Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2837
Revised: January 16, 2012
Accepted: April 13, 2012
Published online: June 14, 2012
AIM: To investigate the usefulness of tumor markers and adenosine deaminase in differentiating between tuberculous peritonitis (TBP) and peritoneal carcinomatosis (PC).
METHODS: A retrospective analysis of data was performed on consecutive patients who underwent peritoneoscopic and abdominal computed tomography (CT) evaluations. Among 75 patients at the Seoul National University Hospital from January 2000 to June 2010 who underwent both tests, 27 patients (36.0%) and 25 patients (33.3%) were diagnosed with TBP and PC, respectively. Diagnosis was confirmed by peritoneoscopic biopsy.
RESULTS: Serum c-reactive protein (7.88 ± 6.62 mg/dL vs 3.12 ± 2.69 mg/dL, P = 0.01), ascites adenosine deaminase (66.76 ± 32.09 IU/L vs 13.89 ± 8.95 IU/L, P < 0.01), ascites lymphocyte proportion (67.77 ± 23.41% vs 48.36 ± 18.78%, P < 0.01), and serum-ascites albumin gradient (0.72 ± 0.49 g/dL vs 1.05 ± 0.50 g/dL, P = 0.03) were significantly different between the two groups. Among tumor markers, serum and ascites carcinoembryonic antigen, serum carbohydrate antigen 19-9 showed significant difference between two groups. Abdominal CT examinations showed that smooth involvement of the parietal peritoneum was more common in the TBP group (77.8% vs 40.7%) whereas nodular involvement was more common in the PC group (14.8% vs 40.7%, P = 0.04). From receiver operating characteristic (ROC) curves ascites adenosines deaminase (ADA) showed better discriminative capability than tumor markers. An ADA cut-off level of 21 IU/L was found to yield the best results of differential diagnosis; sensitivity, specificity, positive predictive value, and negative predictive value were 92.0%, 85.0%, 88.5% and 89.5%, respectively.
CONCLUSION: Besides clinical and radiologic findings, ascitic fluid ADA measurement is helpful in the differential diagnosis of TBP and PC.
- Citation: Kang SJ, Kim JW, Baek JH, Kim SH, Kim BG, Lee KL, Jeong JB, Jung YJ, Kim JS, Jung HC, Song IS. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J Gastroenterol 2012; 18(22): 2837-2843
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2837.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2837
Tuberculous peritonitis (TBP) and peritoneal carcinomatosis (PC) are two of the most common causes of exudative ascites in South Korea and both diseases require rapid recognition for the appropriate therapeutic management[1-3]. In a clinical situation, etiological diagnosis of the two diseases is very difficult because of the lack of specific differential clinical, radiological, or laboratory findings. Peritoneoscopy is thought to be the method of choice in the diagnosis of the two diseases[4-6]. However, the diagnostic failure rate of peritoneoscopy can reach as high as approximately 14%; the main reason for failure is interference from adhesions due to tumor, tuberculosis or previous surgery[4].
The purpose of this study was to clarify the differences in clinical, radiological, laboratory and peritoneoscopic findings between TBP and PC and to evaluate the diagnostic capacity of ascites adenosine deaminase (ADA) and tumor markers for the differentiating the two diseases.
Between January 2000 and June 2010, patients over 18 years of age with exudative ascites of unknown etiology who underwent abdominal computed tomography (CT) scan and peritoneoscopy for diagnosis were enrolled in this study. All patients had laboratory tests such as complete blood count, serum biochemical tests, tumor markers from blood and ascites fluid, ascites cytology, ascites cell count and biochemical tests, and ascites ADA. Diagnosis of TBP was made if one of the following criteria was met: (1) ascites was positive for acid-fast bacilli stain and culture; (2) tuberculosis polymerase chain reaction test from ascites or peritoneoscopic biopsy specimen was positive; or (3) caseating granuloma was noted in the peritoneal biopsy specimen. PC was diagnosed if cancer cells from ascites cytology were detected or cancer cells were documented from the peritoneoscopic biopsy specimen. This study protocol was approved by the Ethics Committee at Seoul National University Hospital.
Patients underwent peritoneoscopy under local anesthesia with systemic analgesics. A lidocaine injection was done from skin to fascia at about 1 cm left side from umbilicus. After local anesthesia, a Veress needle was inserted, and then air insufflation was performed through the needle. After removal of the Veress needle, a trochar was inserted into peritoneum and peritoneoscope (Olympus; Tokyo, Japan) was inserted through the trochar into the peritoneum. After ascites fluid was drained for examination, a detailed observation of the peritoneum and intra-abdominal organs was performed. Experienced endoscopists performed all peritoneoscopic procedures and observation. Pictures of all important peritoneoscopic findings were taken and stored in a picture archiving and communication system. Two endoscopists (Kang SJ, Kim JW) reviewed the peritoneoscopic images to assess the nature of the ascites fluid and look for abnormalities such as nodules, patches, and adhesions. Nodules were classified according to size as ≤ 1 cm or > 1 cm. Patches were classified according to their location (parietal or visceral peritoneum). Membranous patches were defined as plaques.
All patients had abdominal CT scan examination. The following CT scanners were used in this study: Hi Speed/RP single channel CT scanner (GE Healthcare, Milwaukee, Wisconsin, United States) (n = 14), MX 8000 four-channel CT scanner (Marconi Medical Systems, Cleveland, Ohio, United States) (n = 32), LightSpeed eight-channel CT scanner (GE Healthcare) (n = 12), Sensation 16 16-channel CT scanner (Siemens Medical Solutions, Erlangen, Germany) (n = 13), and Brilliance 64 64-channel CT scanner (Philips Healthcare, Cleveland, Ohio, United States) (n = 4). Section thickness and reconstruction interval were both 7mm for the single channel CT scanner and 5mm for the four-channel and eight-channel CT scanners, for the 16- and 64-channel CT scanners, section thickness and reconstruction interval were 3mm and 2.5 mm, respectively. Scanning was performed from the dome of the diaphragm through the pubic symphysis. Contrast-enhanced CT scan was performed after injection of nonionic contrast material (iopromide, Ultravist 370; Bayer Healthcare, Germany).
All scans were obtained on a GE 9800 (General Electric, Milwaukee, Wisconsin, United States) or a Somatom DR3 (Siemens, Erlangen, Germany) scanner with a slice thickness of 10 mm at 10- to 13-mm intervals from the dome of the diaphragm to the pubic symphysis. Two radiologists (Baek JH, Kim SH) reviewed abdominal CT scans of patients and looked at ascites (presence of loculation), parietal involvement patterns (smooth thickening, irregular or nodular thickening, seeding nodules), mesenteric changes, mesenteric thickening, mesenteric nodules (micronodule, macronodule), omental thickness, omental changes (smudged, nodular, omental cake), and intestinal involvement.
Values for continuous variables were presented as mean ± SD or median with ranges and as the number of individuals (and the percentage in each group) for the categorical variables. Nominal data were compared by using the Fisher exact test or Pearson χ2 test, and continuous variables were compared by using the Student t test or Mann-Whitney U-test. A receiver-operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated to determine the predictive ability of different ascites ADA and tumor markers level cutoff values for differentiation. For all analyses, a P value of < 0.05 (two-tailed) was taken as statistically significant. All statistical analyses were performed with SPSS 15.0K for Windows (SPSS South Korea, Seoul, South Korea).
A total of 75 patients underwent abdominal CT scan and diagnostic peritoneoscopy. Of that population, 27 patients were diagnosed with TBP and 25 patients were diagnosed with PC according to the definition stated in the methods section. Other patients were diagnosed with various diseases such as pelvic inflammatory disease, continuous ambulatory peritoneal dialysis peritonitis, systemic lupus erythematosus (SLE), or peritoneal lymphomatosis. PC group included only adenocarcinomas from the various origins (6 pancreatic cancers, 4 ovary cancers, 5 malignancies of unknown origin, 4 colorectal cancers, 3 advanced gastric cancers and 3 other cancers). Clinical characteristics of patients are shown in Table 1. In the TBP group, there were 11 men (40.7%) and 16 women (59.3%), ranging from 28 to 83 years of age (mean ± SD = 58.04 ± 12.61 years). The PC group had more males (16 men and 9 women), but no significant difference in gender was found between the two groups (P = 0.09). There were 11 patients (40.7%) with duration of symptoms < 1 mo in the TBP group, while most patients in the PC group (n = 23, 92.0%) developed symptoms > 1 mo before they were diagnosed (P = 0.01). In the TBP group, 3 patients had night sweats, whereas no patients in the PC group complained of that symptom (P = 0.09). Fever was the predominant manifestation in the TBP group (16, 59.3%), whereas no patient in the PC group developed fever (P < 0.01).
| Tuberculous peritonitis (n = 27) | Peritoneal carcinomatosis (n = 25) | P value | |
| Age (yr) | 58.04 ± 12.61 | 61.12 ± 11.67 | 0.37 |
| Gender (M:F) | 11:16 | 16:9 | 0.09 |
| Duration of symptoms | 0.01 | ||
| < 1 mo | 11 (40.7) | 2 (8.0) | |
| ≥ 1 mo | 16 (59.3) | 23 (92.0) | |
| Symptoms | |||
| Abdominal pain | 12 (44.4) | 15 (60.0) | 0.26 |
| Abdominal distension | 25 (92.6) | 21 (84.0) | 0.33 |
| Weight loss | 8 (29.6) | 11 (44.0) | 0.28 |
| Loss of appetite | 12 (44.4) | 8 (32.0) | 0.36 |
| Night sweating | 3 (11.1) | 0 (0.0) | 0.09 |
| Fever | 16 (59.3) | 0 (0.0) | < 0.01 |
| Diarrhea | 2 (7.4) | 3 (12.0) | 0.58 |
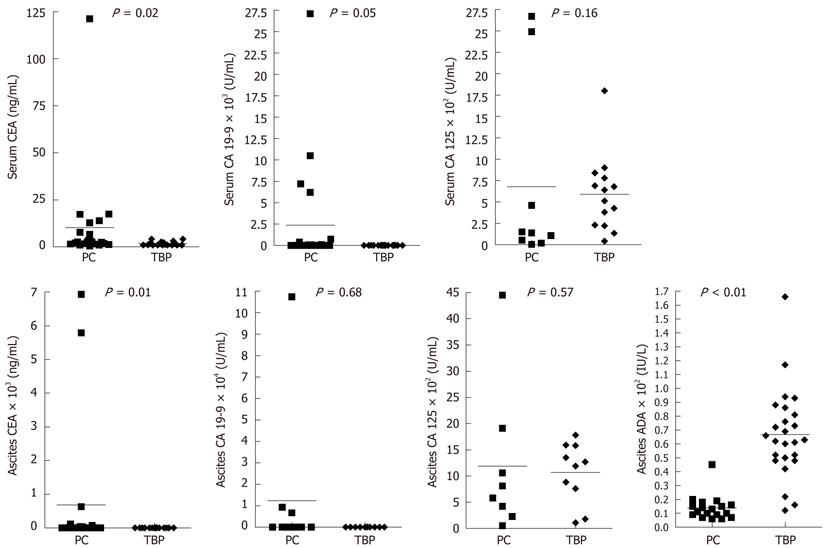
Results of blood and ascites laboratory tests including tumor markers are summarized in Table 2. Serum c-reactive protein (CRP) was significantly higher in the TBP group. Among ascites laboratory findings, TBP group showed severer lymphocytosis, lower serum ascites albumin gradient, and higher ascites ADA.
| Tuberculous peritonitis(n = 27) | Peritoneal carcinomatosis(n = 25) | P value | |
| Serum lab | |||
| Serum WBC (/mm3) | 6083.7 ± 3115.9 | 6429.6 ± 2466.6 | NS |
| Serum lymphocyte (%) | 16.70 ± 8.39 | 22.01 ± 7.19 | 0.02 |
| Serum CRP (mg/dL) | 7.88 ± 6.62 | 3.12 ± 2.69 | 0.03 |
| Serum CEA (ng/mL) | 1.79 ± 1.09 | 10.26 ± 25.38 | 0.02 |
| Serum CA 19-9 (U/mL) | 10.63 ± 8.52 | 2283.56 ± 6211.99 | 0.05 |
| Serum CA 125 (U/mL) | 591.36 ± 440.95 | 676.73 ± 1088.28 | NS |
| Ascites lab | |||
| Ascites WBC (/mm3) | 1325.9 ± 955.1 | 951.1 ± 773.1 | NS |
| Ascites lymphocyte (%) | 67.77 ± 23.41 | 48.36 ± 18.78 | < 0.01 |
| Ascites albumin (g/dL) | 2.30 ± 0.75 | 2.32 ± 0.76 | NS |
| SAAG | 0.72 ± 0.49 | 1.05 ± 0.50 | 0.03 |
| Ascites ADA (IU/L) | 66.76 ± 32.09 | 13.89 ± 8.95 | < 0.01 |
| Ascites CEA (ng/mL) | 1.36 ± 0.83 | 682.77 ± 1955.34 | 0.01 |
| Ascites CA 19-9 (U/mL) | 17.53 ± 24.15 | 12344.10 ± 33569.78 | NS |
| Ascites CA 125 (U/mL) | 1069.20 ± 578.74 | 1188.56 ± 1439.06 | NS |
Tumor markers [cercinoembryonic antigen (CEA), CA 19-9, CA 125] in serum and ascites in both groups are also presented in Table 2 and theirs scatter plots are presented in Figure 1. In PC group, serum and ascites CEA and serum CA 19-9 were higher than TBP group. Serum and ascites CA 125 were elevated in both groups and showed no significant differences.
Ascites was found in the abdominal CT scan of all patients. The parietal involvement pattern was significantly different between the PC and TBP groups as shown in Table 3. In the TBP group, 21 patients (77.8%) showed smooth thickening of the parietal peritoneum, whereas smooth thickening was found in 10 patients (40.0%) in the PC group. Irregular and nodular parietal involvement was noted in 11 patients (44.0%) in the PC group whereas only 4 patients (14.8%) showed irregular or nodular involvement in the TBP group (P = 0.04). Thickening of mesentery was found in 15 (55.6%) and 8 (32.0%) patients in TBP and PC group, respectively (P = 0.09). In the TBP group, mesenteric nodularities were seen in 12 patients (44.4%) and all nodules were micronodules. Twelve patients (48.0%) in the PC group showed mesenteric nodules, which were composed of 10 micronodules and 2 macronodules. There was no discriminative difference in omental thickness and patterns of change between the two groups.
| Tuberculous peritonitis(n = 27) | Peritoneal carcinomatosis(n = 25) | P value | |
| Ascites loculation | 11 (40.7) | 10 (40.0) | 0.96 |
| Parietal involvement | 0.04 | ||
| No | 2 (7.4) | 3 (12.0) | |
| Smooth thickening | 21 (77.8) | 10 (40.0) | |
| Irregular or nodular | 4 (14.8) | 11 (44.0) | |
| Seeding nodule | 0 (0.0) | 1 (4.0) | |
| Mesenteric change | 23 (85.2) | 17 (68.0) | 0.14 |
| Thickening of mesentery | 15 (55.6) | 8 (32.0) | 0.09 |
| Mesenteric nodule | 0.31 | ||
| No | 15 (55.6) | 12 (48.0) | |
| Micronodule | 12 (44.4) | 10 (40.0) | |
| Macronodule | 0 (0.0) | 2 (8.0) | |
| Omental thickness (mm) | 20.48 ± 11.03 | 23.00 ± 15.77 | 0.51 |
| Omental change | 0.56 | ||
| No | 2 (7.4) | 2 (8.0) | |
| Smudged | 8 (29.6) | 4 (16.0) | |
| Nodular | 3 (11.1) | 2 (8.0) | |
| Omental cake | 13 (48.1) | 17 (68.0) | |
| Intestinal involvement | 3 (11.1) | 5 (20.0) | 0.53 |
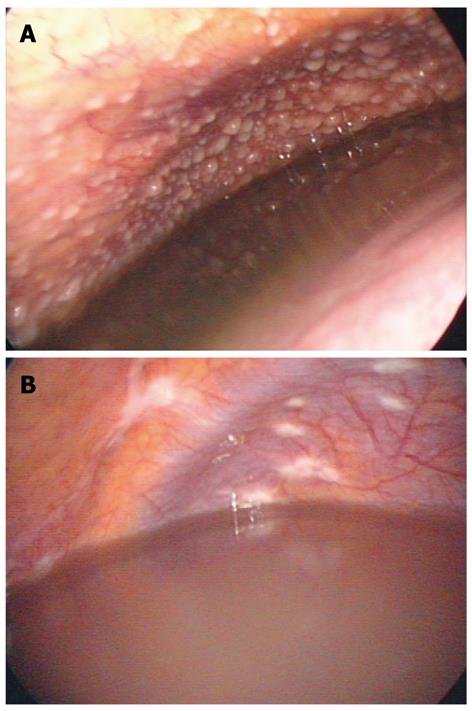
Peritoneoscopic images were reviewed by 2 experienced endoscopists (Kang SJ and Kim JW), and findings are summarized in Table 4. Twenty patients (74.1%) in the TBP group were found to have micronodules on the peritoneum (example of micronodule was shown in Figure 2A), and this number was greater than the number of TBP patients found to have micronodules on CT scan. Macronodules were not observed in TBP group. In the PC group, micronodules were detected in 8 patients, and macronodules were observed in 9 patients (P = 0.01). The distribution of whitish patches and presence of plaques were not significantly different (P = 0.41) between the two groups (example of patch lesion in Figure 2B). Adhesions between the peritoneum and abdominal organs were seen in 14 patients in the TBP group and 10 patients in the PC group (P = 0.41).
| Tuberculous peritonitis(n = 27) | Peritoneal carcinomatosis(n = 25) | P value | |
| Nodules on peritoneum | < 0.01 | ||
| No | 7 (25.9) | 7 (28.0) | |
| < 1 cm micronodule | 20 (74.1) | 8 (32.0) | |
| > 1 cm macronodule | 0 (0.0) | 9 (36.0) | |
| Peritoneum | 0.41 | ||
| Multiple whitish patches on parietal peritoneum | 2 (7.4) | 5 (20.0) | |
| Multiple whitish patches on parietal and visceral peritoneum | 0 (0.0) | 1 (4.0) | |
| No patches | 19 (70.4) | 15 (60.0) | |
| Whitish plaques | 4 (14.8) | 3 (12.0) | |
| Adhesion | 14 (51.9) | 10 (40.0) | 0.41 |
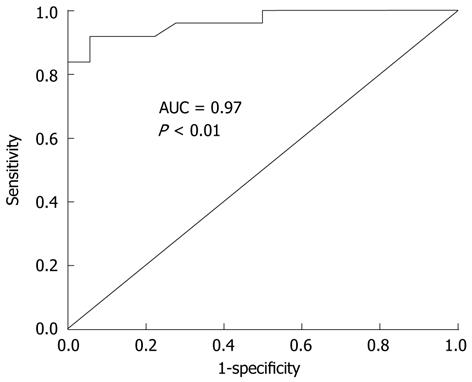
Among the evaluated laboratory parameters, parameters that showed significant difference between two groups were serum CRP, CEA, CA 19-9 and ascites ADA, CEA. AUC was calculated using ROC curves and these results were presented in Table 5. Among these markers, ascites ADA level was the strongest factor that differentiated TBP and PC patients. The ability to consider ascites ADA levels as a biomarker for differentiating TBP and PC patients was evaluated using ROC curve analysis. The AUC for TBP was 0.966 [95% confidence interval (CI): 0.916-1.00; P < 0.01] (Figure 3). The sensitivity, specificity and positive and negative predictive values of ascites ADA were 92.0%, 94.4%, 95.8% and 89.5% at cut-off level of 21.0 IU/L and 88.0%, 94.4%, 95.7% and 85.0% at 32.0 IU/L. When compared to cut-off level of 32.0, cut-off level of 21.0 shows higher sensitivity and negative predictive value and same specificity and positive predictive value.
| AUC | 95% CI | P value | |
| Serum CRP | 0.705 | 0.537-0.872 | 0.033 |
| Serum CEA | 0.721 | 0.562-0.880 | 0.017 |
| Serum CA 19-9 | 0.693 | 0.527-0.860 | 0.044 |
| Ascites ADA | 0.966 | 0.916-1.000 | < 0.001 |
| Ascites CEA | 0.823 | 0.686-0.960 | 0.002 |
TBP and PC are two of the most common causes of exudative ascites[1]. Differentiating between the two disease entities is difficult, and laparoscopy is thought to be the gold standard for diagnosis. Laparoscopy with biopsy has shown impressive sensitivity and specificity rates of 93% and 98% respectively[7]. Yet even with laparoscopy, some patients cannot be diagnosed, because the biopsy findings can be insufficient for diagnosis. Furthermore, some patients cannot be evaluated by laparoscopy because of their poor general condition or technical failure. In those cases, clinical and laboratory findings can be helpful for differentiating between TBP and PC. This study demonstrates that ascites ADA is valuable laboratory finding in differentiating between TBP and PC. To the best of our knowledge, this is the first comprehensive series to date that compares tumor markers, abdominal CT scan, and laparoscopic findings between tuberculous peritonitis and PC patients.
It is well known that ascites ADA has high sensitivity and specificity in the early diagnosis of TBP[8-10]. When used for diagnosing exudative ascites, at the cut-off level of 30 IU/L, ADA has demonstrated sensitivity of approximatley 94%[4]. One study about pediatric patients report 100% sensitivity and 97% specificity for diagnosis of TBP[11]. Of interest, in our study ADA also had the high diagnostic value in differentiating tuberculosis peritonitis and PC and showed highest differentiating value (largest AUC) at the cut-off level of 21 IU/L. This suggests that when two diseases, TBP and PC, are strongly suspected from clinical, laboratory and radiological findings, and other diseases causing exudative ascites are reliably excluded, an ADA cut-off level of 21 IU/L that is lower than the usual cut-off level used for diagnosis could be used for discrimination. But this study only includes 27 TBP patients and 25 PC patients, about the precise cut-off level of ascites ADA for discrimination of two diseases, further verification with large numbers of patients are required.
Ascites ADA is accurate but insensitive for detecting TBP in liver cirrhosis patients living in the United States where the prevalence of tuberculosis is low. In the United States, which has a low tuberculous burden, 59% of TBP patients have liver cirrhosis[12]. This means that in developed country where tuberculosis burden is low, TBP is mainly developed in immune compromised patients such as liver cirrhosis. In South Korea, the tuberculous burden is intermediate, so in our study only 14.8% (4 out of 27) of TBP patients had liver cirrhosis[13]. Low portion of cirrhotic patients partly explains high ascites ADA performance. Furthermore, 75% of TBP patients with liver cirrhosis (3 out of 4 patients) had a higher ascites ADA level than normal. So even cirrhotic patients, ascites of TBP patients shows high ADA. This also explains the sensitivity of ascites ADA for diagnosing TBP in our study.
Increased serum and ascites CA -125 levels, detected in up to 80% of women with late-stage ovarian cancer, have demonstrated great value in treatment monitoring and recurrence detection of ovary cancer[14-16]. In TBP, serum CA 125 levels are as high as ovarian cancer associated with peritoneal infiltration, and, by the end of the fourth month of anti-tuberculous therapy in a patient with TBP, serum CA 125 levels have returned to normal[17-19]. Similar to previous reports, this study demonstrated that both TBP and PC patients have elevated serum and ascites CA 125 levels[20-22]. As a results, ascites and serum CA 125 are cautiously interpreted in differentiating between TBP and PC.
Radiological findings of TBP are similar to PC. With a model of multivariate logistic analysis using mesenteric macronodules, omental lining, irregularity of omentum, and splenic abnormalities, the sensitivity for predicting tuberculous peritonitis was 69%, whereas the sensitivity for PC was 91%[23]. In our study, only the parietal involvement pattern was significantly different between the TBP and PC groups. A thickened mesentery and loss of normal mesenteric configuration are known to be characteristics of TBP and helpful for diagnosis, but our results did not show these findings to be helpful in differentiating between two disease[24]. When parietal involvement patterns were used as marker for differentiation, the sensitivity and specificity for diagnosing TBP in this study were 60.5% and 75.0%, respectively. This characteristics is insufficient as a marker for differentiation of diseases but can be used in adjunct to other examinations.
Peritoneoscopy is the diagnostic tool of choice in patients with exudative ascites of unknown origin[2,4]. Peritoneal tubercles and ascites are the main features of TBP and appear in more than 90% of TBP cases[25-27]. In our study, micronodules on the peritoneum and ascites were seen in 20 of 27 patients (74.1%). The low micronodule detection rate compared to previous studies may be due to the discovery of patch and plaque lesions in 6 patients (2 patch lesions and 4 plaque lesion, 22.2%) of the TBP group during peritoneoscopy, because nearly all suspicious patch lesions were biopsied whenever possible. For diagnosis of PC, peritoneoscopy has higher sensitivity and specificity than helical CT scan with 5-mm slice thickness, and similar results were found in this study[28]. The diagnostic failure rate of peritoneoscopy is substantially high, however, reaching about 14%; the main reason for diagnostic failure is interference from adhesions from previous surgery or tumor adhesion[7].
Interpretation of our findings requires careful consideration of several aspects. First, the positive and negative predictive values are usually affected by pretest probability, so those values of the ascites ADA in this study may be variable in countries of low tuberculosis prevalence. Second, PC group is composed of various cancers. So discriminative ability of each tumor markers such as CEA for specific cancer could not fully tested in this study, so larger numbers of patients are needed to assess the predicting ability of each ascites tumor marker for each type of cancer.
In conclusion, clinical findings such as duration of symptom less than 1 mo and fever are helpful for differentiating TBP and PC of various causes. Among laboratory findings, ascites ADA was the most valuable discriminative marker. For differentiation of two diseases, in addition to clinical findings and radiologic characteristics, ascites ADA should also be considered.
Tuberculous peritonitis (TBP) and peritoneal carcinomatosis (PC) are two of the most common causes of exudative ascites but in a clinical situation, etiological diagnosis of the two diseases is very difficult. Peritoneoscopy with biopsy is thought to be the method of choice in the differential diagnosis of the two diseases but the morbidity related with peritoneoscopic procedure and diagnostic failure rate reaches as high as approximately 8% and 14%, respectively. So we investigate the diagnostic capacities of adenosines deaminase (ADA) and tumor markers for differentiating the two diseases.
Diagnostic abilities of serum ADA and tumor markers in differentiating two diseases are hotspots in recent studies. Serum c-reactive protein, serum cercinoembryonic antigen (CEA), serum CA 19-9, ascites ADA and ascites CEA were significantly different between two disease groups. Among them, ascites ADA showed largest area under the curves (AUC) in receiver operating characteristic curves (AUC = 0.966; 95% confidence interval: 0.916-1.00; P < 0.01). In addition to ascites ADA, clinical findings such as symptom duration less than 1 mo and fever was more frequent finding in tuberculosis peritonitis. In abdomen computed tomography findings, smooth thickening was the most common in TBP whereas in PC group nodular pattern was the most common finding.
This study showed that among laboratory findings, ascites ADA was the most valuable marker for discriminating TBP and PC.
This analysis for the diagnostic capabilities of ADA implicates that ascites ADA may be helpful for differentiation of two diseases.
It is a very nice paper with an excellent statistic work and very interesting findings.
Peer reviewer: Bernabe Matias Quesada, MD, Department of Surgery, Hospital Cosme Argerich, Talcahuano 944 9°A, Buenos Aires 1013, Argentina
S- Editor Cheng JX L- Editor A E- Editor Zhang DN
| 1. | Hwangbo Y, Jung JH, Shim J, Kim BH, Jung SH, Lee CK, Jang JY, Dong SH, Kim HJ, Chang YW. [Etiologic and laboratory analyses of ascites in patients who underwent diagnostic paracentesis]. Korean J Hepatol. 2007;13:185-195. [PubMed] |
| 2. | Bedioui H, Ksantini R, Nouira K, Mekni A, Daghfous A, Chebbi F, Rebai W, Fteriche F, Jouini M, Kacem M. Role of laparoscopic surgery in the etiologic diagnosis of exsudative ascites: a prospective study of 90 cases. Gastroenterol Clin Biol. 2007;31:1146-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Vardareli E, Kebapci M, Saricam T, Pasaoglu O, Açikalin M. Tuberculous peritonitis of the wet ascitic type: clinical features and diagnostic value of image-guided peritoneal biopsy. Dig Liver Dis. 2004;36:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Al-Mulhim AA. Laparoscopic diagnosis of peritoneal tuberculosis. Surg Endosc. 2004;18:1757-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Krishnan P, Vayoth SO, Dhar P, Surendran S, Ponnambathayil S. Laparoscopy in suspected abdominal tuberculosis is useful as an early diagnostic method. ANZ J Surg. 2008;78:987-989. [PubMed] |
| 7. | Coupland GA, Townend DM, Martin CJ. Peritoneoscopy--use in assessment of intra-abdominal malignancy. Surgery. 1981;89:645-649. [PubMed] |
| 8. | Martinez-Vazquez JM, Ocaña I, Ribera E, Segura RM, Pascual C. Adenosine deaminase activity in the diagnosis of tuberculous peritonitis. Gut. 1986;27:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Sathar MA, Simjee AE, Coovadia YM, Soni PN, Moola SA, Insam B, Makumbi F. Ascitic fluid gamma interferon concentrations and adenosine deaminase activity in tuberculous peritonitis. Gut. 1995;36:419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Dinler G, Sensoy G, Helek D, Kalayci AG. Tuberculous peritonitis in children: report of nine patients and review of the literature. World J Gastroenterol. 2008;14:7235-7239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med. 2009;24:337-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Sevinc A, Camci C, Turk HM, Buyukberber S. How to interpret serum CA 125 levels in patients with serosal involvement? A clinical dilemma. Oncology. 2003;65:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC. Accuracy of CA 125 in the diagnosis of ovarian tumors: a quantitative systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;142:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer. 2000;82:1535-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Mas MR, Cömert B, Sağlamkaya U, Yamanel L, Kuzhan O, Ateşkan U, Kocabalkan F. CA-125; a new marker for diagnosis and follow-up of patients with tuberculous peritonitis. Dig Liver Dis. 2000;32:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ulusoy AN, Karabicak I, Dicle K, Kefeli M, Tosun M, Cetinkaya M, Alper T, Ustun C. Peritoneal tuberculosis in premenopausal patients with elevated serum CA 125. Arch Gynecol Obstet. 2010;282:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Younossian AB, Rochat T, Favre L, Janssens JP. Ascites and highly elevated CA-125 levels in a case of peritoneal tuberculosis. Scand J Infect Dis. 2006;38:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | O'Riordan DK, Deery A, Dorman A, Epstein OE. Increased CA 125 in a patient with tuberculous peritonitis: case report and review of published works. Gut. 1995;36:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Thakur V, Mukherjee U, Kumar K. Elevated serum cancer antigen 125 levels in advanced abdominal tuberculosis. Med Oncol. 2001;18:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Candocia SA, Locker GY. Elevated serum CA 125 secondary to tuberculous peritonitis. Cancer. 1993;72:2016-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |
| 23. | Ha HK, Jung JI, Lee MS, Choi BG, Lee MG, Kim YH, Kim PN, Auh YH. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am J Roentgenol. 1996;167:743-748. [PubMed] |
| 24. | Denton T, Hossain J. A radiological study of abdominal tuberculosis in a Saudi population, with special reference to ultrasound and computed tomography. Clin Radiol. 1993;47:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Demir K, Okten A, Kaymakoglu S, Dincer D, Besisik F, Cevikbas U, Ozdil S, Bostas G, Mungan Z, Cakaloglu Y. Tuberculous peritonitis--reports of 26 cases, detailing diagnostic and therapeutic problems. Eur J Gastroenterol Hepatol. 2001;13:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Poyrazoglu OK, Timurkaan M, Yalniz M, Ataseven H, Dogukan M, Bahcecioglu IH. Clinical review of 23 patients with tuberculous peritonitis: presenting features and diagnosis. J Dig Dis. 2008;9:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Apaydin B, Paksoy M, Bilir M, Zengin K, Saribeyoglu K, Taskin M. Value of diagnostic laparoscopy in tuberculous peritonitis. Eur J Surg. 1999;165:158-163. [PubMed] |
| 28. | Weickert U, Jakobs R, Riemann JF. Diagnostic laparoscopy. Endoscopy. 2005;37:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |