Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2132
Revised: March 3, 2012
Accepted: March 9, 2012
Published online: May 7, 2012
AIM: To investigate the effect of emodin on pancreatic claudin-5 and occludin expression, and pancreatic paracellular permeability in acute pancreatitis (AP).
METHODS: Experimental pancreatitis was induced by retrograde injection of 5% sodium taurocholate into the biliopancreatic duct. Emodin was injected via the external jugular vein 0 or 6 h after induction of AP. Rats from sham operation and AP groups were injected with normal saline at the same time. Samples of pancreas were obtained 6 or 12 h after drug administration. Pancreatic morphology was examined with hematoxylin and eosin staining. Pancreatic edema was estimated by measuring tissue water content. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 level were measured by enzyme-linked immunosorbent assay. Pancreatic paracellular permeability was assessed by tissue dye extravasation. Expression of pancreatic claudin-5 and occludin was examined by immunohistology, quantitative real-time reverse transcriptase polymerase chain reaction and western blotting.
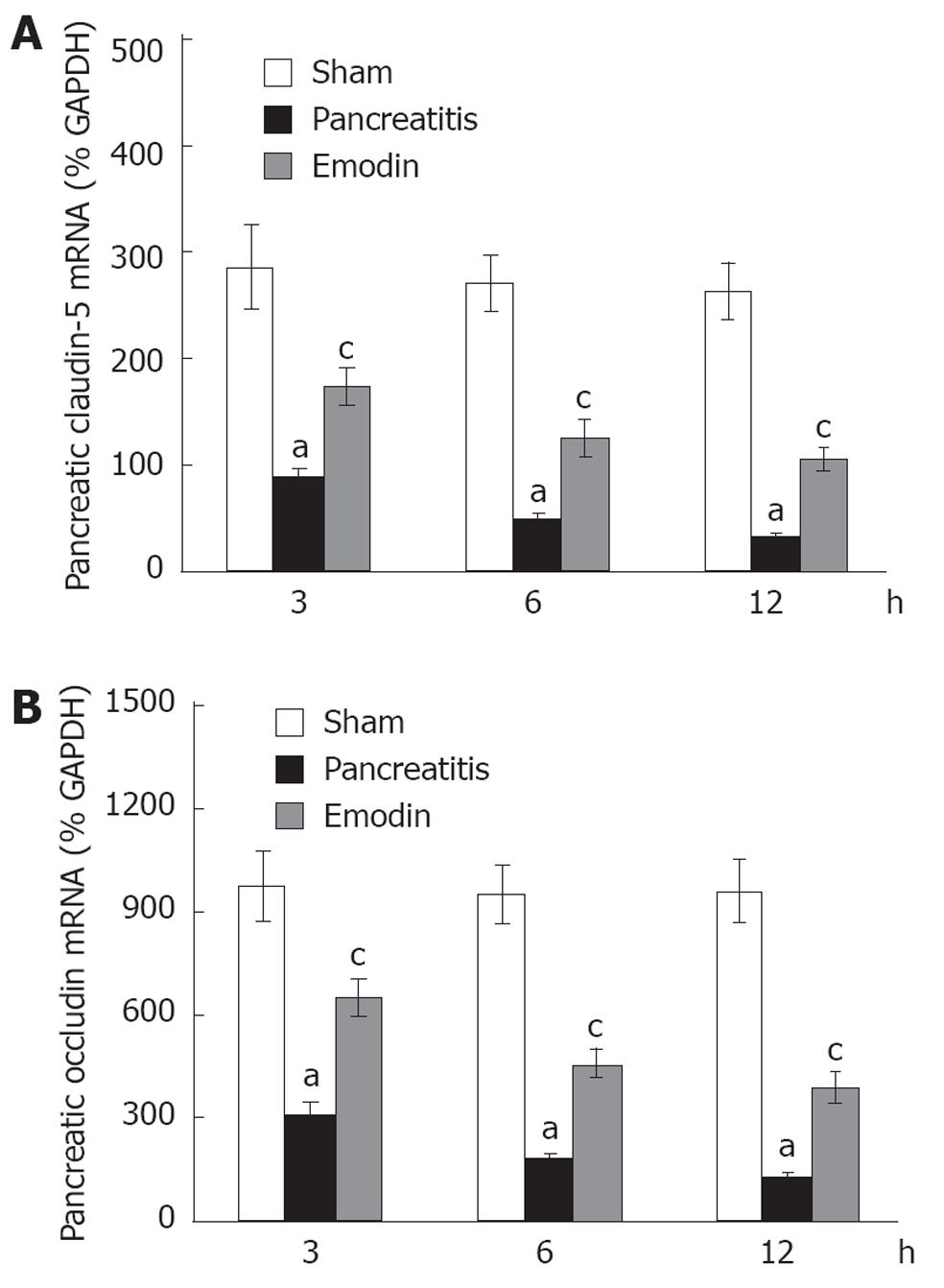
RESULTS: Pancreatic TNF-α and IL-6 levels, wet/dry ratio, dye extravasation, and histological score were significantly elevated at 3, 6 and 12 h following sodium taurocholate infusion; treatment with emodin prevented these changes at all time points. Immunostaining of claudin-5 and occludin was detected in rat pancreas, which was distributed in pancreatic acinar cells, ductal cells and vascular endothelial cells, respectively. Sodium taurocholate infusion significantly decreased pancreatic claudin-5 and occludin mRNA and protein levels at 3, 6 and 12 h, and that could be promoted by intravenous administration of emodin at all time points.
CONCLUSION: These results demonstrate that emodin could promote pancreatic claudin-5 and occludin expression, and reduce pancreatic paracellular permeability.
- Citation: Xia XM, Li BK, Xing SM, Ruan HL. Emodin promoted pancreatic claudin-5 and occludin expression in experimental acute pancreatitis rats. World J Gastroenterol 2012; 18(17): 2132-2139
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2132
Acute pancreatitis (AP) is an inflammatory disease characterized by interstitial edema, acinar necrosis, hemorrhage, and inflammatory infiltration in the pancreas[1]. Increased paracellular permeability and loss of barrier function in pancreas have been demonstrated at early stages of AP[2-4], but the molecular basis for these phenomena is poorly understood.
Tight junctions, the major apical structures in epithelium and endothelium, have been recently reported to play important roles in barrier function by forming cell-to-cell contacts and sealing paracellular pathway[5,6]. Tight junctions comprise the integral transmembrane proteins occludin, junctional adhesion molecules, and members of the claudin multigene family[4-7]. In mammals, the claudin family of 20-24 kDa integral membrane proteins includes at least 24 members; most of them have been shown to control the permeability of the paracellular pathway[6-8]. Sharing similar membrane location with claudins, occludin also plays an important role in maintaining epithelial and endothelial barriers[9]. Previous studies have demonstrated that claudin-1-5 and occludin are expressed in the pancreas[3,10-12]. Schmitt et al[3] have reported that claudin-1 and occludin expression in pancreas is significantly decreased in caerulein-induced AP, suggesting a possible role of tight junctions disruption in interstitial edema formation.
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone), an anthraquinone derivative from the Chinese herb Radix et Rhizoma Rhei, has been reported to inhibit production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1[13,14]. Our previous study has demonstrated that emodin significantly reduces serum amylase and inflammatory cytokines and attenuates pancreatic damage in AP rats[15]. However, the effects of emodin on claudin and occludin expression, as well as pancreatic paracellular permeability remain largely undefined.
We have previously found that 6 h after duct infusion of sodium taurocholate, pancreatic claudin-1 and claudin-4 were slightly elevated, claudin-5 and occludin were significantly decreased, whereas claudin-2 and claudin-3 remained unchanged (data not shown). Thus, in the present study, we assessed the effects of emodin on claudin-5 and occludin expression. Time course of pancreatic paracellular permeability, edema, and cytokines were also determined.
All chemicals were purchased from Sigma (St. Louis, MO, United States) unless otherwise indicated. TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Jingmei Biotech (Beijing, China). TRIzol kit and SYBR Green SuperMix-UDG were purchased from Invitrogen (Carlsbad, CA, United States). A first-strand cDNA synthesis kit was purchased from Fermentas (Burlington, Ont., Canada). Antibodies for claudin-5 and occludin were obtained from Zymed Laboratories (South San Francisco, CA, United States). The Power Vision Two-Step Histostaining Reagent was purchased from ImmunoVision Technologies (Norwell, MA, United States). Glyceraldehyde phosphate dehydrogenase (GAPDH) antibody was purchased from Abcam (Cambridge, United Kingdom). Horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from Kangchen Biotech (Shanghai, China). Immobilon western chemiluminescent HRP substrate was purchased from Millipore (Boston, MA, United States).
Adult male Sprague-Dawley rats (200-250 g body weight) were obtained from the Animal Facility of Anhui Medical University (Hefei, China). Animals were housed under controlled temperature, humidity and day-night cycles, with free access to standard laboratory feed and water. The Animal Studies Ethics Committee of Anhui Medical University approved all of the experiments.
AP was induced as described by Pereda et al[16]. Briefly, animals were anesthetized with intraperitoneal administration of ketamine (80 mg/kg body weight) and acepromazine (2.5 mg/kg body weight). The biliopancreatic duct was cannulated through the duodenum, and the hepatic duct was closed by a small bulldog clamp. Pancreatitis was induced by retrograde injection into the biliopancreatic duct of 5% sodium taurocholate in a volume of 1 mL/kg body weight, at a constant infusion pressure of 20 mmHg. Presenting as controls, sham group received retrograde infusion of sterile saline.
AP rats were randomly allocated into two groups: the model group and emodin group (2.5 mg/kg body weight). Emodin was injected via the external jugular vein immediately after duct infusion of sodium taurocholate. Both the sham group and model group were injected with normal saline of equivalent volume. Samples were obtained 3, 6 and 12 h after duct infusion. For animals that were euthanized at the 12-h time point, a second administration of emodin or saline was adopted, 6 h after duct infusion of sodium taurocholate.
Samples of pancreas were obtained at 3, 6 and 12 h after intraductal infusion, immediately frozen and maintained at -80 °C until assayed. Blood samples were obtained from the inferior cava vein by direct puncture. For histological examination, the central body of the pancreas was fixed in 4% neutral phosphate-buffered formalin and then embedded in paraffin wax. Serum amylase activity was measured to confirm the appropriate induction of pancreatitis.
An additional experiment was adopted to assess the effect of emodin on pancreatic dye extravasation (marker of paracellular permeability). Animals were distributed in the same groups as in the previous series.
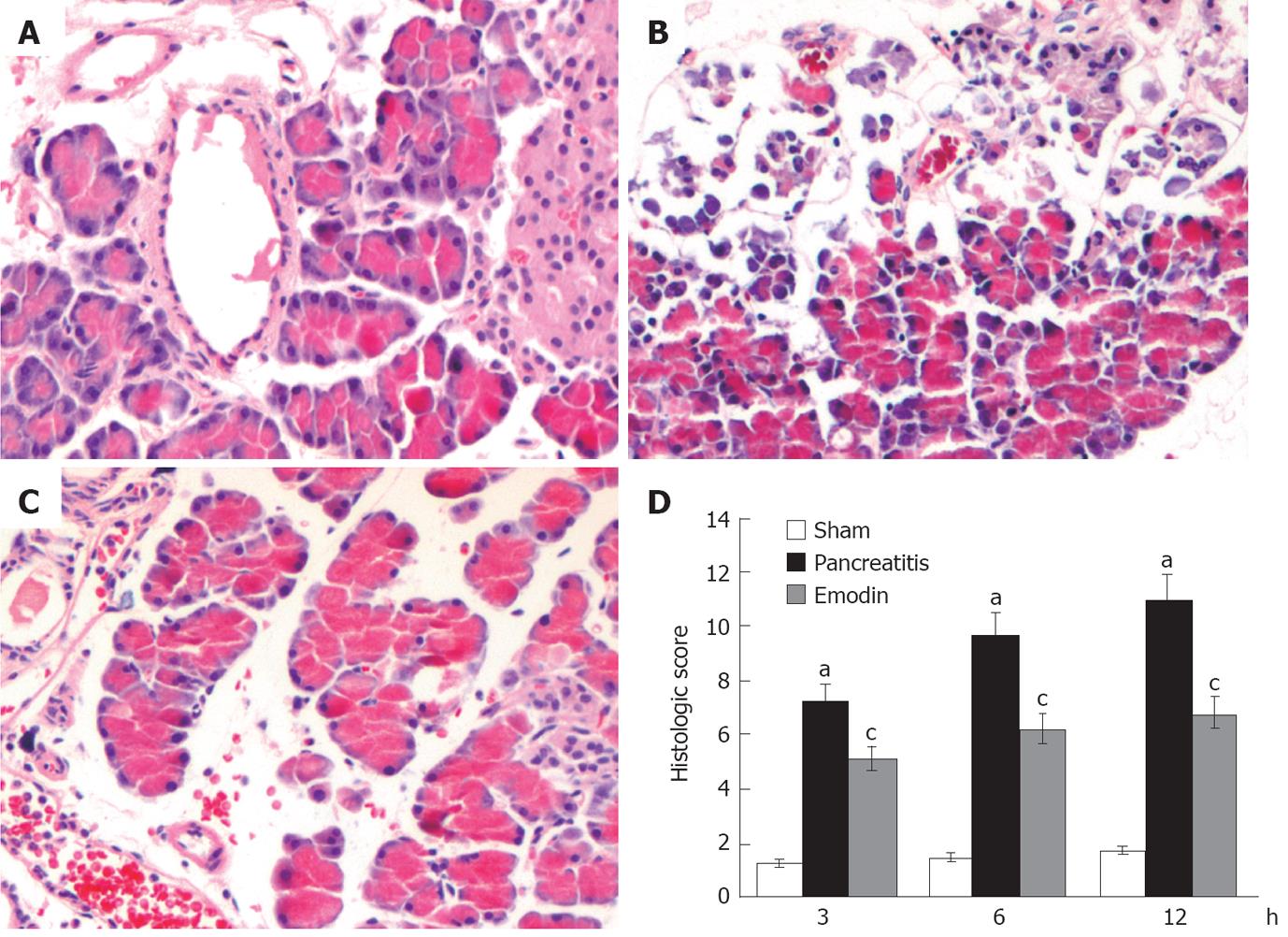
Rat pancreas was washed in phosphate buffered saline (PBS), fixed in 10% neutral-buffered formalin, and embedded in paraffin wax. Five-micrometer sections were deparaffinized with xylene, stained with hematoxylin and eosin, and examined by two experienced pathologists in blinded fashion. Pancreatic damage was scored using a grading system described by Ryan et al[17]. The grading was based on the number of acinar cell ghosts, the presence of vacuolization, interstitial edema and interstitial inflammation, and to what extent these characteristics affected the pancreas (0 being normal and 3 being severe), giving a maximum score of 12 (Table 1).
| Condition | Score | Description |
| Edema | 0 | Absent |
| 1 | Diffuse expansion of interlobular septa | |
| 2 | 1 + diffuse expansion of interlobular septa | |
| 3 | 2 + diffuse expansion of interlobular septa | |
| Inflammation (%) | 0 | Absent |
| 1 | In parenchyma (< 50 of lobules) | |
| 2 | In parenchyma (51-75 of lobules) | |
| 3 | In parenchyma (> 75 of lobules) | |
| Vacuolization (%) | 0 | Absent |
| 1 | Focal (5-20) | |
| 2 | Diffuse (21-50) | |
| 3 | Severe (> 50) |
The extent of pancreatic edema was estimated by measuring tissue water content. Freshly obtained blotted samples of pancreas were weighed on aluminum foil, dried for 24 h at 95 °C, and reweighed. The difference between the wet and dry tissue weights was calculated and expressed as wet/dry ratio.
Pancreatic TNF-α and IL-6 were examined using a sandwich ELISA according to the manufacturer’s instructions. The tissue homogenate ELISA was corrected by the concentration of protein, and expressed as the content per protein of the tissue (pg/mg protein).
Paracellular permeability of the pancreas was evaluated by the measurement of Evans blue extravasation[18]. Briefly, Evans blue (20 mg/kg) was injected into the jugular vein of rats, 30 min before duct infusion. Samples of pancreas were obtained 3, 6 and 12 h after duct infusion. A portion of the splenic segment was sectioned and immersed in formamide solution, and homogenized for 2 min. After incubation at room temperature for 24 h, the suspension was centrifuged at 4000 g for 30 min. The quantity of dye extracted was determined spectrophotometrically at 620 nm and calculated from a standard curve established with known amounts of Evans blue. Results were corrected by the wet/dry ratio of the pancreas and expressed as the dye content per dry weight of the pancreatic tissue (μg/g tissue).
Western blotting was performed as described by Hietaranta et al[19]. From each sample, 20 μg total protein was separated on 4%-20% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride membranes. Membranes were blocked in blocking solution, incubated overnight with primary antibodies, and developed with an HRP-conjugated secondary antibody (1:1000 dilution). Dilutions for primary antibody were as follows: claudin-5, 1:100; and occludin, 1:300. The immune complexes were then visualized using chemiluminescent HRP substrate and X-ray film. Additional immunoblots were performed using GAPDH antibody as the primary antibody to evaluate equal loading.
Pancreas sections (4 μm) were dewaxed in graded alcohols, and finally washed in tap water. Endogenous peroxidase activity was blocked by 3% (v/v) H2O2, and the antigen was retrieved by microwave in 0.01 mol/L citrate buffer. Sections were then washed in PBS (0.1 mol/L). Mouse anti-rat claudin-5, and rabbit anti-rat occludin polyclonal antibodies were applied at 1:100 and incubated overnight at 4 °C. Sections were washed four times in PBS for 20 min. The Power Vision Two-Step Histostaining Reagent was used for detection. All sections were developed using diaminobenzidine, and subsequently counterstained with hematoxylin.
Total RNA was extracted using TRIzol Kit and converted to first-strand cDNA according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Green SuperMix-UDG in Prism 7000 Q real-time PCR detection system (Applied Biosystems, Foster City, CA, United States). The primer sequences used for PCR were as follows: claudin-5 (forward 5’-TACTCAGCACCAAGGCGAACCAC-3’, reverse 5’-GCGGCTT CCCACATCG-GTC-3’), occludin (forward 5’-AGTACATGGCTGCTGCTGAT G-3’, reverse 5’-CCCACCATCCTCTTGAT GTGT-3’), GAPDH (forward 5’-CA GTGCCAGCC-TCGTCTCATA-3’, reverse 5’-TGCCGTGGGTAGAGTCAT A-3’). Amplification was performed with use of the following cycles: 50 °C for 2 min (UDG incubation), 95 °C for 2 min, followed by 40 cycles of denaturing at 95 °C for 15 s and annealing at 60 °C for 30 s. All reactions were performed in triplicate. Melting curve analysis was performed to ensure the specificity of quantitative PCR. Data analysis was performed using the 2-ΔΔCT method described by Livak et al[20], where GAPDH was used as reference gene.
Results are presented as mean ± SE. One-way repeated-measures analysis of variance (followed by multiple pair-wise comparisons using Student-Newman-Keuls method) was used for the analysis of differences between the experimental and control groups. All statistical analysis were carried out using SPSS for Windows version 11.5, with statistical significance set at P < 0.05.
Histological sections from representative pancreas are shown in Figure 1. Pancreatic damage was characterized by leukocyte infiltrate, acinar cell necrosis, hemorrhage and fat necrosis (Figure 1B), and the histological score was significantly elevated as compared with the sham operation group at all time points (Figure 1D). Treatment with emodin obviously ameliorated pancreatic damage (Figure 1C), thus decreasing pancreatic pathological scores.
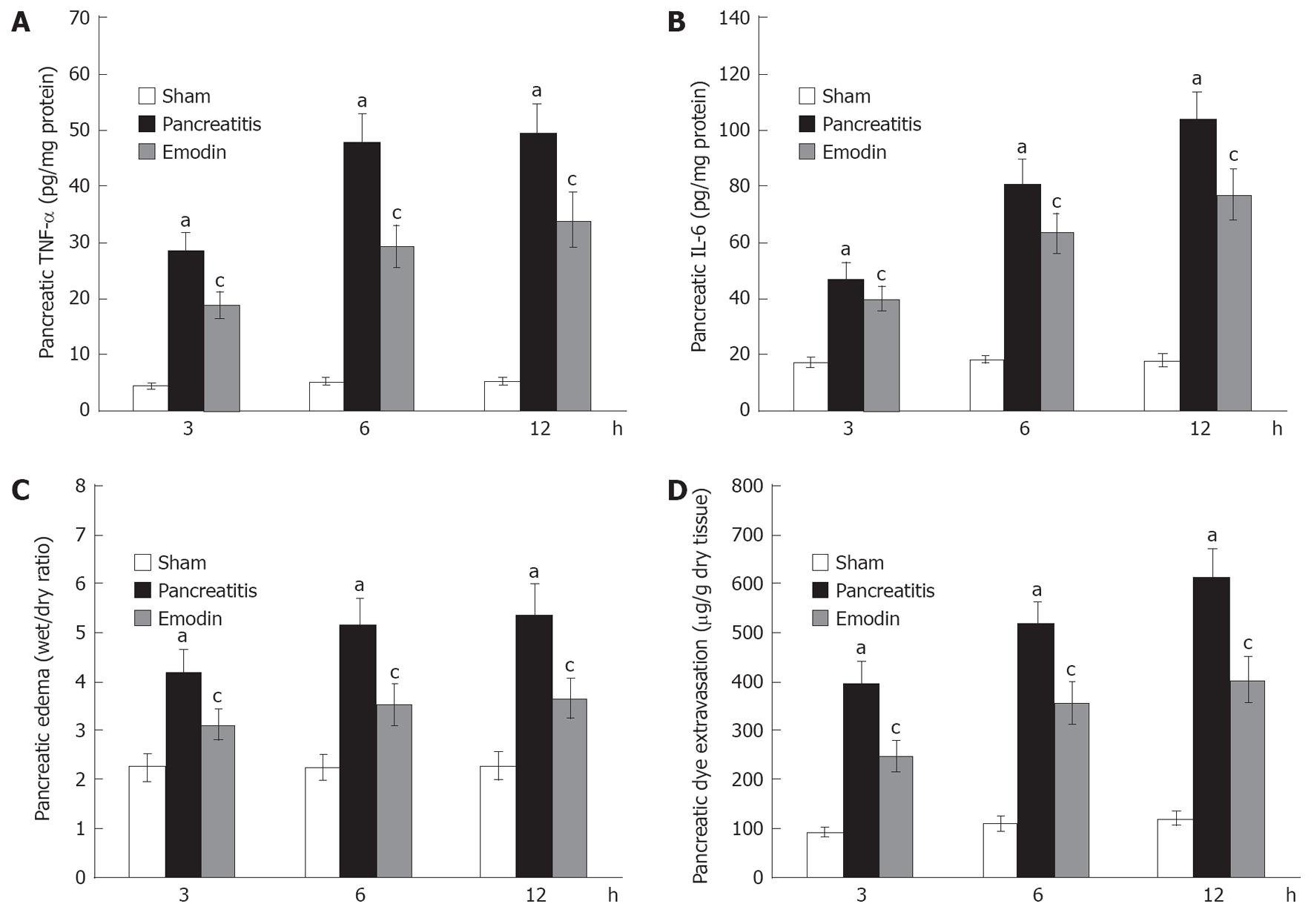
Time course of pancreatic TNF-α and IL-6 was also examined. Pancreatic TNF-α and IL-6 levels were significantly elevated at 3, 6 and 12 h following sodium taurocholate infusion, and treatment with emodin significantly reduced pancreatic TNF-α and IL-6 level at all time points (Figure 2A and B).
Pancreatic edema was evaluated by measuring the pancreatic water content, expressed as wet/dry ratio. As shown in Figure 2C, pancreatic wet/dry ratio was significantly elevated at 3, 6 and 12 h following sodium taurocholate infusion; emodin treatment significantly decreased pancreatic wet/dry ratio at all time points.
Pancreatic dye extravasation, as a marker of paracellular permeability, was examined in the present study. Figure 2D showed that pancreatic dye extravasation was significantly elevated at 3, 6 and 12 h after induction of AP; emodin treatment significantly inhibited pancreatic dye extravasation at all time points. These findings indicated that emodin could reduce pancreatic paracellular permeability.
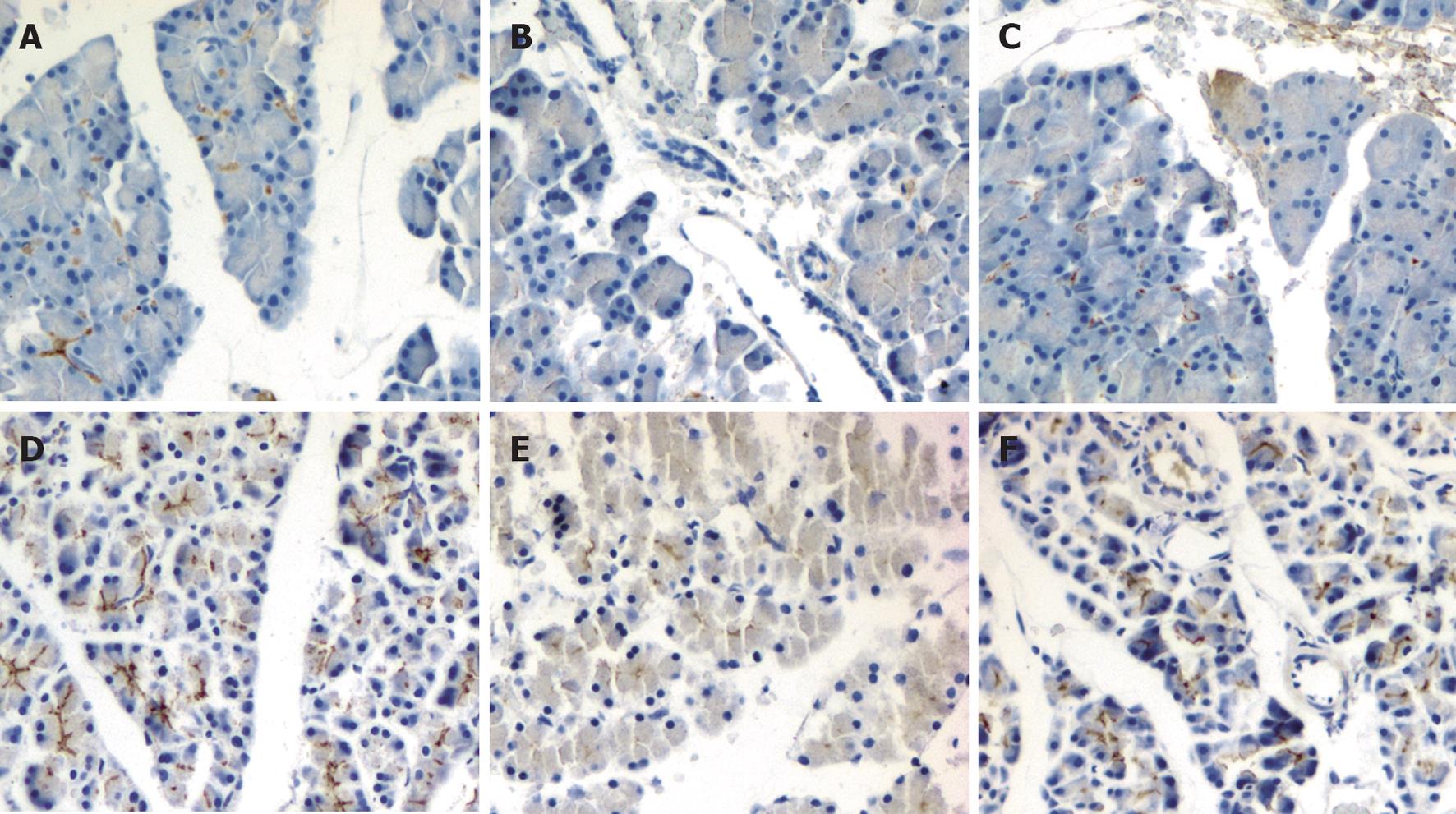
We further evaluated the effect of emodin on pancreatic claudin-5 and occludin expression in sodium taurocholate induced AP rats. Immunolocalization of claudin-5 and occludin in pancreas was investigated using immunohistochemical staining. In sham rats, moderate claudin-5 immunostaining was detected in pancreatic acinar cells and vascular endothelial cells (Figure 3A), and intense occludin immunostaining was detected in pancreatic acinar cells, ductal cells and vascular endothelial cells (Figure 3D). Duct infusion of sodium taurocholate markedly decreased the immunostaining of claudin-5 and occludin (Figure 3B and E), and the immunostaining was enhanced when treated with emodin (Figure 3C and F).
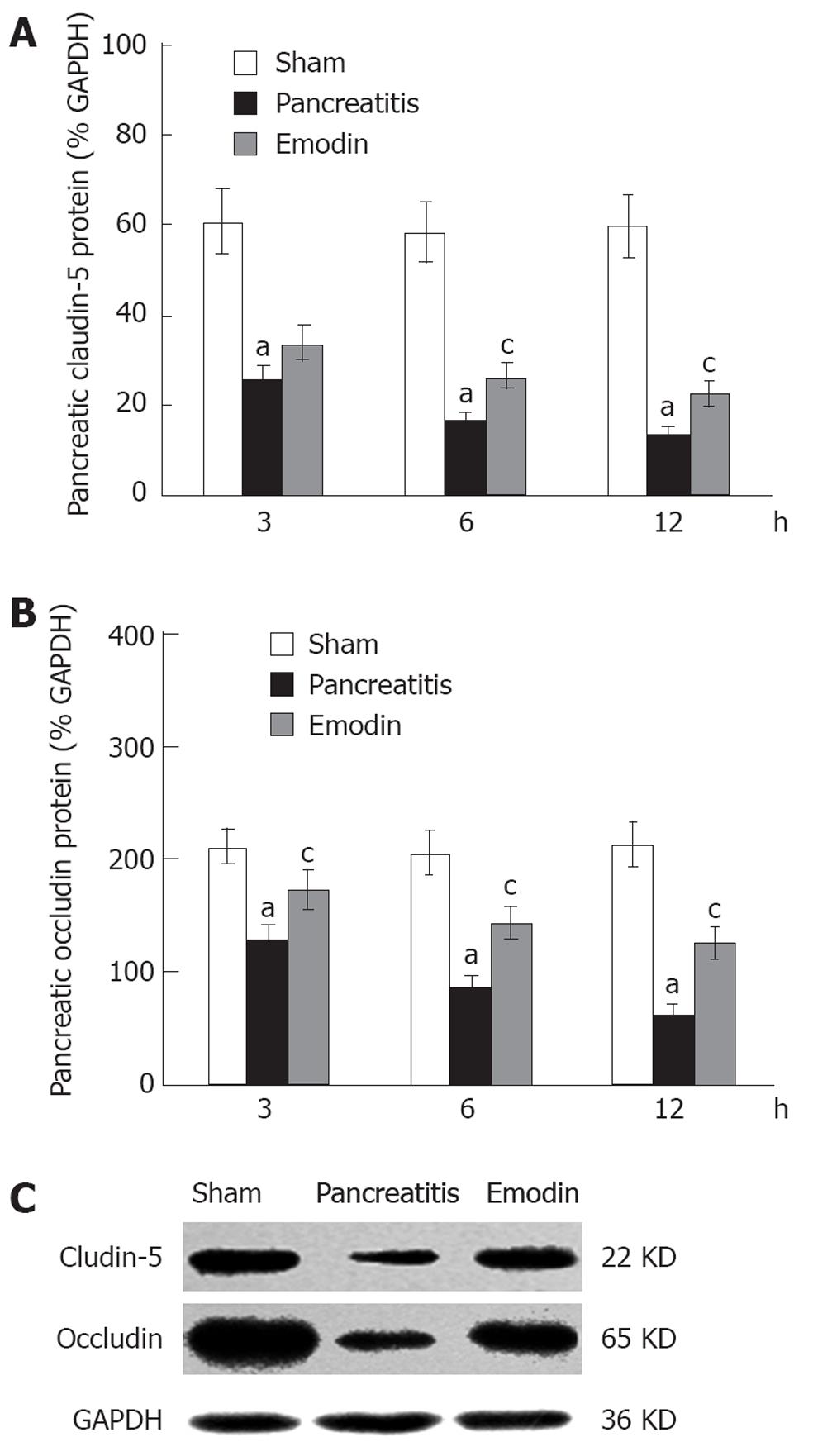
Claudin-5 and occludin protein levels in pancreas were evaluated using western blotting. As shown in Figure 4, sodium taurocholate infusion significantly decreased pancreatic claudin-5 and occludin levels at 3, 6 and 12 h, as compared with sham rats; treatment with emodin markedly arrested the decline at all time points.
Kinetics of claudin-5 and occludin mRNA expression in pancreas was examined using quantitative real-time reverse transcription (RT)-PCR analysis. Sodium taurocholate infusion significantly downregulated pancreatic claudin-5 and occludin mRNA expression at 3, 6 and 12 h, and that could be upregulated by intravenous administration of emodin at all time points (Figure 5). Results from present study demonstrated that emodin promoted pancreatic claudin-5 and occludin expression at the level of mRNA transcription and protein synthesis.
The present study investigated the kinetic expression of claudin-5 and occludin in sodium-taurocholate-induced AP, and identified the effects of emodin on pancreatic claudin-5 and occludin expression, as well as pancreatic paracellular permeability in AP rats.
The fundamental functions of epithelia and endothelia in pancreas are to separate distinct compartments and regulate the exchange of small solutes and other substances between them[4]. Increased paracellular permeability may allow noxious contents from the luminal ductal system to enter the interstitium of the pancreatic gland, which results in local inflammation and early edema formation in AP[3]. Lerch et al[21] have pointed out that the sealing junctions of the interstitial space in the pancreas are the tight junctions and their proteins. They play crucial roles in the barrier function by constituting tight junction strands and by regulating the tightness of the paracellular pathway[6].
Previous studies have reported that claudin-5 is expressed in the pancreas and localized to the tight junctions[10,22,23]. The role of claudin-5 in barrier function has been investigated in several inflammatory models. Decreased expression and redistribution of claudin-5 is found in acute colitis[24]. Downregulation of claudin-5 has also been demonstrated in experimental autoimmune encephalomyelitis, correlated with breakdown of the blood-brain barrier; recombinant claudin-5 protected brain microvascular endothelial cell cultures from vascular-endothelial-growth-factor-induced increase in paracellular permeability, showing claudin-5 to be a key determinant at the blood-brain barrier[25]. However, in experimental AP, Meriläinen et al[26] have reported that the expression of claudin-5 is not changed during pancreatitis. Whether claudin-5 plays a role in pancreatic paracellular permeability therefore needs further investigation.
Occludin shares very similar membrane location with claudins. Based on the staining feature of claudins and occludin along endothelial cell borders in and outside the central nervous system, Persidsky et al[8] have speculated that claudins formed the primary make-up of the tight junctions, and occludin further enhances tight junction tightness. Tai et al[9] have previously reported that increased paracellular permeability is associated with a specific decrease in occludin, both at the protein and mRNA levels, in hCMEC/D3 cells. Drugs prevent downregulation of occluding, which could decrease paracellular permeability, suggesting an important role of occludin in the blood-brain barrier. In caerulein-induced AP rats, the disintegration of occludin precedes the increase of serum lipase and amylase, accompanied by increased paracellular permeability, indicating a possible role of occludin in pancreatic barrier function[3].
In the present study, we identified the localization of claudin-5 and occludin in rat pancreas. In normal rats, intense occludin immunostaining was detected in pancreatic acinar cells, ductal cells and vascular endothelial cells, and this was consistent with results from Schmitt et al[3] and Borka et al[11]. In addition, we also detected moderate claudin-5 immunostaining in pancreatic acinar cells and vascular endothelial cells. Using RT-PCR and Western blotting, our present study confirmed the downregulation of occludin in pancreas of AP rats, which was in keeping with the results of Schmitt et al[3]. Our present study also identified the decrease of claudin-5 expression in pancreas of AP rats. It was found that the increase of pancreatic edema and paracellular permeability (marked by extravasation of Evans blue) was accompanied by a decrease of pancreatic claudin-5 and occludin. As demonstrated in previous studies in which paracellular permeability was associated with changes of occludin and claudin multigenes[4-7,23-25], our results suggested possible roles of claudin-5 and occludin in pancreatic barrier function.
Our present study also investigated the effects of emodin on pancreatic edema and paracellular permeability, as well as pancreatic claudin-5 and occludin expression in AP rats. Emodin significantly increased pancreatic claudin-5 and occludin expression at the level of mRNA transcription and protein synthesis, accompanied with decreased pancreatic edema and paracellular permeability. Based on results from previous and present studies, we speculate that the amelioration of pancreatic damage by emodin may contribute, in part at least, to the promotion of claudin-5 and occludin expression.
Disruption of tight junctions results in leakage of amylase and lipase, which could increase production of proinflammatory cytokines; cytokines further impair epithelial barrier function by regulation of expression of the barrier-builders such as the claudin family and occludin[27-29]. In agreement, our present study showed that elevated pancreatic TNF-α and IL-6 levels were paralleled with increased paracellular permeability and decreased expression of claudin-5 and occludin. Emodin could reduce pancreatic TNF-α and IL-6 levels, decrease paracellular permeability, and promote claudin-5 and occludin expression in AP rats, thus it plays an important role in pancreas protection.
In conclusion, our results demonstrate that emodin treatment could ameliorate pancreatic inflammation and edema, reduce paracellular permeability, and promote pancreatic claudin-5 and occludin expression. The decrease of pancreatic paracellular permeability by emodin may contribute, in part at least, to the promotion of claudin-5 and occludin expression.
Increased paracellular permeability and loss of barrier function in pancreas have been demonstrated at early stages of acute pancreatitis (AP), but the molecular basis for these phenomena is poorly understood.
Claudin and occludin, the major components of tight junctions in epithelium and endothelium, have been reported to play important roles in barrier function by sealing paracellular pathway. Emodin, an anthraquinone derivative from the Chinese herb Radix et Rhizoma Rhei, has been used for anti-inflammatory purposes. Weather emodin has effects on pancreatic tight junction expression and pancreatic paracellular permeability has not been defined.
A recent report has demonstrated that claudin-1 and occludin expression in pancreas is significantly decreased in caerulein-induced AP, suggesting a possible role of tight junction disruption in interstitial edema formation. This is the first study to report that decreased pancreatic claudin-5 and occludin expression is parallel with increased pancreatic edema and paracellular permeability. Emodin can promote pancreatic claudin-5 and occludin expression, decrease pancreatic paracellular permeability, and inhibit pancreatic inflammation.
The results of this study may improve the understanding of the pathogenesis of AP, and also provide evidence for emodin in treatment of AP.
This is an observational study representing an incremental advance in treatment of acute pancreatitis with emodin. The discussion is adequately developed and focused on the experimental results.
Peer reviewer: Dr. Naoaki Sakata, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Gou SX L- Editor Kerr C E- Editor Li JY
| 1. | Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97:13126-13131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 182] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 3. | Schmitt M, Klonowski-Stumpe H, Eckert M, Lüthen R, Häussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas. 2004;28:181-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 548] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Oliveira SS, Morgado-Díaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Troy TC, Arabzadeh A, Yerlikaya S, Turksen K. Claudin immunolocalization in neonatal mouse epithelial tissues. Cell Tissue Res. 2007;330:381-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 574] [Cited by in F6Publishing: 592] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 9. | Tai LM, Holloway KA, Male DK, Loughlin AJ, Romero IA. Amyloid-beta-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J Cell Mol Med. 2010;14:1101-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Borka K, Kaliszky P, Szabó E, Lotz G, Kupcsulik P, Schaff Z, Kiss A. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch. 2007;450:549-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G124-G133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Chang CP, Huang WT, Cheng BC, Hsu CC, Lin MT. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology. 2007;52:1024-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Lee J, Jung E, Lee J, Huh S, Hwang CH, Lee HY, Kim EJ, Cheon JM, Hyun CG, Kim YS. Emodin inhibits TNF alpha-induced MMP-1 expression through suppression of activator protein-1 (AP-1). Life Sci. 2006;79:2480-2485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Li Z, Xia X, Zhang S, Zhang A, Bo W, Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed Pharmacother. 2009;63:120-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J, Cerdá M, Lledó S. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg. 2004;240:108-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Ryan CM, Schmidt J, Lewandrowski K, Compton CC, Rattner DW, Warshaw AL, Tompkins RG. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993;104:890-895. [PubMed] [Cited in This Article: ] |
| 18. | Muhs BE, Patel S, Yee H, Marcus S, Shamamian P. Inhibition of matrix metalloproteinases reduces local and distant organ injury following experimental acute pancreatitis. J Surg Res. 2003;109:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Hietaranta A, Mustonen H, Puolakkainen P, Haapiainen R, Kemppainen E. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-kappaB. Biochem Biophys Res Commun. 2004;323:192-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117419] [Cited by in F6Publishing: 122062] [Article Influence: 5307.0] [Reference Citation Analysis (0)] |
| 21. | Lerch MM, Lutz MP, Weidenbach H, Müller-Pillasch F, Gress TM, Leser J, Adler G. Dissociation and reassembly of adherens junctions during experimental acute pancreatitis. Gastroenterology. 1997;113:1355-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | D'Souza T, Sherman-Baust CA, Poosala S, Mullin JM, Morin PJ. Age-related changes of claudin expression in mouse liver, kidney, and pancreas. J Gerontol A Biol Sci Med Sci. 2009;64:1146-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Comper F, Antonello D, Beghelli S, Gobbo S, Montagna L, Pederzoli P, Chilosi M, Scarpa A. Expression pattern of claudins 5 and 7 distinguishes solid-pseudopapillary from pancreatoblastoma, acinar cell and endocrine tumors of the pancreas. Am J Surg Pathol. 2009;33:768-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140-G1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 353] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 25. | Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Meriläinen S, Mäkelä J, Anttila V, Koivukangas V, Kaakinen H, Niemelä E, Ohtonen P, Risteli J, Karttunen T, Soini Y. Acute edematous and necrotic pancreatitis in a porcine model. Scand J Gastroenterol. 2008;43:1259-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 806] [Cited by in F6Publishing: 838] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 29. | Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Baty C, Beer-Stolz D, Guo F, Murray SA, Hackam DJ. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132:2395-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |