated tumor with characteristic CD31, cytokeratin, and vimentin expression, which led to the diagnosis of small intestinal angiosarcoma. Consequently, the infiltrated part of the jejunum was successfully resected in a subsequent operation, and adjuvant chemotherapy with paclitaxel was planned. Angiosarcoma of the small intestine is an extremely rare malignant neoplasm that presents with bleeding and high mortality. Early diagnosis and treatment are essential to improve outcome. A small intestinal angiosarcoma is a challenging diagnosis to make because of its rarity, nonspecific symptoms of altered intestinal function, nonspecific abdominal pain, severe melena, and acute abdominal signs. Therefore, a quick clinical and histological diagnosis and decisive measures including surgery and adjuvant chemotherapy should be the aim.
- Citation: Zacarias Föhrding L, Macher A, Braunstein S, Knoefel WT, Topp SA. Small intestine bleeding due to multifocal angiosarcoma. World J Gastroenterol 2012; 18(44): 6494-6500
- URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6494.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6494
Primary malignant tumors of the small intestine are rare neoplasms, which comprise < 2% of all gastrointestinal tumors[1], including adenocarcinoma, carcinoid, sarcoma, gastrointestinal stromal tumors, and lymphoma. The reason for the poor prognosis of small bowel malignant tumors is partly due to a late diagnosis. The difficulty diagnosing this type of tumor is associated with the nonspecific symptoms, including nausea, vomiting, abdominal pain, constipation, generalized weakness, fatigue, malaise, weight loss, anemia, diarrhea, ileus, intestinal perforation, or hemorrhage[2], as well as limited diagnostic methods for the small intestine.
Angiosarcoma is a rare mesenchymal tumor that most often arises from skin and subcutaneous tissues[3-7] but can ultimately arise anywhere in the body. Angiosarcomas have been described in the liver[8-11], spleen[12,13], adrenal glands[14-16], ovaries[17-19], heart[20-22], lung[23,24], breast[25-27] and, very rarely, in the gastrointestinal tract[28-32]. Consequently, an intestinal angiosarcoma that is located in the small bowel rather than the upper or lower gastrointestinal tract is a very rare medical condition. Angiosarcomas are aggressive tumors with a high rate of lymph node and peripheral metastases. This tumor arises as a de novo primary tumor or secondary to irradiation or chemical exposure. Angiosarcoma of the small intestine presents unique diagnostic challenges and is often discovered late, leading to a very poor prognosis.
Additionally, the histological diagnosis is difficult and can be confused with other neoplasms such as poorly differentiated carcinoma[4,33,34]. Diagnosis is facilitated by immunohistochemical expression analysis of the endothelial markers CD31 and CD34, as well as factor VIII-associated antigens.
Herein, we describe the case of an 84-year-old man with the first episode of gastrointestinal bleeding due to angiosarcoma of the small intestine.
The patient was transferred to the Department of Internal Medicine of a peripheral hospital with gastrointestinal bleeding, which required a blood transfusion. Three lesions with coagulum and vessels necessitating application of two clips were found by endoscopy of the distal duodenum and upper segment of the jejunum. A colonoscopy revealed old blood, so bleeding in the small intestine was suspected. The patient had been diagnosed with an aortic aneurysm 3 years previously, which was treated with juxtarenal stent-graft prosthesis. A prosthetic-enteric fistula was suspected on the emergency abdominal aortic computed tomography (CT) scan. With this suspected diagnosis, the patient was transferred to the University Hospital Düsseldorf, and emergency vascular surgery was performed. The stent-graft prosthesis was removed and desobliteration of the saccular aortic aneurysm and the renal arteries was implemented, followed by implantation of an aorto-biiliacal silver-graft prosthesis. However, a prosthetic-enteric fistula was not revealed intraoperatively. The gastrointestinal bleeding did not arrest postoperatively, and Forrest IIb bleeding in the proximal jejunum was endoscopically diagnosed and treated. The local bleeding was stopped with hemoclips.
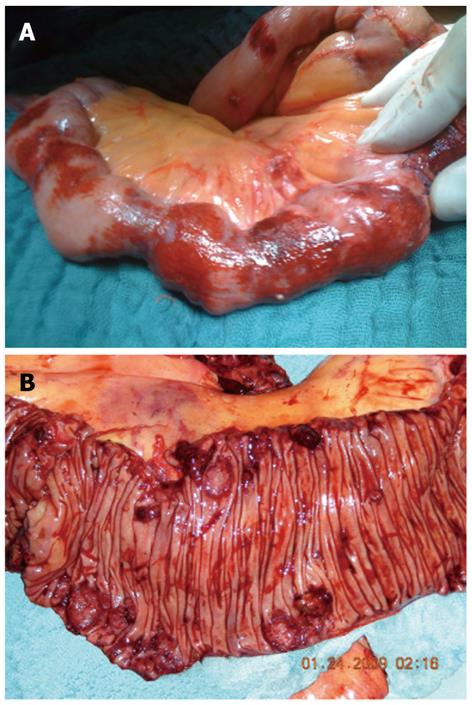
An exploratory laparotomy was performed due to persistent gastrointestinal hemorrhage, which showed multiple intra-abdominal hemorrhagic lesions in the intestinal wall of the jejunum (Figure 1). Three segmental resections and a distal loop jejunostomy were performed for a histological evaluation but achieved only temporal arrest of bleeding, which again became visible postoperatively after a loop jejunostomy. As no transanal bleeding was observed, and the histological analysis suggested a malignant angiosarcoma, a small bowel resection proximal to the loop jejunostomy with an end-to-end duodenoileostomy was subsequently performed.
Only approximately 1 m of small intestine could be preserved to achieve bleeding control. Adjuvant therapy was intended with paclitaxel, due to histological evidence of angiosarcoma. Unfortunately, a spontaneous intracranial hemorrhage with ventricular bleeding led to death of the patient, and the cause could not be determined. During hospitalization, the patient had received 75 erythrocyte concentrates, 49 units of fresh frozen plasma, 12 thrombocyte concentrates, and coagulation factors.
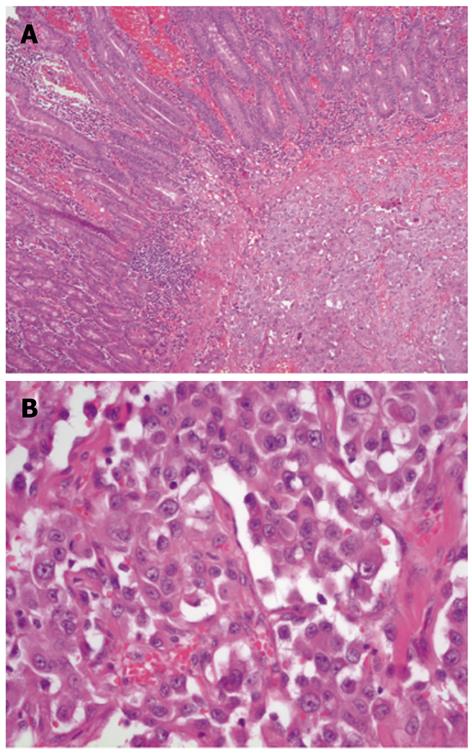
The small bowel showed an epithelium partly ulcerated with hemorrhage and infiltration of a mesenchymal fusiform tumor with parts of high-grade cells and nuclear polymorphism, including several mitoses and apoptosis formation (Figure 2). The tumor cells formed slit-shaped hollows, and they were predominantly grouped together with a solid appearance. The neoplastic cells were multifocal with macronucleoli. Eight mitoses were identified per 10 high-power fields. The tumor cells stained positive for CD31, cytokeratin, and vimentin and slightly weaker for CD34. The tumor cells were also focally positive for factor VIII. The MIB-1 marker of proliferation was expressed in approximately 40% of nuclei. The Berlin blue reaction indicated siderin deposits and Elastica-van-Gieson staining revealed collagen fibers. In summary, the tumor showed a less differentiated, multifocally growing epithelioid angiosarcoma (degree of malignancy III Coindre) in the submucosa with infiltration of the subserosal fat tissue and extensive lymphatic spread.
A gastrointestinal hemorrhage is a potentially dangerous condition that warrants a quick diagnosis and decisive treatment. The vast majority of these bleeding events are due to either upper or lower gastrointestinal bleeding, and only 5% cannot be localized endoscopically[35]. These bleeding events typically occur from the small intestine. The most common cause of small intestinal bleeding is a vascular abnormality such as angioectasia, followed by tumors and, more infrequently, small bowel ulcers and aortoenteric fistulas[36]. Angiosarcoma of the small intestine is an extremely rare but potentially life-threatening cause of such bleeding.
Angiosarcomas typically occur in skin and superficial soft tissue, rather than in the gastrointestinal tract, and compromise < 2% of all sarcomas[37,38]. Consequently, only 33 cases of small intestinal angiosarcoma have been reported in the English literature over the past 42 years (Table 1).
| Patient[age (yr)/sex] | Tumor manifestation | Histology | Radiation/ pre-disposition | Symptoms | Therapy | Outcome | Ref. |
| 46/M | Duodenum, ileum, and stomach | Epithelioid | None | Abdominal pain, melena | Resection | Died after 6 mo | [50] |
| 65/F | Ileum | Well-differentiated | Radiation | Abdominal pain, nausea, vomiting | Resection, chemotherapy | Died after 14 mo | [39] |
| 64/M | NA | Epithelioid | None | Gastrointestinal bleeding | Resection | Died after 1 yr | [29] |
| 57/F | Small intestine | Epithelioid | None | NA | Resection | Died after 4 mo | [29] |
| 47/F | Ileum | NA | Radiation | Abdominal pain | Resection, chemotherapy, radiation | NA | [40] |
| 64/M | Small intestine | Well-differentiated tumor | None | Gastrointestinal bleeding | Resection | Died with disseminated disease after approximately 1 yr | [30] |
| 57/M | Ileocecal valve, small bowel, and mesentery | Well-differentiated tumor | None | NA | Resection | Died after several days | [30] |
| 76/M | Ileum | Mixed, well-differentiated and epithelioid | None | Abdominal pain, poor appetite, fatigue | Resection | Died after 9 d | [59] |
| 74/F | Jejunum | Well-differentiated | NA | Melena | Resection | Died due to multiple complications | [60] |
| 51/M | NA | Well-differentiated | Radiation | Abdominal pain | Resection, chemotherapy | Died after 5 mo | [48] |
| 76/F | Ileum | Well-differentiated | Radiation | Abdominal pain, weight loss, vomiting, diarrhea | Resection | Died after 5 mo | [45] |
| 48/F | Ileum | NA | Radiation | Abdominal pain | Resection | Died from sepsis after 23 d | [43] |
| 60/F | Small intestine | Well-differentiated | Radiation | Acute abdomen, and a distal jejunal perforation | Resection | Died after 3 mo | [44] |
| 80/F | Small and large bowel | Well-differentiated | Radiation | Altered intestinal function | Resection | Died after 2 wk | [42] |
| 69/F | Small and large bowel | Well-differentiated | Radiation | Weight loss, abdominal distention, hematochezia | Resection | Died after 23 d | [42] |
| NA/M | Duodenum, stomach | Epithelioid | None | Severe melena | Resection | Died of respiratory failure, metastases were found in various organs, including the lungs, bones, liver, gall-bladder, and lymph nodes | [61] |
| 78/F | Small intestine | High-grade | Radiation | Relative bowel obstruction | Resection | Died after 2 yr | [41] |
| 50/F | Ileum | Multifocal and infiltrating | Radiation | Repeated symptoms of intestinal obstruction | Resection, chemotherapy | Died after 21 mo | [41] |
| 61/F | Ileum | Well-differentiated | Radiation | Fullness, abdominal pain | Resection | Died after 10 mo | [46] |
| 67/M | Jejunum, ileum | Epithelioid | None | Weight loss, Intermittent severe abdominal pain, and melena | Resection | Died after 3 mo | [51] |
| 85/M | Small intestine | High-grade | None | Weight loss, decreased appetite, generalized weakness, left upper quadrant abdominal pain | Resection, chemotherapy | Survived at least 1 yr | [52] |
| 59/M | Ileum | Mixed epithelioid and well-differentiated | None | Gastrointestinal bleeding | Resection | Died after 11 d | [37] |
| 70/M | Duodenum | Epithelioid | None | Melena, anemia | Chemotherapy | Died after 4 mo | [49] |
| 84/F | Jejunum | Epithelioid | None | Melena, anemia, shortness of breath | NA | Died after 17 mo | [49] |
| 47/M | Jejunum | Epithelioid | None | Melena, anemia, shortness of breath | NA | Died after 4 mo | [49] |
| 25/M | Small intestine | Epithelioid | None | Gastrointestinal bleeding, hemoptysis, anemia | Chemotherapy, radiation | Alive 18 mo after diagnosis, palliative situation | [49] |
| 70/M | Ileum | Mixed, well-differentiated | None | Abdominal pain, vomiting | Resection, chemotherapy, radiation | Survived at least 4 yr | [53] |
| 68/M | Ileocecal | High-grade | Radiation, polyvinyl chloride | Gastrointestinal bleeding, melena | Resection | Died before starting chemotherapy | [31] |
| 51/F | Ileum | Well-differentiated | Radiation | Decreased appetite and vague abdominal pain of several months duration | Resection | Died after 10 mo | [47] |
| 87/M | Duodenum, jejunum | Epithelioid | None | Lethargy, weakness, and nonspecific chest pain | Endoscopy, argon plasma coagulation | Died after 6 wk | [62] |
| 73/M | Duodenum, jejunum | Epithelioid | Radiation | Weakness, dizziness, constipation, and melena | Resection | Died after 4 mo | [32] |
| NA/M | Jejunum | NA | NA | Acute abdominal signs | Resection, chemotherapy | Survived at least 3 yr | [2] |
| 25/F | Small and large bowl | NA | None | Intermittent abdominal pain, weight loss, and progressive abdominal distension, a 7-wk history of shortness of breath, hematemesis, and melena | Resection | Died after 2 wk | [63] |
The precise predisposing factors remain unknown. Exposure to vinyl chloride, thorotrast, arsene, and radiation have been associated with the pathogenesis[9,31,39-41]. Of the 33 cases reported, 14 describe patients developing an angiosarcoma after being treated with radiation for a malignant tumor, including ovarian carcinoma[39,42], ovarian dysgerminoma[40], squamous cell carcinoma of the uterine cervix[43-46], endometrial adenocarcinoma of the uterus[41,47], and Hodgkin’s disease[48]. The first report of an angiosarcoma of the small intestine after postoperative irradiation was published in 1979[39]. That patient developed an angiosarcoma in the terminal ileum 8 years after irradiation for an ovarian carcinoma. Since then, 13 more angiosarcoma cases following radiation have been published (Table 1). In one case, an angiosarcoma occurred after exposure to irradiation and polyvinyl chloride[31], but predisposing factors could not be identified in the remaining 19 cases. The patient presented in this report also did not have any known malignancy or exposure to irradiation, vinyl chloride, or other chemicals known to induce angiosarcomas such as thorotrast or arsene.
Categorization by sex and age does not reveal any clear-cut distribution. The average age of patients with this type of angiosarcoma was 62 years (range, 25-87 years), and 18 patients were male and 15 were female (Table 1).
The clinical manifestations of patients with angiosarcomas of the small intestine include lethargy, weakness, altered intestinal function, nonspecific abdominal pain, severe melena, anemia, acute abdominal signs and/or ileus symptoms, and even nonspecific chest pain (Table 1). In 15 of the 33 cases, the patient had signs of gastrointestinal bleeding[30,31,37,49,50], similar to the patient described in this report. This variability in clinical manifestations makes it even more difficult to reach a quick and correct diagnosis. Furthermore, currently available diagnostic modalities, including CT, capsule endoscopy, double-balloon enteroscopy, magnetic resonance imaging, and positron emission tomography-CT all fail to detect the bleeding site, let alone lead to a diagnosis.
Angiosarcomas are classified as well-differentiated, poorly differentiated, and epithelioid tumors. A histological diagnosis can be challenging because angiosarcoma of the small intestine shows high architectural and cytological variability. The epithelioid morphology is typical but can be easily confused with other entities such as a poorly differentiated carcinoma[4,47]. Immunohistochemical expression analysis for the endothelial markers CD31, CD34, and factor VIII-associated antigen is crucial. The majority of cases listed in Table 1 were positive for these antigens. Other antigens show limited relevance and can cause confusion with other carcinomas. There is some controversy about the relevance of cytokeratin, which has been reported positive by some authors[32,37,49,51]. However, most authors have reported no such expression by intestinal angiosarcomas[29-31,48,52].
The current therapy for angiosarcoma includes bleeding control and symptomatic therapy to stabilize the patient, followed by radical tumor resection.
Six patients in the literature received adjuvant chemotherapy[39,41,48,49,52], and three patients were treated with combination chemotherapy and radiation[40,49,53]. Adjuvant therapy with paclitaxel was intended in the present case; however, the patient died before starting chemotherapy. All adjuvant therapy protocols are generally empiric and based on studies of cutaneous angiosarcoma, as randomized clinical studies on gastrointestinal angiosarcomas are lacking due to their rarity. The first case published received combination chemotherapy consisting of doxorubicin, vincristine, dacarbazine, and cyclophosphamide, after operative resection of the terminal ileum. That patient survived 14 mo[39]. Another combination therapy that has been used is doxorubicin and dacarbazine, which led to 5 mo survival after diagnosis[48]. Monotherapy with doxorubicin showed survival of 21 mo, at which time the tumor was widely disseminated[41]. Furthermore, thalidomide therapy was initiated as an experimental measure after operative resection in one case[52]. That patient was still alive 1 year after the initial diagnosis. No recommendation can usually be made, but paclitaxel and/or thalidomide are currently commonly considered[52,54,55]. The newest studies suggest administering doxorubicin and paclitaxel weekly for cutaneous angiosarcoma, which seem to provide longer progression-free survival[56-58].
Despite all efforts, survival of patients with small bowel angiosarcoma is generally poor. Survival usually ranges from several days after surgical intervention to 2 years. The majority of patients die within 6 mo to 1 year after being diagnosed (Table 1). Only two reported patients survived > 2 years after resection and adjuvant (radio-) chemotherapy[2,53].
One major cause of this poor outcome seems to be that the diagnosis is difficult, and many tumors are diagnosed only in the late stages of the disease. Therefore, a quick diagnosis using endoscopy and imaging procedures, as well as fast and decisive surgical intervention and adjuvant chemotherapy are necessary.
Peer reviewer: Yu-Yuan Li, Professor, Department of Medicine, First Municipal People’s Hospital of Guangzhou, Guangzhou 510180, Guangdong Province, China
S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6112] [Cited by in RCA: 5983] [Article Influence: 332.4] [Reference Citation Analysis (0)] |
| 2. | Turan M, Karadayi K, Duman M, Ozer H, Arici S, Yildirir C, Koçak O, Sen M. Small bowel tumors in emergency surgery. Ulus Travma Acil Cerrahi Derg. 2010;16:327-333. [PubMed] |
| 3. | Mendenhall WM, Mendenhall CM, Werning JW, Reith JD, Mendenhall NP. Cutaneous angiosarcoma. Am J Clin Oncol. 2006;29:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Mobini N. Cutaneous epithelioid angiosarcoma: a neoplasm with potential pitfalls in diagnosis. J Cutan Pathol. 2009;36:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Vogt T. [Angiosarcoma]. Hautarzt. 2008;59:237-48; quiz 249-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921. [PubMed] |
| 8. | Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore). 1979;58:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 147] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92:349-376. [PubMed] |
| 10. | Maluf D, Cotterell A, Clark B, Stravitz T, Kauffman HM, Fisher RA. Hepatic angiosarcoma and liver transplantation: case report and literature review. Transplant Proc. 2005;37:2195-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 11. | Chien CY, Hwang CC, Yeh CN, Chen HY, Wu JT, Cheung CS, Lin CL, Yen CL, Wang WY, Chiang KC. Liver angiosarcoma, a rare liver malignancy, presented with intraabdominal bleeding due to rupture--a case report. World J Surg Oncol. 2012;10:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 167] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, Abbondanzo SL. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol. 2000;13:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Wenig BM, Abbondanzo SL, Heffess CS. Epithelioid angiosarcoma of the adrenal glands. A clinicopathologic study of nine cases with a discussion of the implications of finding "epithelial-specific" markers. Am J Surg Pathol. 1994;18:62-73. [PubMed] |
| 15. | Kareti LR, Katlein S, Siew S, Blauvelt A. Angiosarcoma of the adrenal gland. Arch Pathol Lab Med. 1988;112:1163-1165. [PubMed] |
| 16. | Krüger S, Kujath P, Johannisson R, Feller AC. Primary epithelioid angiosarcoma of the adrenal gland case report and review of the literature. Tumori. 2001;87:262-265. [PubMed] |
| 17. | Nielsen GP, Young RH, Prat J, Scully RE. Primary angiosarcoma of the ovary: a report of seven cases and review of the literature. Int J Gynecol Pathol. 1997;16:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Nucci MR, Krausz T, Lifschitz-Mercer B, Chan JK, Fletcher CD. Angiosarcoma of the ovary: clinicopathologic and immunohistochemical analysis of four cases with a broad morphologic spectrum. Am J Surg Pathol. 1998;22:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Bradford L, Swartz K, Rose S. Primary angiosarcoma of the ovary complicated by hemoperitoneum: a case report and review of the literature. Arch Gynecol Obstet. 2010;281:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 20. | Glancy DL, Morales JB, Roberts WC. Angiosarcoma of the heart. Am J Cardiol. 1968;21:413-419. [PubMed] |
| 22. | Luk A, Nwachukwu H, Lim KD, Cusimano RJ, Butany J. Cardiac angiosarcoma: a case report and review of the literature. Cardiovasc Pathol. 2010;19:e69-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Patel AM, Ryu JH. Angiosarcoma in the lung. Chest. 1993;103:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Pandit SA, Fiedler PN, Westcott JL. Primary angiosarcoma of the lung. Ann Diagn Pathol. 2005;9:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Marchal C, Weber B, de Lafontan B, Resbeut M, Mignotte H, du Chatelard PP, Cutuli B, Reme-Saumon M, Broussier-Leroux A, Chaplain G. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys. 1999;44:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Sher T, Hennessy BT, Valero V, Broglio K, Woodward WA, Trent J, Hunt KK, Hortobagyi GN, Gonzalez-Angulo AM. Primary angiosarcomas of the breast. Cancer. 2007;110:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol. 2009;13:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Barclay TH, Schapira DV. Malignant tumors of the small intestine. Cancer. 1983;51:878-881. [PubMed] |
| 29. | Ordóñez NG, del Junco GW, Ayala AG, Ahmed N. Angiosarcoma of the small intestine: an immunoperoxidase study. Am J Gastroenterol. 1983;78:218-221. [PubMed] |
| 30. | Taxy JB, Battifora H. Angiosarcoma of the gastrointestinal tract. A report of three cases. Cancer. 1988;62:210-216. [PubMed] |
| 31. | Khalil MF, Thomas A, Aassad A, Rubin M, Taub RN. Epithelioid Angiosarcoma of the Small Intestine After Occupational Exposure to Radiation and Polyvinyl Chloride: A case Report and Review of Literature. Sarcoma. 2005;9:161-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Grewal JS, Daniel AR, Carson EJ, Catanzaro AT, Shehab TM, Tworek JA. Rapidly progressive metastatic multicentric epithelioid angiosarcoma of the small bowel: a case report and a review of literature. Int J Colorectal Dis. 2008;23:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Lin CF, DeFrias D, Lin X. Epithelioid angiosarcoma: a neoplasm with potential diagnostic challenges. Diagn Cytopathol. 2010;38:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Fletcher CD, Beham A, Bekir S, Clarke AM, Marley NJ. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol. 1991;15:915-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 200] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Bashir RM, al-Kawas FH. Rare causes of occult small intestinal bleeding, including aortoenteric fistulas, small bowel tumors, and small bowel ulcers. Gastrointest Endosc Clin N Am. 1996;6:709-738. [PubMed] |
| 37. | Chami TN, Ratner LE, Henneberry J, Smith DP, Hill G, Katz PO. Angiosarcoma of the small intestine: a case report and literature review. Am J Gastroenterol. 1994;89:797-800. [PubMed] |
| 38. | Bardwil JM, Mocega EE, Butler JJ, Russin DJ. Angiosarcomas of the head and neck region. Am J Surg. 1968;116:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Chen KT, Hoffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048. [PubMed] |
| 41. | Aitola P, Poutiainen A, Nordback I. Small-bowel angiosarcoma after pelvic irradiation: a report of two cases. Int J Colorectal Dis. 1999;14:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Wolov RB, Sato N, Azumi N, Lack EE. Intra-abdominal "angiosarcomatosis" report of two cases after pelvic irradiation. Cancer. 1991;67:2275-2279. [PubMed] |
| 43. | Su CC, Jin YT, Chien CH, Yu CY, Lin PW. Postirradiation angiosarcoma of the terminal ileum. Zhonghua Yi Xue Za Zhi (Taipei). 1991;48:147-152. [PubMed] |
| 44. | Hwang TL, Sun CF, Chen MF. Angiosarcoma of the small intestine after radiation therapy: report of a case. J Formos Med Assoc. 1993;92:658-661. [PubMed] |
| 45. | Hansen SH, Holck S, Flyger H, Tange UB. Radiation-associated angiosarcoma of the small bowel. A case of multiploidy and a fulminant clinical course. Case report. APMIS. 1996;104:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Suzuki F, Saito A, Ishi K, Koyatsu J, Maruyama T, Suda K. Intra-abdominal angiosarcomatosis after radiotherapy. J Gastroenterol Hepatol. 1999;14:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Policarpio-Nicolas ML, Nicolas MM, Keh P, Laskin WB. Postradiation angiosarcoma of the small intestine: a case report and review of literature. Ann Diagn Pathol. 2006;10:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Berry GJ, Anderson CJ, Pitts WC, Neitzel GF, Weiss LM. Cytology of angiosarcoma in effusions. Acta Cytol. 1991;35:538-542. [PubMed] |
| 49. | Allison KH, Yoder BJ, Bronner MP, Goldblum JR, Rubin BP. Angiosarcoma involving the gastrointestinal tract: a series of primary and metastatic cases. Am J Surg Pathol. 2004;28:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Vrind HM, Becker AE. Multiple malignant (haem) angioendotheliomas of the stomach and small intestine. Arch Chir Neerl. 1970;22:15-23. [PubMed] |
| 51. | Delvaux V, Sciot R, Neuville B, Moerman P, Peeters M, Filez L, Van Beckevoort D, Ectors N, Geboes K. Multifocal epithelioid angiosarcoma of the small intestine. Virchows Arch. 2000;437:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Fraiman G, Ganti AK, Potti A, Mehdi S. Angiosarcoma of the small intestine: a possible role for thalidomide? Med Oncol. 2003;20:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Butrón Vila T, García Villar O, Alonso García S, Bonachia Naranjo O, Pérez Espejo G, Lomas Espadas M, Hidalgo Pascual M. Angiosarcoma in the small intestine. Apropos of a particular case. Hepatogastroenterology. 2005;52:1139-1142. [PubMed] |
| 54. | Raina V, Sengar M, Shukla NK, Deo SS, Mohanty BK, Sharma D, Ray R, Das P, Rath GK. Complete response from thalidomide in angiosarcoma after treatment of breast cancer. J Clin Oncol. 2007;25:900-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Vakkalanka B, Milhem M. Paclitaxel as neoadjuvant therapy for high grade angiosarcoma of the spleen: a brief report and literature review. Clin Med Insights Oncol. 2010;4:107-110. [PubMed] |
| 56. | Penel N, Marréaud S, Robin YM, Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol. 2011;80:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Penel N, Italiano A, Ray-Coquard I, Chaigneau L, Delcambre C, Robin YM, Bui B, Bertucci F, Isambert N, Cupissol D. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Italiano A, Cioffi A, Penel N, Levra MG, Delcambre C, Kalbacher E, Chevreau C, Bertucci F, Isambert N, Blay JY. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118:3330-3336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Kelemen K, Yu QQ, Howard L. Small intestinal angiosarcoma leading to perforation and acute abdomen: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:95-98. [PubMed] |
| 60. | Cilursu AM. Massive hemorrhage due to angiosarcomatosis diagnosed by intraoperative small bowel endoscopy. Endoscopy. 1991;23:245. [PubMed] |
| 61. | Usuda H, Naito M. Multicentric angiosarcoma of the gastrointestinal tract. Pathol Int. 1997;47:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Al Ali J, Ko HH, Owen D, Steinbrecher UP. Epithelioid angiosarcoma of the small bowel. Gastrointest Endosc. 2006;64:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Mohammed A, Aliyu HO, Liman AA, Abdullahi K, Abubakar N. Angiosarcoma of the small intestine. Ann Afr Med. 2011;10:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |