Published online Apr 28, 2012. doi: 10.3748/wjg.v18.i16.1884
Revised: December 20, 2011
Accepted: December 31, 2011
Published online: April 28, 2012
AIM: To investigate the effects of antithrombin III (AT III) injection via the portal vein in acute liver failure.
METHODS: Thirty rats were intraperitoneally challenged with lipopolysaccharide (LPS) and D-galactosamine (GalN) and divided into three groups: a control group; a group injected with AT III via the tail vein; and a group injected with AT III via the portal vein. AT III (50 U/kg body weight) was administrated 1 h after challenge with LPS and GalN. Serum levels of inflammatory cytokines and fibrin degradation products, hepatic fibrin deposition, and hepatic mRNA expression of hypoxia-related genes were analyzed.
RESULTS: Serum levels of alanine aminotransferase, tumor necrosis factor-α and interleukin-6 decreased significantly following portal vein AT III injection compared with tail vein injection, and control rats. Portal vein AT III injection reduced liver cell destruction and decreased hepatic fibrin deposition. This treatment also significantly reduced hepatic mRNA expression of lactate dehydrogenase and heme oxygenase-1.
CONCLUSION: A clinically acceptable dose of AT III injection into the portal vein suppressed liver damage, probably through its enhanced anticoagulant and anti-inflammatory activities.
-
Citation: Miyazaki M, Kato M, Tanaka M, Tanaka K, Takao S, Kohjima M, Ito T, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Antithrombin III injection
via the portal vein suppresses liver damage. World J Gastroenterol 2012; 18(16): 1884-1891 - URL: https://www.wjgnet.com/1007-9327/full/v18/i16/1884.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i16.1884
In some patients with acute liver injury (ALI), the liver disease proceeds to acute liver failure (ALF); a severe condition associated with a high mortality rate. Liver transplantation is an effective treatment for patients with severe ALF[1], whereas artificial liver support systems such as plasma exchange and hemodiafiltration are less effective[2,3]. The difficulties associated with the development of effective treatments for ALF may be attributed to the incomplete understanding of the mechanisms involved in disease progression.
ALF is pathologically characterized by massive hepatocellular necrosis, therefore, intrahepatic microcirculatory disturbances are involved in the pathogenesis and progression of liver disease. Observation of sinusoidal fibrin deposition, increased fibrinogen catabolism and decreased platelet counts suggest that the intrahepatic coagulation system may be activated in ALF, and the following microcirculatory disturbances may play a role in the formation of massive hepatocellular necrosis[4]. The hypothesis that activation of the intrahepatic coagulation system is a fundamental pathogenic factor underlying the development of ALF has prompted researchers to develop new treatments using anticoagulants in experimental animal models and in clinical trials. Intravenous injection of antithrombin III (AT III), which inhibits serine proteases involved in the coagulation cascade, has been reported to attenuate the progression of liver disease in animal models of ALF induced by concanavalin A, dimethylnitrosamine (DMN) and endotoxin[5-8]. However, the need for high doses of AT III (200-400 U/kg body weight) to suppress liver damage makes it difficult to apply this treatment in clinical practice. Therefore, the development of regimens using clinically acceptable doses of AT III is necessary.
It has been shown that direct drug delivery into the target organs is more efficient than systemic administration. Direct delivery of 5-fluorouracil and cisplatin into the hepatic artery has been reported to control tumor progression and to extend the median survival time of patients with unresectable hepatocellular carcinoma, which is resistant to systemic chemotherapy[9]. Similarly, we have reported that the progression of severe ALI toward fulminant liver failure is inhibited by transcatheter arterial steroid injection, in which methylprednisolone is directly delivered into the diseased liver via the hepatic artery[10]. The effectiveness of direct steroid delivery into the liver has been confirmed in an experimental animal model. Injection of steroids via the portal vein in rats with lipopolysaccharide (LPS)- and D-galactosamine (GalN)-induced ALF more effectively suppresses hepatic inflammation and improves survival than injection via the tail vein[11]. These observations suggest that the direct delivery of AT III into the liver, via the hepatic artery or portal vein, may improve liver damage more effectively than peripheral injection of AT III.
In this study, we administered a clinically acceptable dose of AT III via the portal vein or a peripheral vein (tail vein) in rats with LPS/GalN-induced ALF. The suppressive effects of AT III on hepatic inflammation were estimated based on the serum levels of transaminase and inflammatory cytokines, and hepatic histology. The extent of damage to the intrahepatic coagulation system was estimated by determining sinusoidal fibrin deposition. Hypoxia in the diseased liver, which is caused by hepatic microcirculatory disturbances, was estimated by analyzing the hepatic mRNA expression of hypoxia-related genes. These parameters were compared among three groups: a control group; rats injected with AT III via the tail vein; and rats injected via the portal vein. Our observations suggest that the injection of AT III via the portal vein suppresses liver damage more effectively than via the tail vein, because of its enhanced anticoagulant and anti-inflammatory activities.
Human concentrated AT III (Anthrobin P500) was purchased from CSL Bering (King of Prussia, PA, United States). LPS (Escherichia coli, 055:B5), GalN and other chemicals were purchased from Sigma (St. Louis, MO, United States). All experiments were performed using the same lot of LPS.
Eight-week-old male Wistar rats weighing 200 g were purchased from Japan SLC (Hamamatsu, Japan). Rats were maintained under controlled conditions with free access to standard chow and water. All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health) and approved by the Animal Care Committee of Kyushu University.
LPS (5 μg/kg body weight) and GalN (500 mg/kg body weight) dissolved in 500 μL phosphate buffered solution (PBS) were injected intraperitoneally into rats. One hour after the injections, the animals were anesthetized with pentobarbital sodium, and AT III (50 U/kg body weight) dissolved in 200 μL of PBS was injected into the portal or tail vein. Control animals underwent sham injections. Each group consisted of 10 rats.
Blood samples were taken from the tail vein at 6 h, 12 h and 24 h after injection of LPS and GalN. The serum levels of alanine aminotransferase (ALT) were estimated by Transaminase C-test (Wako Pure Chemical Industry, Osaka, Japan). Tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-6 (IL-6) were measured using enzyme linked immunosorbent assay (ELISA) kits (Endogen, Rockford, IL, United States).
Blood samples were taken from the tail vein 24 h after injection of LPS and GalN. Serum fibrin degradation products (FDPs) levels were measured using an ELISA kit (Cusabio Biotech, Barksdale, DE, United States).
Liver tissue samples were collected 24 h after injecting LPS and GalN, fixed in 10% formalin, and embedded in paraffin. The sections were stained with hematoxylin and eosin to assess hepatic damage. To determine intrasinusoidal fibrin deposition, the sections were stained with phosphotungstic acid-hematoxylin[12].
Total RNA from liver tissue was prepared with TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and cDNA was synthesized from 1.0 μg RNA by GeneAmp RNA polymerase chain reaction (PCR) (Applied Biosystems, Branchburg, NJ, United States) using random hexamers. Real-time PCR was performed using LightCycler FastStart DNA Master SYBR Green I (Roche, Basel, Switzerland). The reaction mixture (20 μL) contained Master SYBR Green I, 4 mmol MgCl2, 0.5 μmol upstream and downstream PCR primers, and 2 μL first-strand cDNA as a template. To control variations in reactions, all PCR data were normalized against glyceraldehyde 3-phosphate dehydrogenase expression. The forward and reverse PCR primers were 5’-ACTTTCAGAAGGGTCAGGTGTCC-3’ and 5’-TTGAGCAGGAAGGCGGTCTTAG-3’, respectively, for heme oxygenase-1 (HO-1) and 5’-AGACTGCCGTCCCGAACAAC-3’ and 5’-ACATCCACCAGGGCAAGCTC-3’, respectively, for lactate dehydrogenase (LDH), respectively.
All results are expressed as means ± SD. Significant differences between two groups were assessed using Wilcoxon’s rank-sum test. A value of P < 0.05 was considered to be statistically significant.
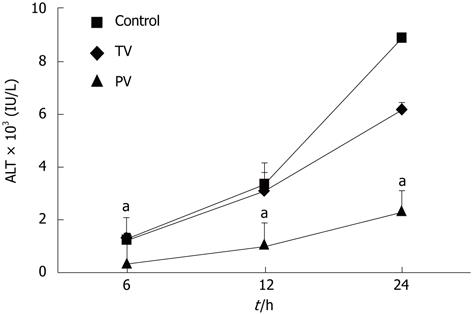
In the control group, the serum levels of ALT increased over time, reaching 1262 ± 240, 3381 ± 808 and 8906 ± 766 U/L (Figure 1) at 6, 12 and 24 h, respectively. Injection of AT III into the tail vein did not affect ALT levels at 6 h or 12 h after the injection of LPS and GalN. However, at 24 h, the ALT levels in the tail vein injection group were significantly lower than those in the control group (8906 ± 766 U/L vs 6181 ± 823 U/L, P < 0.01). This suggests that the suppressive effects of AT III injected via the tail vein may be limited to the late stage of liver disease. In contrast, in rats injected with AT III via the portal vein, ALT levels were reduced during the early stage (i.e., 6 h, 369 ± 141 U/L), which was maintained at all time-points. At 24 h, the ALT levels in this group were significantly lower than those in the control group (2352 ± 760 U/L vs 8906 ± 766 U/L, P < 0.01).
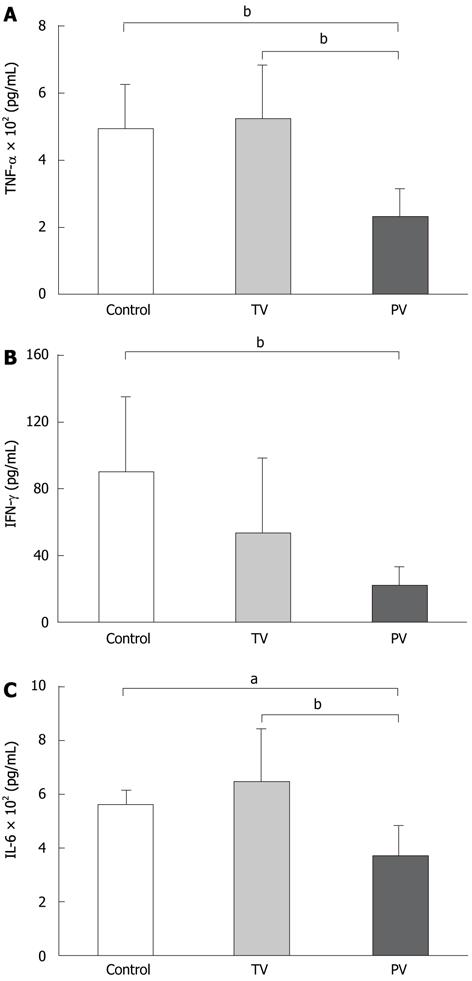
To support the effects of these treatments on the suppression of liver damage, the serum levels of inflammatory cytokines were measured. The cytokine levels demonstrate the greater anti-inflammatory effects of AT III injected via the portal vein. TNF-α levels in the tail vein injection group were similar to those in the control group. In contrast, TNF-α levels in the portal vein injection group were significantly lower than those in the control group (235 ± 79 pg/mL vs 500 ± 127 pg/mL, P < 0.01, Figure 2A). AT III injection via the tail vein reduced the IFN-γ levels compared with the controls, but the difference was not significant. As with other cytokines, IFN-γ was significantly reduced in the portal vein group compared with the control group (21 ± 12 pg/mL vs 89 ± 45 pg/mL, P < 0.05, Figure 2B). The IL-6 levels showed similar trends to those observed for TNF-α. AT III injection via the tail vein did not suppress IL-6 levels, whereas its injection via the portal vein significantly reduced IL-6 levels compared with those in the control group (368 ± 120 pg/mL vs 572 ± 47 pg/mL, P < 0.01, Figure 2C).
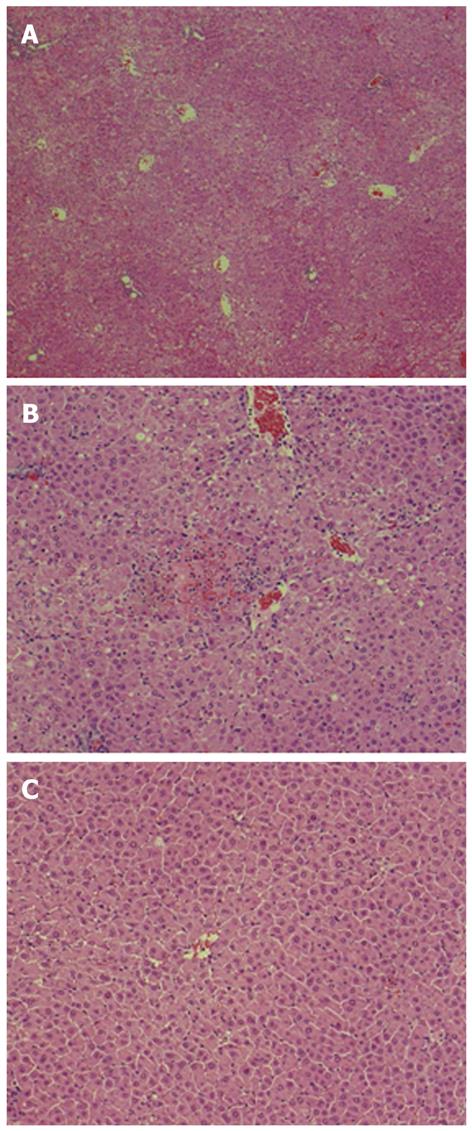
Histological examination showed extensive hepatocellular necrosis and hemorrhaging in the control liver (Figure 3A). In tail-vein-injected rats, extensive hepatocellular necrosis was not found but areas with confluent necrosis were scattered throughout the liver (Figure 3B). Consistent with the suppression of ALT and inflammatory cytokine levels, prominent histological improvement was noted in the liver of rats injected with AT III via the portal vein, because relatively few, scattered areas of necrosis with disordered hepatic cords were observed (Figure 3C).
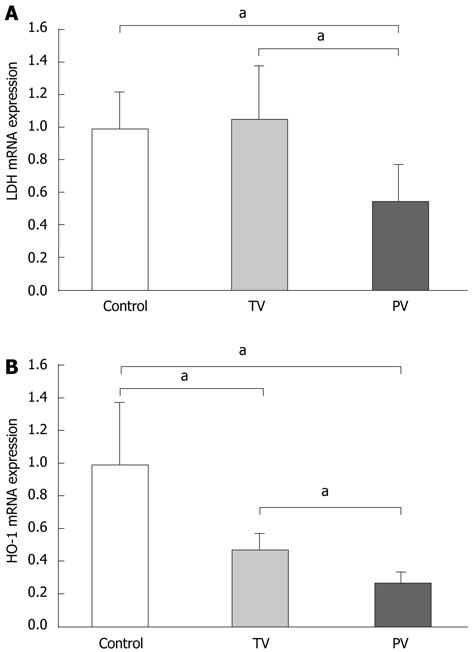
To evaluate the extent of hepatic hypoxia, as induced by microcirculatory disturbances, we determined the hepatic expression of hypoxia-related genes such as LDH and HO-1. LDH is an essential enzyme for anaerobic respiration, and its expression increases in cells exposed to hypoxia[13-15]. The transcriptional expression of HO-1 is also increased in hypoxia, resulting in increased production of carbon monoxide, a vasodilator, and bilirubin, an antioxidant[16,17]. Increased serum LDH levels and hepatic expression of HO-1 have been reported in patients with ALF and might be useful to predict prognosis[18,19]. Therefore, we speculated that the expression of these genes could reflect the extent of hypoxia in the diseased liver. Tail vein AT III injection did not affect the expression of LDH, suggesting that peripheral administration of AT III does not improve hepatic hypoxia. In contrast, LDH expression was significantly reduced in rats injected with AT III via the portal vein compared with the tail vein, and the control group (Figure 4A). The expression patterns of HO-1 differed from those of LDH. AT III injection via the tail vein significantly reduced HO-1 expression levels compared with those in the control group, and its expression was further reduced by AT III injection via the portal vein (Figure 4B). These observations suggest that AT III injection via the portal vein reduces intrahepatic hypoxia, probably by controlling the microcirculatory disturbances.
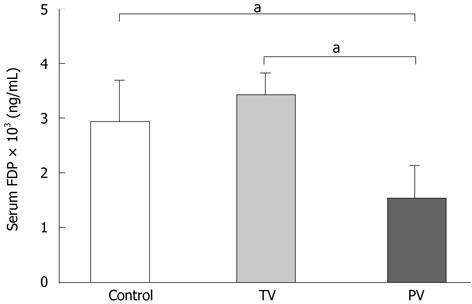
Serum levels of FDPs are a marker for the extent of deterioration in the coagulation system in ALF[20,21]. Injection of AT III via the tail vein did not change FDP levels, whereas injection of AT III via the portal vein significantly reduced FDP levels compared with those in the control and tail vein groups (Figure 5). Taken together, improvements in the coagulation system were only achieved by injecting AT III directly into the diseased liver.
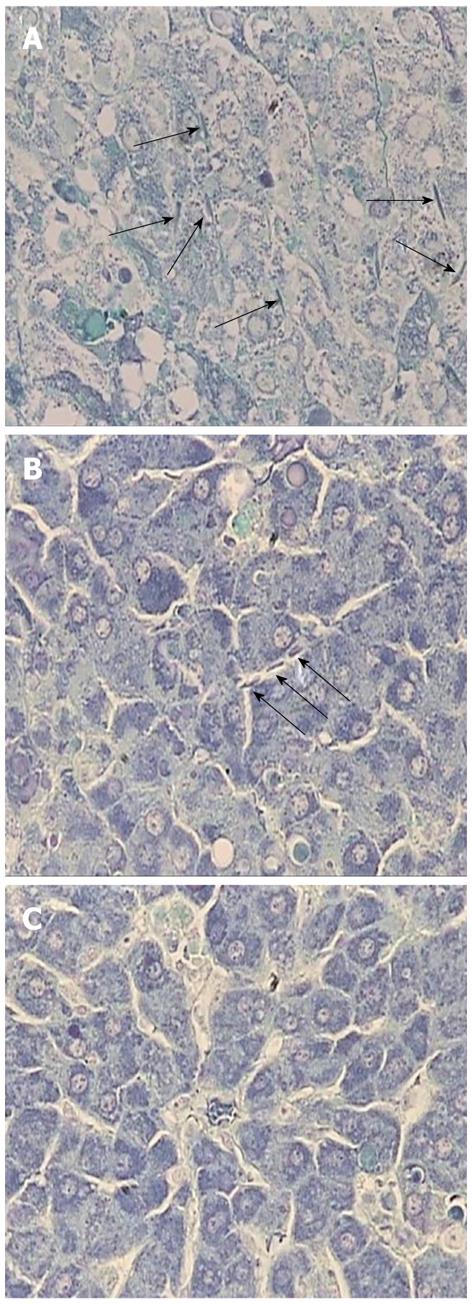
Fibrin deposition in hepatic sinusoids has been observed in ALF. It is recognized as a manifestation of disturbances in the intrahepatic coagulation system and is mainly caused by sinusoidal endothelial cell injury[22]. In our study, phosphotungstic acid-hematoxylin staining revealed that fibrin was diffusely deposited in the sinusoids in the control liver, suggesting intrahepatic coagulation (Figure 6A). In rats treated with AT III via the tail vein, hepatic fibrin deposition was reduced but it was still sparsely distributed (Figure 6B). Meanwhile, injection of AT III via the portal vein diminished fibrin deposition in the liver parenchyma, which suggests that it may affect the maintenance of the intrahepatic coagulation system (Figure 6C).
We demonstrated that directly injecting AT III into the portal vein improved liver damage in a rat model of ALF induced by LPS and GalN. AT III injection via the portal vein suppressed the increases in serum ALT and inflammatory cytokine levels, and intrahepatic fibrin deposition, and reduced the mRNA expression of hypoxia-related genes associated with ALF. These effects were accomplished by administering a relatively low dose of AT III that should be acceptable in clinical practice.
Microcirculatory disturbances are involved in the pathogenesis of ALF. Activation of the inflammatory cascade may affect the coagulation system and the resulting intrahepatic coagulation may worsen sinusoidal blood flow, leading to massive liver necrosis; a histological feature of ALF[23]. Activation of macrophages, as represented by the elevated serum levels of CD163 and osteopontin in ALF patients, seems to be a trigger for this inflammatory process[24]. Following the onset of inflammation, disturbances in the intrahepatic coagulation system may enhance the destruction of liver cells. In patients with ALF, electron microscopy has revealed disorders in sinusoidal endothelial cells (SECs)[25]. Moreover, reduced mRNA expression of anticoagulants such as tissue factor pathway inhibitor and thrombomodulin are observed in SECs. Following the activation of tissue factor, intrahepatic coagulation causes sinusoidal fibrin deposition and thus restricts blood circulation in the diseased liver[26-28].
Based on these observations, AT III treatment has been tested in animal models of ALF because of its anticoagulant activity. Systemic injection of AT III (500 U/kg body weight) prevented Con-A-induced liver injury by inhibiting macrophage inflammatory protein-2 release and endothelial cell production of prostacyclin[7]. Meanwhile, a similar dose of AT III (400 U/kg body weight) reduced liver damage in an ALF model with coagulopathy induced by DMN, which was characterized by marked intrasinusoidal fibrin deposition and elevated serum fibrin monomer complexes[5]. However, large doses of AT III, which are unacceptable in clinical practice, were used to suppress liver injury in these experimental models.
The effects of AT III for the treatment of ALF patients seem to be limited. Fujiwara et al[29] treated 26 patients with fulminant hepatic failure with daily infusion of 3000 U AT III. Notably, survival time was longer in patients with plasma AT III levels within the normal range compared with levels beyond the normal range. However, the survival rates were not significantly different between patients treated with AT III and control patients. Another research group treated 13 ALF patients with 3000 U AT III, followed by a further 1000 U every 6 h. However, survival time was not improved by AT III and the extent of intravascular coagulation was similar between AT-III-treated and control patients[30]. These different outcomes of clinical trials and animal experiments might be due to the insufficient concentration of AT III in the patients’ liver. Indeed, the relative doses of AT III per body weight used in patients were < 10% of those used in animals. Moreover, even though the plasma concentrations of AT III were maintained within the normal ranges, the survival rate was not improved, which suggests that the intrahepatic concentration of AT III did not reach the levels needed to maintain the integrity of the coagulation system in the diseased liver because of impaired sinusoidal circulation.
From our previous experience of treating ALF rats with methylprednisolone, we have demonstrated that direct delivery of steroid into the liver suppresses liver damage more effectively than does systemic injection. We found that injecting methylprednisolone via the portal vein significantly increased the survival rate, reduced serum cytokine levels, and decreased the number of apoptotic liver cells compared with tail vein injection[11]. Therefore, we speculated that the anticoagulant activity of AT III would be more effective when injected via the portal vein than via a peripheral vein. In our study, significant reductions in the serum FDP levels and fibrin deposition were only observed in rats injected with AT III via the portal vein, suggesting that direct drug delivery is necessary to achieve therapeutic concentrations of AT III in the diseased liver. Of particular interest is that, using this method, we reduced the dose of AT III to levels acceptable for clinical practice.
Anti-inflammatory activities of AT III have been reported in addition to its anticoagulant activity. In septic patients, AT III improves lung injury by suppressing the production of inflammatory cytokines, and prevents liver and kidney failure[31,32]. The mechanisms involved in the anti-inflammatory activities of AT III have been analyzed in rats treated with endotoxin[33]. AT III prevents pulmonary vascular injury by inhibiting leukocyte activation mediated by the enhanced release of prostacyclin from endothelial cells. Additionally, AT III has been reported to inhibit the activation of inflammatory signaling cascades in several cell types, including the activation of nuclear factor (NF)-κB in human monocytes and vascular endothelial cells[34]; the production of TNF-α and IL-6 in LPS-stimulated murine macrophages[35]; and human neutrophil migration[36]. In our study, portal vein injection of AT III significantly reduced serum TNF-α, IFN-γ and IL-6 levels compared with tail vein injection, and the control group. These results support two possible actions of AT III injected via the portal vein to suppress inflammation: (1) the anti-inflammatory activity of AT III was enabled because the tissue concentration reached effective levels following direct drug delivery; and (2) the reduced liver cell destruction mediated by the anticoagulant activity of AT III suppressed the activation of surrounding inflammatory cells. We are currently unable to postulate which action of AT III might be dominant; however, it seems reasonable to suggest that the anticoagulant and anti-inflammatory activities of AT III may act together to suppress tissue inflammation.
In patients with ALF, it has been reported that microcirculatory disturbances induce hypoxia in the liver[18,19]. Increased serum LDH levels and hepatic HO-1 expression are markers for the extent of hypoxia, reflecting the damage to the hepatic microcirculation. In this study, serum LDH levels were significantly reduced in rats injected with AT III via the portal vein, whereas hepatic HO-1 expression was decreased in both groups of rats injected with AT III compared with the control group. Expression of LDH and HO-1 are induced by hypoxia-inducible factor-1, therefore, the different expression patterns of these genes are unlikely to be due to hypoxia-mediated transcriptional regulation[37]. In contrast, the expression of HO-1 is transactivated by activator protein-1 and NF-κB, which are transcriptional factors that can activate various inflammatory signals[38]. In this context, we speculate that the reduced HO-1 expression in rats injected with AT III via the tail vein may partly reflect decreased hepatic inflammation; however, the hypoxia may only be improved by injecting AT III via the portal vein.
In conclusion, we demonstrated that injecting AT III via the portal vein suppressed liver damage in a rat model of ALF. The increased concentration of AT III in the diseased liver following direct drug delivery might enhance its anticoagulant and anti-inflammatory activities. Furthermore, the dose of AT III used in this method was < 10% of that used in previous studies where AT III was injected via peripheral veins. We believe that further studies are needed to establish this method as an effective treatment for ALF.
Acute liver damage occasionally progresses to acute liver failure (ALF) with extremely high mortality. Liver transplantation is the only effective treatment for patients with ALF. Plasma exchange and hemodiafiltration have been used as artificial liver support systems for affected patients but are only partially effective.
Intravascular coagulation is thought to be involved in the pathogenesis of ALF. Anticoagulation therapy using antithrombin (AT) III effectively suppresses liver damage in experimental models of ALF; however, extremely high doses of AT III (200-500 U/kg body weight) are necessary. In this study, the authors demonstrated that injection of AT III into the portal vein may help to improve the efficiency of AT III compared with injected into peripheral vein.
The authors found that injection of AT III via the portal vein showed superior effects to those achieved by tail vein injection in terms of lowering the serum levels of transaminase and inflammatory cytokines, reducing damage to the intrahepatic coagulation system, and improving hypoxia in the diseased liver. A clinically acceptable dose of AT III injected via the portal vein suppressed liver damage, therefore, direct delivery of AT III into the diseased liver could enhance the anticoagulant and anti-inflammatory activities of AT III.
The development of injection of AT III via the portal vein might be useful to enhance the effects of AT III and ultimately improve the outcomes of patients with ALF, such as increasing the survival rate and reducing the number of patients who need liver transplantation.
AT III inhibits serine proteases involved in the coagulation cascade. Additionally, AT III is reported to inhibit activation of inflammatory signaling cascades, including the activation of nuclear factor-κB, production of tumor necrosis factor-α and interleukin-6 in various cell types.
The manuscript is clearly written and the authors discuss their findings in an adequate way. Moreover, the reduction of the dose of AT III via injection in the portal vein has clinical implications.
Peer reviewer: Dr. Georg Alexander Roth, Medical University of Vienna, General Anesthesia and Critical Care, Waehringer Guertel 18-20, Level 9I, Vienna A-1090, Austria
S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
| 1. | Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Hakala TR, Rosenthal JT. Evolution of liver transplantation. Hepatology. 1982;2:614-636. [PubMed] [Cited in This Article: ] |
| 2. | Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis. 2008;28:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Rake MO, Flute PT, Pannell G, Williams R. Intravascular coagulation in acute hepatic necrosis. Lancet. 1970;1:533-537. [PubMed] [Cited in This Article: ] |
| 5. | Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, Sato Y, Masaki N, Oka H. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103-1108. [PubMed] [Cited in This Article: ] |
| 6. | Mochida S, Ogata I, Hirata K, Ohta Y, Yamada S, Fujiwara K. Provocation of massive hepatic necrosis by endotoxin after partial hepatectomy in rats. Gastroenterology. 1990;99:771-777. [PubMed] [Cited in This Article: ] |
| 7. | Nakamura K, Ito T, Yoneda M, Takamoto S, Nakade Y, Okamoto S, Okada M, Yokohama S, Aso K, Makino I. Antithrombin III prevents concanavalin A-induced liver injury through inhibition of macrophage inflammatory protein-2 release and production of prostacyclin in mice. J Hepatol. 2002;36:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Yamada S, Ogata I, Hirata K, Mochida S, Tomiya T, Fujiwara K. Intravascular coagulation in the development of massive hepatic necrosis induced by Corynebacterium parvum and endotoxin in rats. Scand J Gastroenterol. 1989;24:293-298. [PubMed] [Cited in This Article: ] |
| 9. | Lin CP, Yu HC, Cheng JS, Lai KH, Lo GH, Hsu PI, Lin CK, Chen HH, Lo CC, Liang HL. Clinical effects of intra-arterial infusion chemotherapy with cisplatin, mitomycin C, leucovorin and 5-flourouracil for unresectable advanced hepatocellular carcinoma. J Chin Med Assoc. 2004;67:602-610. [PubMed] [Cited in This Article: ] |
| 10. | Kotoh K, Enjoji M, Nakamuta M, Yoshimoto T, Kohjima M, Morizono S, Yamashita S, Horikawa Y, Yoshimitsu K, Tajima T. Arterial steroid injection therapy can inhibit the progression of severe acute hepatic failure toward fulminant liver failure. World J Gastroenterol. 2006;12:6678-6682. [PubMed] [Cited in This Article: ] |
| 11. | Higuchi N, Kato M, Kotoh K, Kohjima M, Aishima S, Nakamuta M, Fukui Y, Takayanagi R, Enjoji M. Methylprednisolone injection via the portal vein suppresses inflammation in acute liver failure induced in rats by lipopolysaccharide and d-galactosamine. Liver Int. 2007;27:1342-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Puchtler H, Waldrop FS, Meloan SN. On the mechanism of Mallory's phosphotungstic acid-haematoxylin stain. J Microsc. 1980;119:383-390. [PubMed] [Cited in This Article: ] |
| 13. | Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci USA. 1994;91:6496-6500. [PubMed] [Cited in This Article: ] |
| 14. | Kay HH, Zhu S, Tsoi S. Hypoxia and lactate production in trophoblast cells. Placenta. 2007;28:854-860. [PubMed] [Cited in This Article: ] |
| 15. | Shi LB, Huang JH, Han BS. Hypoxia inducible factor-1alpha mediates protective effects of ischemic preconditioning on ECV-304 endothelial cells. World J Gastroenterol. 2007;13:2369-2373. [PubMed] [Cited in This Article: ] |
| 16. | Kourembanas S. Hypoxia and carbon monoxide in the vasculature. Antioxid Redox Signal. 2002;4:291-299. [PubMed] [Cited in This Article: ] |
| 17. | Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553-555. [PubMed] [Cited in This Article: ] |
| 18. | Kotoh K, Enjoji M, Kato M, Kohjima M, Nakamuta M, Takayanagi R. A new parameter using serum lactate dehydrogenase and alanine aminotransferase level is useful for predicting the prognosis of patients at an early stage of acute liver injury: a retrospective study. Comp Hepatol. 2008;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Fujii H, Takahashi T, Matsumi M, Kaku R, Shimizu H, Yokoyama M, Ohmori E, Yagi T, Sadamori H, Tanaka N. Increased heme oxygenase-1 and decreased delta-aminolevulinate synthase expression in the liver of patients with acute liver failure. Int J Mol Med. 2004;14:1001-1005. [PubMed] [Cited in This Article: ] |
| 20. | Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, Roth RA. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46:1177-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Ohta S, Nakamuta M, Fukushima M, Kohjima M, Kotoh K, Enjoji M, Nawata H. Beraprost sodium, a prostacyclin (PGI) analogue, ameliorates concanavalin A-induced liver injury in mice. Liver Int. 2005;25:1061-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Seto S, Kaido T, Yamaoka S, Yoshikawa A, Arii S, Nakamura T, Niwano M, Imamura M. Hepatocyte growth factor prevents lipopolysaccharide-induced hepatic sinusoidal endothelial cell injury and intrasinusoidal fibrin deposition in rats. J Surg Res. 1998;80:194-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Mochida S, Arai M, Ohno A, Yamanobe F, Ishikawa K, Matsui A, Maruyama I, Kato H, Fujiwara K. Deranged blood coagulation equilibrium as a factor of massive liver necrosis following endotoxin administration in partially hepatectomized rats. Hepatology. 1999;29:1532-1540. [PubMed] [Cited in This Article: ] |
| 24. | Matsui A, Mochida S, Ohno A, Nagoshi S, Hirose T, Fujiwara K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatol Res. 2004;29:202-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Le Bail B, Bioulac-Sage P, Senuita R, Quinton A, Saric J, Balabaud C. Fine structure of hepatic sinusoids and sinusoidal cells in disease. J Electron Microsc Tech. 1990;14:257-282. [PubMed] [Cited in This Article: ] |
| 26. | Arai M, Mochida S, Ohno A, Ogata I, Obama H, Maruyama I, Fujiwara K. Blood coagulation equilibrium in rat liver microcirculation as evaluated by endothelial cell thrombomodulin and macrophage tissue factor. Thromb Res. 1995;80:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Hirata K, Ogata I, Ohta Y, Fujiwara K. Hepatic sinusoidal cell destruction in the development of intravascular coagulation in acute liver failure of rats. J Pathol. 1989;158:157-165. [PubMed] [Cited in This Article: ] |
| 28. | Yamanobe F, Mochida S, Ohno A, Ishikawa K, Fujiwara K. Recombinant human tissue factor pathway inhibitor as a possible anticoagulant targeting hepatic sinusoidal walls. Thromb Res. 1997;85:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Fujiwara K, Okita K, Akamatsu K, Abe H, Tameda Y, Sakai T, Inoue N, Kanai K, Aoki N, Oka H. Antithrombin III concentrate in the treatment of fulminant hepatic failure. Gastroenterol Jpn. 1988;23:423-427. [PubMed] [Cited in This Article: ] |
| 30. | Langley PG, Hughes RD, Forbes A, Keays R, Williams R. Controlled trial of antithrombin III supplementation in fulminant hepatic failure. J Hepatol. 1993;17:326-331. [PubMed] [Cited in This Article: ] |
| 31. | Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock. 1997;8:328-334. [PubMed] [Cited in This Article: ] |
| 32. | Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock. 1998;10:90-96. [PubMed] [Cited in This Article: ] |
| 33. | Okajima K, Uchiba M. The anti-inflammatory properties of antithrombin III: new therapeutic implications. Semin Thromb Hemost. 1998;24:27-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Oelschläger C, Römisch J, Staubitz A, Stauss H, Leithäuser B, Tillmanns H, Hölschermann H. Antithrombin III inhibits nuclear factor kappaB activation in human monocytes and vascular endothelial cells. Blood. 2002;99:4015-4020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. High dose antithrombin III inhibits HMGB1 and improves endotoxin-induced acute lung injury in rats. Intensive Care Med. 2008;34:361-367. [PubMed] [Cited in This Article: ] |
| 36. | Dunzendorfer S, Kaneider N, Rabensteiner A, Meierhofer C, Reinisch C, Römisch J, Wiedermann CJ. Cell-surface heparan sulfate proteoglycan-mediated regulation of human neutrophil migration by the serpin antithrombin III. Blood. 2001;97:1079-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 857] [Cited by in F6Publishing: 841] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 38. | Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479-491. [PubMed] [Cited in This Article: ] |