Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6106
Revised: August 1, 2012
Accepted: August 26, 2012
Published online: November 14, 2012
AIM: To compare program costs of chronic hepatitis B (CHB) screening and treatment using Australian and other published CHB treatment guidelines.
METHODS: Economic modeling demonstrated that in Australia a strategy of hepatocellular cancer (HCC) prevention in patients with CHB is more cost-effective than current standard care, or HCC screening. Based upon this model, we developed the B positive program to optimize CHB management of Australians born in countries of high CHB prevalence. We estimated CHB program costs using the B positive program algorithm and compared them to estimated costs of using the CHB treatment guidelines published by the Asian-Pacific, American and European Associations for the Study of Liver Disease (APASL, AASLD, EASL) and those suggested by an independent United States hepatology panel. We used a Markov model that factored in the costs of CHB screening and treatment, individualized by viral load and alanine aminotransferase levels, and calculated the relative costs of program components. Costs were discounted by 5% and calculated in Australian dollars (AUD).
RESULTS: Using the B positive algorithm, total program costs amount to 13 979 224 AUD, or 9634 AUD per patient. The least costly strategy is based upon using the AASLD guidelines, which would cost 34% less than our B positive algorithm. Using the EASL and the United States Expert Group guidelines would increase program costs by 46%. The largest expenditure relates to the cost of drug treatment (66.9% of total program costs). The contribution of CHB surveillance (20.2%) and HCC screening and surveillance (6.6%) is small - and together they represent only approximately a quarter of the total program costs.
CONCLUSION: The significant cost variations in CHB screening and treatment using different guidelines are relevant for clinicians and policy makers involved in designing population-based disease control programs.
- Citation: Robotin M, Patton Y, Kansil M, Penman A, George J. Cost of treating chronic hepatitis B: Comparison of current treatment guidelines. World J Gastroenterol 2012; 18(42): 6106-6113
- URL: https://www.wjgnet.com/1007-9327/full/v18/i42/6106.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i42.6106
Although hepatocellular cancer (HCC) remains relatively uncommon in Australia, its incidence has increased approximately fivefold since 1972, and based on current trends, a threefold increase is expected by 2020[1]. HCC rates are highest in Southwestern Sydney, where its incidence (7.7 per 100 000 persons, 95% CI: 7.0-8.4) is significantly higher than the state average (5.2 per 100 000 persons, 95% CI: 5.0-5.5)[1].
Nearly 90% of hepatitis-B-related HCC in NSW occurs in people born overseas, with approximately 70% affecting Australians born in countries of high hepatitis B prevalence[2]. Migrants born in these countries are 6-12 times more likely to develop HCC than other Australians[3], explained by the strong association between hepatitis B infection acquired early in life and the subsequent development of hepatic cirrhosis and HCC[4]. In recent years, effective treatments for chronic hepatitis B (CHB) infection have achieved sustained suppression of viral replication and significant reductions in disease progression to cirrhosis, end-stage liver disease and HCC[5-7]. This opens unique opportunities to reduce CHB-related morbidity and mortality among the 350 million chronically infected people worldwide[8], provided that treatment costs are affordable at a population level. This is particularly relevant in the developing world, where the great majority of people with CHB reside[4].
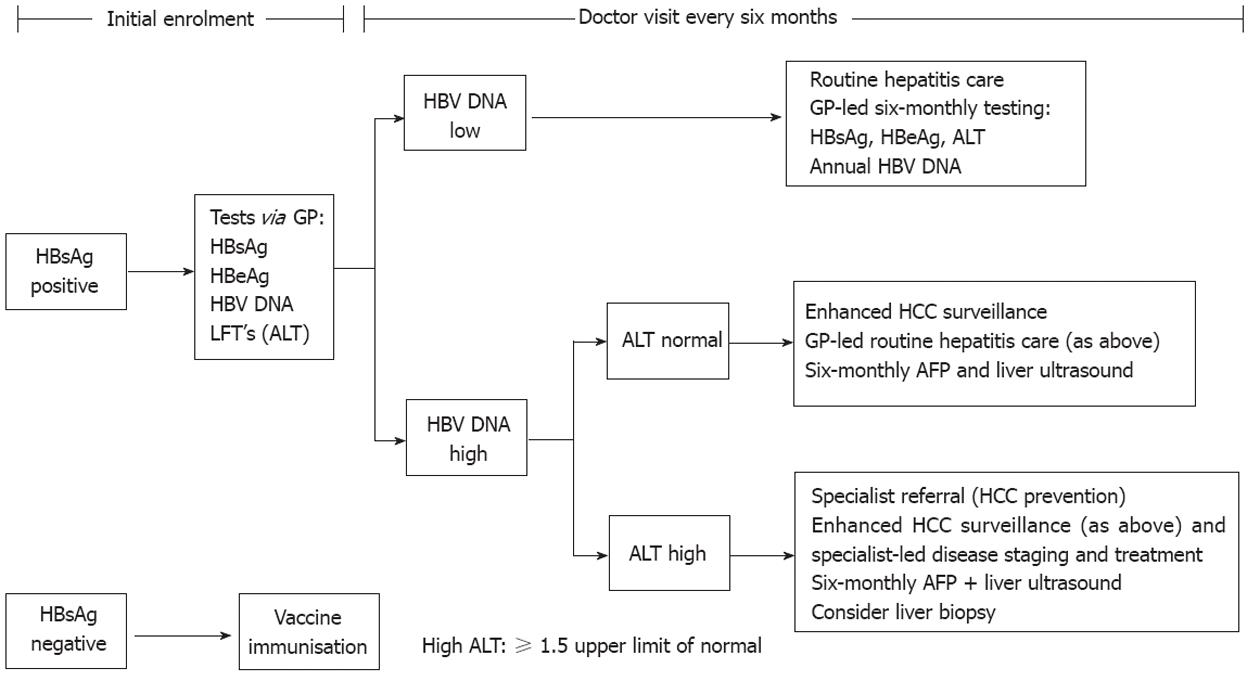
Our previous modeling work showed that in Asian populations with CHB residing in Australia (representing > 50% of people diagnosed with CHB in Australia[9]), a strategy of HCC prevention is more cost-effective than HCC screening[10]. For people with CHB, we defined HCC prevention as an intervention comprising regular (6-monthly) patient follow-up, the institution of antiviral therapy in those with active disease, and HCC surveillance. Following the confirmation of CHB diagnosis, general practitioners (GPs) order the relevant investigations and stratify participants into discrete risk categories, based upon hepatitis B virus (HBV) DNA and alanine aminotransferase (ALT) levels (Figure 1). Low-risk patients [those with low HBV DNA (defined as < 20 000 IU/mL for participants aged < 50 years and < 2000 IU/mL for those aged > 50 years) and normal ALT levels [defined as < 1.5 times upper limit of normal (ULN)] are offered routine CHB surveillance, consisting of 6-monthly GP follow-up visits and testing for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), viral load and ALT levels. Patients with normal ALT levels, but elevated viral loads (as defined above) are followed up by their local medical practitioners under a program of enhanced CHB surveillance (which in addition to routine blood tests also includes 6-monthly HCC surveillance using α fetoprotein (AFP) measurements and liver ultrasound (US) examinations. Patients with elevated ALT and viral load levels are referred to tertiary care for assessment and consideration of antiviral therapy (Figure 1). The general assumptions of the Markov model are summarized in Table 1.
| Assumption | How addressed and rationale |
| Participant recruitment | Target population age ≥ 35 yr, HBsAg +ve for ≥ 6 mo, born in China, Hong Kong, Vietnam |
| Contact testing and immunisation | Not factored into the model |
| Seroprevalence in target populations | 10.7% for people born in China |
| 10.5% for people born in Vietnam | |
| 7.7% for people born in Hong Kong (Nguyen et al[16]) | |
| Initial testing to confirm chronic hepatitis B | Not factored in the GP consultation calculations |
| Program participation rates | Base case assumption: 25% of eligible people are enrolled |
| HCC screening | All participants have AFP and liver US at enrolment |
| Participants receiving enhanced surveillance have 6-monthly AFP and US | |
| Participants receiving treatment also have liver biopsy | |
| Follow up requirements | Routine surveillance arm: 2 GP appointments/yr |
| Enhanced HCC surveillance arm: 2 GP appointments/yr | |
| Interferon treatment: 6 specialist appointments/yr | |
| Entecavir treatment: 4 specialist appointments/yr | |
| Patients with HCC: assumed two monthly follow up | |
| Viral load distribution | Based upon Risk Evaluation of Viral Load Elevation and Associated Liver Disease study data (Chen et al[20]) |
| ALT level distribution | Based upon Hong Kong data (Yuen et al[18]) |
| Progression rates through different disease stages | Constant |
| Treatment protocol | 30% receive first line interferon (weekly for 12 mo); 30% seroconvert and receive no further treatment; 70% commence entecavir the following year 70% receive entecavir as first-line treatment; 20% seroconvert in first year and receive no further treatment; 80% continue lifelong entecavir |
| Patients with liver failure | Receive lifelong entecavir |
These treatment strategies are at slight variance with those described in hepatitis B practice management guidelines, because they factor in age as a consideration for treatment initiation (program participants are aged ≥ 35 years and treatment criteria change at age 50 years) and ALT cutoff levels are ≥ 1.5 × ULN. Additionally, HCC screening is being offered to groups deemed at higher risk, rather than to all Asian males over the age of 40 years and Asian women over the age of 50 years, as it is recommended by the American Association for the Study of Liver Disease (AASLD) guidelines[11].
The modeling work informed the development of a program of CHB management targeting the area of Sydney with the highest burden of hepatitis-B-related HCC in Australia, located in Southwest Sydney. As the Gastroenterology Society of Australia uses viral loads > 2000 IU/mL as a cut-off for treatment initiation irrespective of age[12], we subsequently modified the viral load cut-off for the B positive program, to avoid confusion among primary care providers participating in the program.
In order to inform hepatitis B management and provide data to policy makers, we estimated B positive program costs using the original B positive screening and treatment algorithm and compared them with those incurred using the modified B positive algorithm (viral load cutoff of 2000 IU/mL for treatment initiation, irrespective of patient age) and to costs incurred when applying guidelines published by the American, European and Asia-Pacific Associations for the Study of Liver Disease (AASLD, EASL and APASL)[6,13,14] as well as those developed by an independent panel of hepatologists from the United States (referred to here as the United States Expert Group)[15]. We also determined the relative proportion of program costs attributable to CHB screening, drug treatment, CHB surveillance and HCC screening and surveillance incurred by applying each of these algorithms. Costs were calculated in Australian dollars (AUD).
The B positive project targets Asian migrant communities in Southwest Sydney, but is inclusive of all individuals who meet eligibility criteria. Eligibility criteria include: confirmed CHB, age ≥ 35 years, and attending a general practice in the target local government areas. To estimate the size of the eligible population, we used data provided by the Australian Bureau of Statistics 2006 National Census on the number of local residents born in China, Hong Kong and Vietnam aged ≥ 35 years. We applied HBV seroprevalence data on these numbers, based upon epidemiological estimates from the respective countries of birth[16].
We estimated the proportion of people in different CHB-related disease stages over the 50-year timeframe [CHB without cirrhosis; CHB with cirrhosis; CHB with liver failure; CHB and HCC; spontaneous HBsAg clearance and death due to CHB-related causes (liver failure or HCC), or death from other causes], using the assumptions of our previously published Markov model[10]. The model takes a health care funder perspective and discounts costs at 5% per annum. We estimated a program participation rate of 25%, informed by the experience of the New Zealand HBV screening program, which screened 27% of their eligible population[17]. Table 1 summarizes the key assumptions of our model. We used an ALT level of ≥ 1.5 × ULN to define high-risk patients and estimated that about 50% of the target population have ALT levels < 1 × ULN, 12.5% have ALT levels of 1-1.5 × ULN, 12.5% between 1.5 and 2 × ULN and 25% levels > 2 × ULN, informed by a Hong Kong population-based study of CHB patients[18].
We estimated the proportion of the target population receiving antiviral treatment based upon the cutoffs for ALT and viral load recommended by the different guidelines for patients who are HBeAg negative (Tables 2 and 3). Briefly, in this population group the AASLD guidelines recommend treatment to be initiated when viral load exceeds 20 000 IU/mL and ALT levels are > 2 × ULN[6]. The APASL guidelines use the same ALT level cutoff, but a lower threshold (2000 IU/mL) for viral load[14], while the United States expert group and EASL use the 2000 IU/mL cutoff for viral load, but recommend treatment for patients with ALT levels exceeding the normal range[13,15].
| Treatment guideline | B positive | Modified B positive | EASL, United States experts | APASL | AASLD |
| HBV DNA level to treat | > 2000 if > 50 | > 2000 | > 2000 | > 2000 | > 20 000 |
| > 20 000 if < 50 | |||||
| ALT (ULN) | > 1.5 | > 1.5 | > 1 | > 2 | > 2 |
| Number receiving interferon | 61 (4) | 81 (6) | 108 (8) | 54 (4) | 33 (2) |
| Number receiving entecavir | 143 (10) | 190 (13) | 253 (17) | 126 (9) | 76 (5) |
| Total on treatment | 204 (14) | 271 (19) | 361 (25) | 181 (12) | 109 (8) |
| Total under enhanced surveillance | 340 (23) | 452 (31) | 361 (25) | 542 (37) | 326 (23) |
| Total under routine surveillance | 907 (63) | 728 (50) | 728 (50) | 728 (50) | 1016 (70) |
| Total | 1451 (100) | 1451 (100) | 1451 (100) | 1451 (100) | 1451 (100) |
| Discounted costs of management strategies | B positive | Modified B positive | APASL | EASL, United States experts | AASLD |
| Cost/QALY (discounted) | 13 465 | 15 770 | 11 746 | 19 622 | 8867 |
| Total program cost (discounted) | 13 979 224 | 16 372 320 | 12 194 905 | 20 371 117 | 9 205 680 |
| Cost components | |||||
| Initial CHB screening cost | 767 728 (5.5) | 800 792 (4.9) | 755 971 (6.2) | 845 613 (4.2) | 720 357 (7.8) |
| Drug treatment costs | 9 347 662 (66.9) | 11 493 535 (70.2) | 7 360 940 (60.4) | 15 447 510 (75.8) | 4 951 419 (53.8) |
| CHB surveillance costs | 2 827 093 (20.2) | 2 866 053 (17.5) | 2 866 053 (23.5) | 2 866 053 (14.1) | 2 767 073 (30.1) |
| HCC surveillance costs | 917 783 (6.6) | 1 092 983 (6.7) | 1 092 983 (9.0) | 1 092 983 (5.4) | 647 874 (7.0) |
| Total cost per person in the program | 9634 | 11 283 | 8404 | 14 039 | 6344 |
| % change with equivalent unit costs/QALY | 100 | 117 | 87 | 146 | 66 |
The B positive program (unpublished) data suggested that 94% of those enrolled in the program were HBeAg negative at the time of enrolment (which corroborates the findings of Yuen et al[19], that in an Asian population, HBeAg seroconversion occurs around 35 years of age); consequently we compared estimated CHB program costs using treatment guidelines developed for HBeAg-negative populations.
We factored in the costs of CHB screening and follow-up provided by specialists and/or primary care providers, the costs of HCC screening, and CHB and HCC treatment, but not recruitment costs, or the costs of immunization for those susceptible.
The CHB population targeted by the B positive program in Southwest Sydney was estimated at 5800 patients. Assuming a 25% enrolment rate, about 1500 patients (1451 patients) with CHB in Southwest Sydney would be enrolled in the program. Nearly two-thirds (63%) of these patients would be followed up by their GPs under a program of routine surveillance (Figure 1). Half of the patient population would receive this type of management if using the other guidelines, except for the AASLD guidelines, where 70% of all patients would be receiving routine surveillance. The proportion of patients under enhanced surveillance (which is 23% for B positive and 31% for the modified B positive) would range from a low of 23% using the AASLD guidelines to a high of 37% using the EASL and United States Expert Panel guidelines. The proportion of patients receiving antiviral treatment (14% for B positive) ranges from a low of 8% under AASLD to a high of 25% under the more stringent EASL and United States Expert Panel Group guidelines (Table 2).
Overall, the lowest program costs are associated with the application of AASLD guidelines, because the 20 000 IU/mL viral load cutoff for treatment initiation and ALT levels ≥ 2 × ULN make fewer patients eligible for treatment. Treatment costs are highest when applying EASL and United States Expert Group guidelines, because they recommend treatment for all patients with viral loads > 2000 IU/ mL and ALT levels > 1 × ULN.
The total B positive program costs would amount to 13 979 224 AUD, or 9634 AUD per patient using the original B positive algorithm, ranging from a low of 6344 AUD for the AASLD guidelines to a high of 14 039 for the EASL and United States Expert Group recommendations (Table 3). The largest component of the cost structure relates to antiviral treatment, which represents over three-quarters (75.8%) of program costs if using EASL and United States Expert Group recommendations, approximately 70% (70.2%) for the modified B positive algorithm, 66.9% for the original B positive, 60% for the APASL guidelines, and just over 50% for the AASLD guidelines. Costs of CHB surveillance ranges from 17.5% for the modified B positive protocol to 30.1% of the program expenditure if using AASLD guidelines. The contribution of the cost of HCC screening and surveillance remains relatively small in all scenarios, ranging from a low of 5.4% for the EASL and United States guidelines to 9% for the APASL guidelines.
Compared to the B positive algorithm, the lowest cost to achieve an equivalent unit cost/quality-adjusted life year is incurred by applying the AASLD guidelines (34% cost saving), followed by the APASL guidelines (13% cost saving); costs would be 17% higher with the modified B positive algorithm (because more patients aged 35-50 years would be in receipt of treatment) and 46% higher for the EASL and United States Expert Group recommendations.
This modeling exercise demonstrates that in a population-based CHB management program informed by current treatment guidelines, the majority of patients (ranging from 50 to 70%) have low viral loads (< 2000 IU/mL) and low ALT levels and may be effectively managed at the primary care level. Between a quarter and a third of patients have elevated viral loads and would benefit from more comprehensive follow-up, which we termed “enhanced surveillance”, which could still effectively be delivered at the primary care level and free up specialist resources. These calculations suggest that the proportion of patients requiring tertiary-level assessment for consideration of antiviral therapy is variable, ranging from a low of 8% if the AASLD guidelines are followed to a high of 25% if the more stringent EASL and United States Expert Group guidelines are used. Program costs range from approximately 9 million AUD (if AASLD guidelines are used) to more than double that figure (20 371 117 AUD) if the EASL or United States Expert Group recommendations are applied. Correspondingly, this leads to variations in costs per patient enrolled, ranging from 6344 AUD (for AASLD guidelines) to 14 039 AUD (for EASL and United States Expert Group guidelines). The B positive algorithm steers a mid-course with regards to total (13 979 224-16 372 320 AUD) and per patient costs (9634-11 283 AUD, depending on whether the viral load cut-off is set at 2000 IU/mL for all patients, or only for those aged > 50 years.
The model assumes that all patients with elevated viral loads and ALT levels receive specialist assessment and liver biopsy to assess the degree of fibrosis prior to treatment initiation, although since November 2011, liver biopsy is no longer mandatory for treatment initiation.
In all modeled programs, the greatest contribution to cost is that of antiviral drug treatment (interferon or entecavir), accounting for 50%-75% of the program budget. By comparison, the costs of CHB surveillance are relatively low for a primary-care-based program (ranging from 14% to 30% of total costs); even lower are the costs associated with HCC screening and surveillance (ranging from 5% to 9%). The original B positive modeling did not include HCC surveillance in the subgroup presumed to have inactive disease; this is at variance with current published guidelines, which recommend HCC surveillance for all Asian men aged > 40 years or women aged > 50 years, irrespective of their disease stage or viral load[11]. Although the original intention was to balance cost containment with ensuring that HCC cases are not missed, it appears that the contribution of HCC screening to costs would remain modest even if the program embraces the recommended screening guidelines. Sherman in a recent review concedes that it may be possible that, as more information about HCC risk stratification becomes available, patients with long-term inactive disease may not require the same intensive HCC surveillance[11], something that is supported by the REVEAL data[20].
Our model has a number of limitations related to our assumptions and the lack of clear data in some areas. For example, we used a 1.5 × ULN cut-off for the ALT levels prompting treatment. The different ALT cut-off levels in various guidelines indicate that there is no agreement about what level of ALT should prompt treatment initiation, and information to clarify this is keenly awaited. Similarly, we assumed that all patients with elevated ALT levels would require antiviral therapy, although in reality, ALT elevation may not always relate to disease reactivation, but to other factors, such as high body mass index, non-alcoholic fatty liver disease, chronic alcohol consumption or coexisting hepatitis C infection[21,22]. We also acknowledge that the rate of progression to HCC development is variable in different patient populations, being dependent on the degree of fibrosis, genotype, and other associated risk factors. Although the short-term goals of antiviral therapy have been achieved in recent clinical trials[22], more answers are needed as to the extent to which this affects liver cancer incidence and the number of cancer deaths. As this is also the major assumption that underlies our analysis, more data confirming the impact of treatment on HCC risk reduction would strengthen the economic model findings.
Our original model factored in the cost of liver biopsy for all patients being considered for antiviral therapy, reflecting the recommendations of guidelines referred to in the paper, suggesting that a liver biopsy is helpful for determining the degree of necroinflammation and fibrosis. However the relatively low cost of a liver biopsy and the relatively small numbers of patients in this subgroup (we estimated that only about 12.5% of patients have ALT levels in that range and even fewer also have low–medium viral loads) means that this procedure will have a minimal impact on overall program costs, although it may influence the number of patients willing to accept drug therapy.
The effectiveness and cost-effectiveness of different interventions need to be corroborated by other types of studies and real-life outcomes data utilized to validate the economic models. We are currently collecting program data for this purpose.
Ideally our findings would need to be compared to those of other studies using decision models and against real-life data from clinical studies, but available data are limited. A recent European review[23] identified only two studies addressing the cost-effectiveness of screening high-risk groups for CHB: both a United States[24] and a Dutch study[25] suggested that screening of migrant groups for HBV was both clinically effective and cost-effective findings that were corroborated by our Australian study[10].
A clinical study estimating the efficacy and cost of HCC screening in a clinic population in Australia[26] confirmed our model costs. We need to bear in mind however that direct comparisons between studies are of limited value, due to differences in study design, model assumptions, cut-offs used, and variable cost structures in different countries.
As the stated aim of this short paper was to examine the cost implications of utilizing different guidelines to treat patients with CHB, in order to assist funding decisions, we did not include a broader discussion of cost-effectiveness, effectiveness and efficacy of antiviral treatment, but agree that a more comprehensive review of effectiveness and cost-effectiveness may be warranted.
Although we incorporated a wide range of supporting evidence into our economic model, the relatively limited information available on the composition of cohorts of patients with CHB receiving treatment and the possible differences in treatment response in patients with HBeAg-negative disease (which has been less extensively studied) may limit the generalizability of our findings. In the absence of relevant data, we assumed that the effectiveness and durability of current interventions can be extrapolated over a lifetime horizon, but acknowledge that the lack of long-term evidence precludes confident estimates of treatment outcomes.
From a policy perspective, the high cost of antiviral therapy makes population-based CHB screening and treatment unaffordable for all but well-resourced countries. For example, Hutton et al[27] published their analysis of the cost-effectiveness of interventions aiming to combat hepatitis B in the United States and China. They found that in an American setting it is cost-effective to screen adult Asian and Pacific Islanders (APIs) for CHB, with a view to providing them with appropriate treatment, as well as to vaccinate close contacts. This work led to a change in United States public health policy on hepatitis B screening, with a recommendation that all adult APIs and adults born in areas of intermediate HBV prevalence be screened for CHB. The authors found that in a Chinese setting catch-up adolescent vaccination is cost-effective, but that drug treatment costs would need to be halved (to about 1000 United States dollars/year) before the benefits of vaccination would be surpassed by those of instituting treatment[27]. China’s economic successes makes population level CHB screening and treatment a possibility in the future, but effective and inexpensive treatments are needed to reduce the burden of CHB in developing economies[4].
Our original economic model assumptions attempted to reflect the Australian practice prevalent in 2006 and 2007, when entecavir was becoming first-line treatment for CHB. At that time interferon was still in common use, therefore, we modeled 30% of the cohort as receiving first-line interferon.
However, the therapeutic landscape has changed in recent years, with interferon being used now in only about 10% of patients as first-line treatment for CHB. We therefore repeated our calculations estimating that 10% of patients receive first-line interferon and found the incremental total cost was only about 1.5% higher (data not shown), as a result of interplay between a more intensive and more costly specialist follow-up during interferon treatment, a small proportion of patients who clear the infection, and the overall small number of patients affected.
As both available resources and local clinical preferences guide drug treatment, generic lamivudine may be the only affordable antiviral for low-income countries in Asia and Africa, where CHB is most prevalent, because replacing it with a more potent antiviral agent is associated with a more than 10-fold increase in drug costs[28] .
Consequently, we repeated the analysis, substituting lamivudine for entecavir and assuming that the cost of lamivudine represented only a tenth of the cost of entecavir. This led to significant reductions in drug costs (ranging from 75.3% to 77.6%) and in overall program costs (ranging from 40.5% to 58.9% - data not shown), which further emphasizes the important role played by drug costing in program feasibility.
Our modeling has provided estimates of the cost of CHB management programs that could be useful for policy makers and health care providers in different settings, to inform program development. It would appear that population-based CHB management programs targeting at-risk groups are affordable in high-resource settings, but remain unattainable for many of the world’s population with CHB, living in low- or middle-resourced countries. The high cost of antiviral therapies represents the largest cost component of a CHB management program. It is hoped that reductions in the cost of antiviral drugs will lead to more equitable access to treatment and address the global burden of chronic hepatitis B.
Chronic hepatitis B (CHB) is a leading cause of cirrhosis and hepatocellular carcinoma (HCC), and in Australia, HCC incidence has been rising faster than that of any other cancer, mostly related to changing migration patterns over recent decades. A population-level disease control is predicated upon early disease detection, regular monitoring and timely institution of antiviral treatment for people with active disease, but antiviral treatment is unaffordable for the great majority of people with CHB who live in resource-limited countries. Therefore the cost and cost-effectiveness of CHB management programs is an important consideration for program funders and needs to be factored in by those tasked with guideline development. The authors previously carried out modeling work that showed that screening and treating migrants born in high prevalence countries is cost-effective, which corroborated the findings of research groups in the United States and the Netherlands. This paper examines the cost of treatment of hepatitis B applied to a hypothetical population of people with CHB diagnosis, treated according to CHB screening and treatment guidelines in common use in Asia (issued by the Asia-Pacific Society for the Study of Liver Disease), Australia (issued by the Gastroenterological Society of Australia), Europe (issued by the European Association for the Study of Liver Disease, EASL) and the United States (issued by the American Association for the Study of Liver Disease, AASLD, as well as by an expert United States advisory group).
This work builds the body of evidence suggesting the cost-effectiveness of screening high-risk groups for hepatitis B, by examining the implications of implementing a public health program of screening and treatment using different treatment initiation parameters, as recommended by different guideline developers. The proportion of patients in this hypothetical cohort requiring antiviral therapy ranges from 8% (if the AASLD guidelines are used) to 25% (if EASL guidelines are applied). The most substantial component of program cost relates to antiviral treatment (representing up to 75% of program costs), while screening for CHB and cancer surveillance account for a small part of total costs. Treatment with generic lamivudine (instead of entecavir) leads to substantially lower total program costs, although this must be balanced against the greater suppression of viral replication and lack of drug resistance associated with entecavir treatment.
Here the authors propose and cost a scheme for the diagnosis and subsequent monitoring of patients from countries with high prevalence of hepatitis B, resident in Australia. In the B positive algorithm, antiviral treatment is offered for patients with alanine aminotransferase (ALT) elevation above 1.5 × normal, treading the “middle ground” between treating everyone with an elevated ALT level (which may have significant resource implications for countries with high disease prevalence, but limited resources) and treating only people with advanced disease (and running the risk of limiting the effectiveness of the program).
These findings are relevant for the design of interventions with the potential to make a significant impact on hepatitis B disease burden at a population level, in both well-resourced and low-resource settings. The authors hope that this type of work may be of interest to experts involved in CHB treatment guideline development, policy makers and clinicians working in areas with a large hepatitis B load.
This is a study on the cost of treatment of hepatitis B, using Markov models. The authors propose a scheme for the diagnosis and subsequent monitoring of patients from countries with high prevalence of hepatitis B, resident in Australia. They also compared the relative cost with the proposed guidelines from other major hepatology associations. They found that the AASLD recommendations were more cost-effective. This type of study is important in view of the increasing cost of drug treatment of HBV infection but also of the increasing cost of diagnostic tests related to HCC surveillance.
Peer reviewers: Elias A Kouroumalis, Professor, Department of Gastroenterology, University of Crete, Medical School, Department of Gastroenterology, University Hospital, PO Box 1352, Heraklion, 71110 Crete, Greece; Dr. Yasuji Arase, Gastroenterology, Toranomon Hospital, 2-2-2 Toranomon minato-ku, Tokyo 105-8470, Japan
S- Editor Shi ZF L- Editor Kerr C E- Editor Li JY
| 1. | Alam N, Chen W, Baker D, Bishop J. Liver Cancer in New South Wales. Sydney: Cancer Institute NSW 2009; Available from: http: //www.cancerinstitute.org.au/publications/i/liver-cancer-in-new-south-wales. |
| 2. | Amin J, O'Connell D, Bartlett M, Tracey E, Kaldor J, Law M, Dore G. Liver cancer and hepatitis B and C in New South Wales, 1990-2002: a linkage study. Aust N Z J Public Health. 2007;31:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Supramaniam R, O'Connell DL, Tracey E, Sitas F. Cancer incidence in New South Wales migrants 1991 to 2001. Sydney: The Cancer Council NSW 2006; . |
| 4. | Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22:991-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1738] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 6. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 7. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Dore G, Wallace J, Locarnini S, Desmond P, Gane E, Crawford DH. Hepatitis B in Australia: responding to a diverse epidemic. Sydney: Australasian Society for HIV Medicine 2006; Available from: http://www.ashm.org.au/uploads/Hep-B-in-Australia.pdf. |
| 10. | Robotin MC, Kansil M, Howard K, George J, Tipper S, Dore GJ, Levy M, Penman AG. Antiviral therapy for hepatitis B-related liver cancer prevention is more cost-effective than cancer screening. J Hepatol. 2009;50:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Sherman M. Prevention of hepatocellular carcinoma: the holy grail of hepatitis B treatment. J Hepatol. 2009;50:854-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Digestive Health Foundation. Australian and New Zealand chronic hepatitis B (CHB) recommendations. 2nd ed. Melbourne: Digestive Health Foundation, Gastroenterological Society of Australia 2008; . |
| 13. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1154] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 14. | Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-1341; quiz 1286. [PubMed] |
| 16. | Nguyen VT, Razali K, Amin J, Law MG, Dore GJ. Estimates and projections of hepatitis B-related hepatocellular carcinoma in Australia among people born in Asia-Pacific countries. J Gastroenterol Hepatol. 2008;23:922-929. [PubMed] [DOI] [Full Text] |
| 17. | Robinson T, Bullen C, Humphries W, Hornell J, Moyes C. The New Zealand Hepatitis B Screening Programme: screening coverage and prevalence of chronic hepatitis B infection. N Z Med J. 2005;118:U1345. [PubMed] |
| 18. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Yuen MF, Yuan HJ, Hui CK, Wong DK, Wong WM, Chan AO, Wong BC, Lai CL. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2360] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 21. | Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 22. | Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM. Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology. 2008;48:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, Colombo M, Delarocque-Astagneau E, Dusheiko G, Esmat G. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference*. J Viral Hepat. 2011;18 Suppl 1:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469. [PubMed] |
| 25. | Veldhuijzen IK, Toy M, Hahné SJ, De Wit GA, Schalm SW, de Man RA, Richardus JH. Screening and early treatment of migrants for chronic hepatitis B virus infection is cost-effective. Gastroenterology. 2010;138:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Qian MY, Yuwei J R, Angus P, Schelleman T, Johnson L, Gow P. Efficacy and cost of a hepatocellular carcinoma screening program at an Australian teaching hospital. J Gastroenterol Hepatol. 2010;25:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Hutton DW, Brandeau ML, So SK. Doing Good with Good OR: Supporting Cost-effective Hepatitis B Interventions. Interfaces (Providence). 2011;41:289-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Shouval D. Focus: Long-term treatment with lamivudine in HBeAg negative patients with chronic hepatitis B: to switch or not to switch? J Hepatol. 2012;56:1219-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |