Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. May 7, 2012; 18(17): 2067-2075
Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2067
Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2067
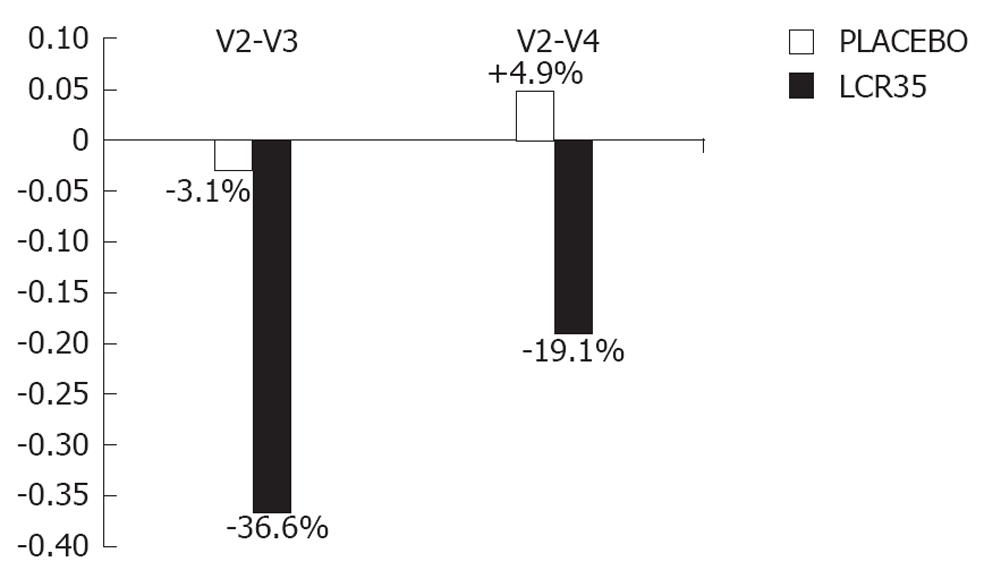
Figure 2 Relative changes in irritable bowel syndrome severity score between V2 and V3, V2 and V4 in irritable bowel syndrome patients with predominance of diarrhoea.
V2: Baseline; V3: At the end of the 4-wk treatment period, a more marked decrease in irritable bowel syndrome (IBS) severity score occurred with the test drug (-36.6% vs -3.1% with placebo); V4: Two weeks later, the IBS severity score was maintained below baseline with the test drug (-19.1%), whilst it slightly increased over baseline (+4.9%) with placebo.
- Citation: Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: A randomized, double-blind study. World J Gastroenterol 2012; 18(17): 2067-2075
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2067