Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 14, 2012; 18(42): 6060-6069
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6060
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6060
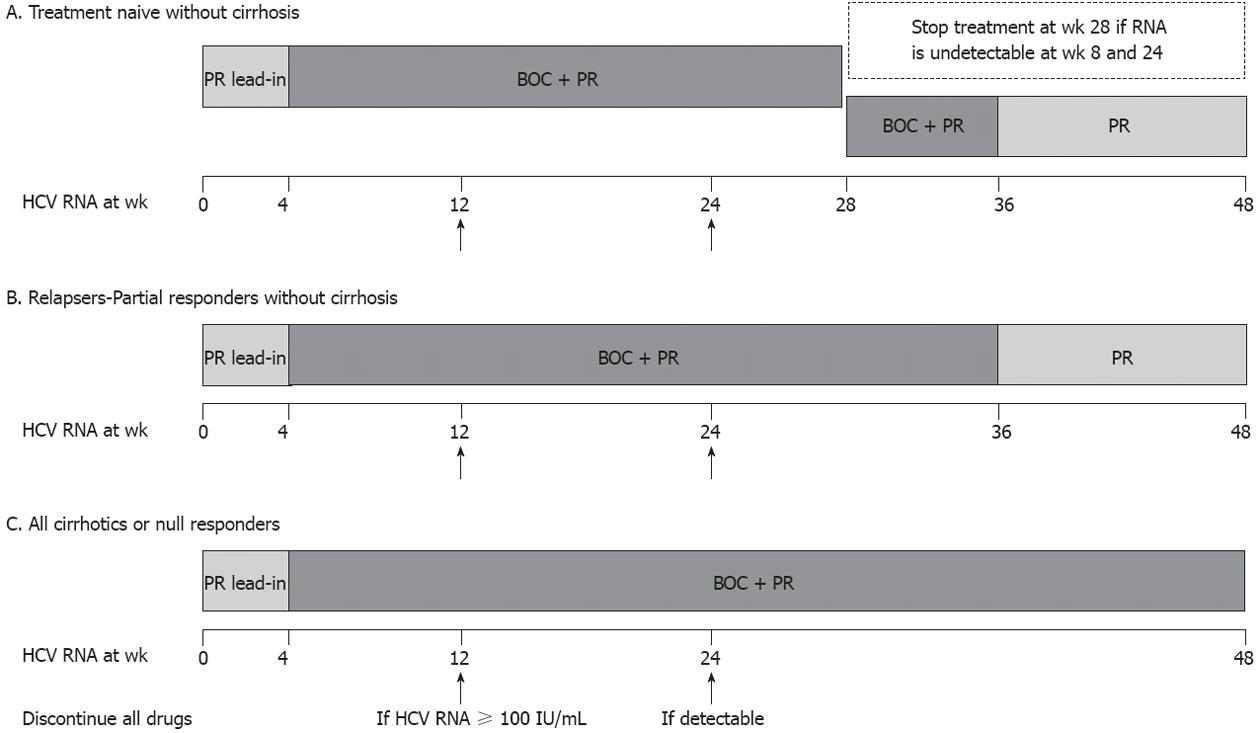
Figure 1 Treatment algorithms of boceprevir-based regimens for genotype 1 chronic hepatitis C virus patients recommended by the European Medicines Agency.
All patients should start with a 4-wk lead-in phase with only pegylated interferon-alfa and ribavirin (PR). After 4 wk, boceprevir (BOC) is added. In treatment-naïve patients without cirrhosis who achieve an extended rapid virological response [eRVR: undetectable hepatitis C virus (HCV) RNA (< 10 IU/mL) at 8 and 24 wk], the triple therapy should last 24 wk and treatment end at 28 wk. In non-cirrhotic treatment-naïve patients who do not achieve such an eRVR and in all previous relapsers or partial responders without cirrhosis, the triple therapy should last 32 wk (until 36 wk of therapy) and should be followed by an additional 12 wk of PR. The United States Food and Drug Administration (FDA) recommendations suggest that prior relapsers or partial responders without cirrhosis who achieve an eRVR under BOC triple therapy can stop therapy at 36 wk without an additional 12-wk course of PR that is suggested by the European Medicines Agency. Finally, in all cirrhotics (treatment-naïve and experienced) and null responders, the triple therapy should last 44 wk (up to 48 wk of treatment). All patients should be tested for HCV RNA levels at 12 and 24 wk of total therapy and treatment should be discontinued for inefficacy if HCV RNA levels are > 100 IU/mL at 12 wk or HCV RNA is still detectable at 24 wk of therapy.
- Citation: Alexopoulou A, Papatheodoridis GV. Current progress in the treatment of chronic hepatitis C. World J Gastroenterol 2012; 18(42): 6060-6069
- URL: https://www.wjgnet.com/1007-9327/full/v18/i42/6060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i42.6060