Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.677
Revised: September 9, 2010
Accepted: September 16, 2010
Published online: February 7, 2011
Recently, the use of confocal laser endomicroscopy (CLE) in the diagnosis of chronic ulcerative colitis (CUC) was reported. In this brief report we aimed to assess the application of probe-based CLE to characterize colonic mucosa and dysplasia in CUC. The study involved a patient presenting long-standing CUC. Confocal imaging of both the inflamed mucosa, a circumscribed lesion (dysplasia-associated lesional mass), and adjacent colonic mucosa are demonstrated and the correlation between the CLE and histological images. Inflamed mucosa and dysplasia showed specific alteration of crypt architecture, cellular infiltration, and vessel architecture with an excellent correlation between CLE and standard histological examination.
- Citation: Palma GDD, Staibano S, Siciliano S, Maione F, Siano M, Esposito D, Persico G. In vivo characterization of DALM in ulcerative colitis with high-resolution probe-based confocal laser endomicroscopy. World J Gastroenterol 2011; 17(5): 677-680
- URL: https://www.wjgnet.com/1007-9327/full/v17/i5/677.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i5.677
The term dysplasia-associated lesional mass (DALM) has been adopted to describe the group of endoscopically visible lesions, within the colitic colon (in the course of IBD), that refers to a heterogeneous population of lesions that demonstrate plaque-like, mass, stricture, sessile, or pedunculated morphology, that have an associated dysplasia in the surrounding mucosa[1-3].
With recent rapid advances in videoendoscopic instrument systems, improved endoscopic skills, and improved detection techniques such as pan-colonic dye spraying, the proportion of lesions that are discovered macroscopically is likely to increase[4].
Recently, the use of confocal laser endomicroscopy (CLE) in the diagnosis of chronic ulcerative colitis (CUC) was reported[5,6].
This report describes the CLE findings in a case of DALM in CUC in correlation with histopathology diagnosis.
A 48-year-old woman with a 23 years history of left-sided ulcerative colitis underwent a surveillance colonoscopy.
A mild anemia (hemoglobin level, 110 g/L) with low serum iron levels (210 μg/L); normal value, 53-167 μg/L) and a high erythrocyte sedimentation rate (37 mm/L per hour; normal value, 1-10 mm/L per hour) were the only blood chemistry abnormalities identified.
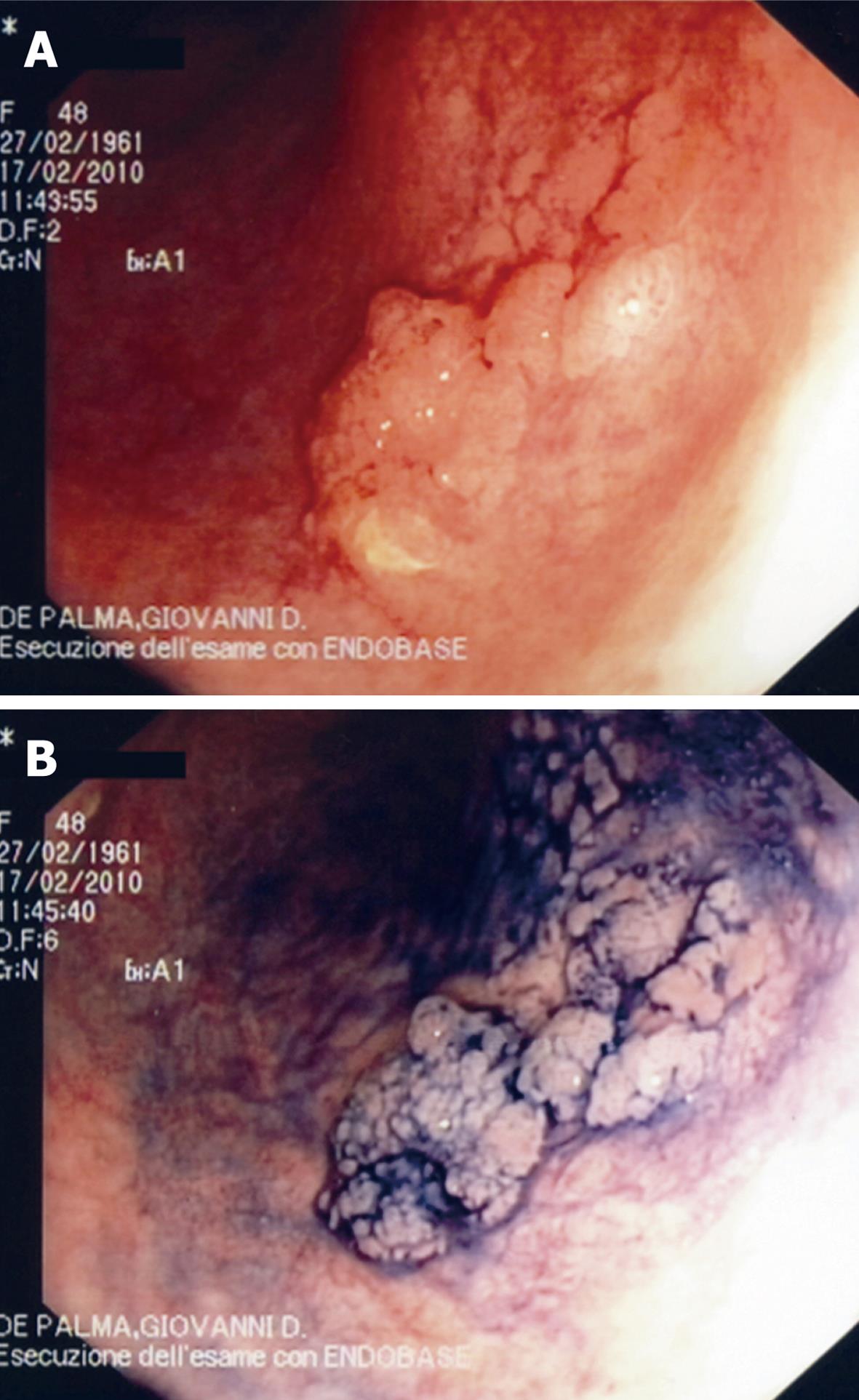
Colonoscopy revealed a left-sided Mayo CU-1 CUC with a 2 cm plaque-like lesion at the sigmoid colon (Figure 1A and B). A morphological characterization of the lesion with CLE was undertaken.
The procedure was performed using the Cellvizio® Endomicroscopy System (Mauna Kea Technologies, Paris, France) by a Coloflex UHD-type probe (1 μm lateral resolution; 12 frames/s).
This system uses a 2.5-mm catheter probe (Coloflex UHD-type probe) that is inserted through the endoscope-working channel to obtain dynamic imaging of the mucosa. This probe has a field of view of 240 μm × 200 μm, with a lateral resolution of 1 μm. Probe-based CLE (pCLE) imaging data were collected at a scan rate of 12 frames/s with a scanning field of 30 000 pixels. Single video frames were reconstructed into 1 larger static image (4 mm × 2 mm) by a special computer software (‘‘mosaicing’’ Mauna Kea Technologies).
Five mililiters of 10% sodium fluorescein were injected intravenously before CLE image acquisition as a contrast agent.
Confocal imaging of both the inflamed mucosa, the circumscribed lesion (DALM), and adjacent colorectal mucosa was performed by placing the tip of the probe in direct contact with the target tissue site.
Mucosal biopsy specimens were collected from the observation sites using biopsy forceps. Fixed samples were embedded in paraffin and sectioned transversely, and stained with hematoxylin-eosin to facilitate the comparison between confocal images and histology.
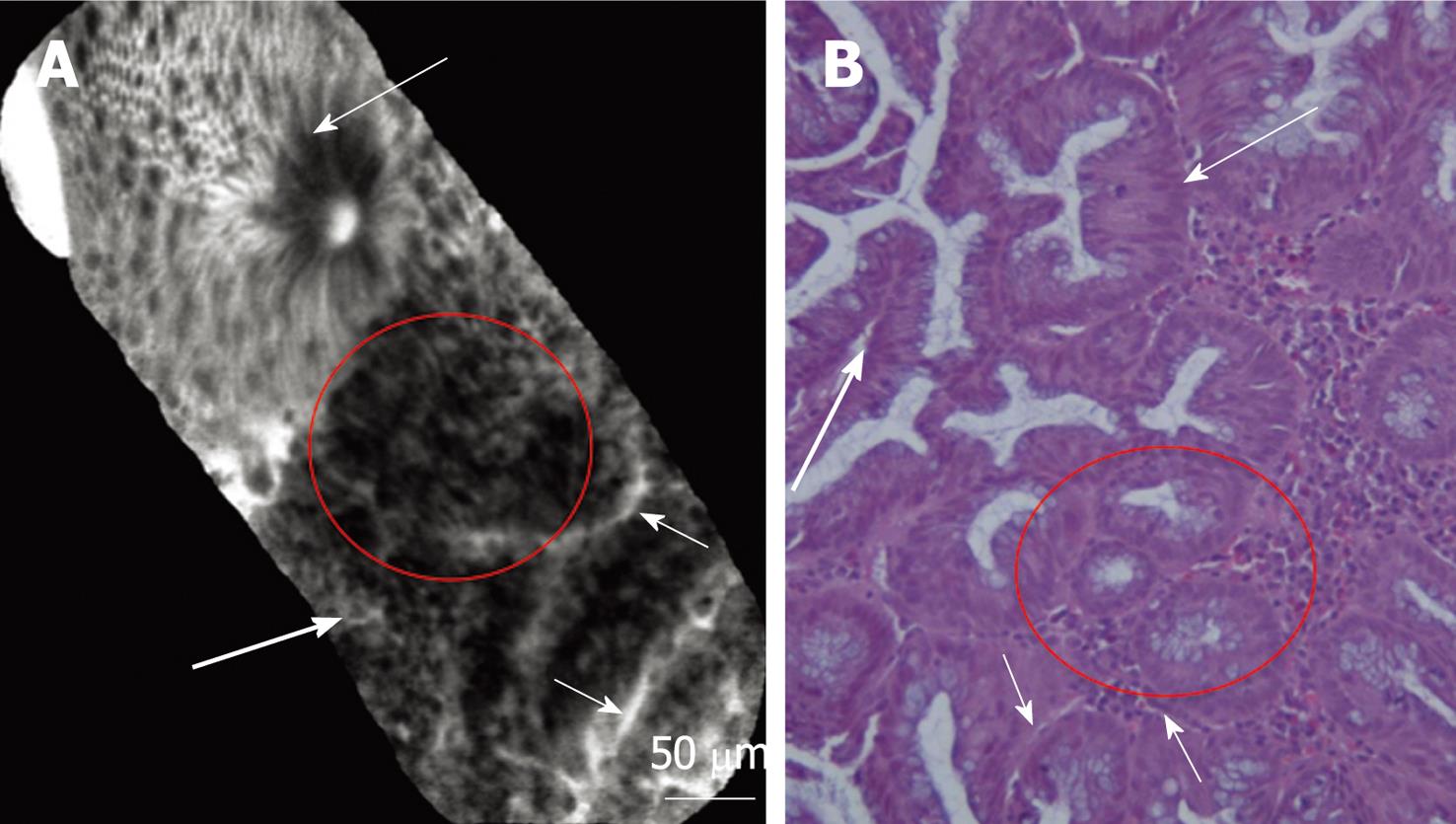
pCLE imaging of inflamed mucosa showed dilation of crypt openings, more irregular arrangement of crypts, and enlarged spaces between crypt, crypt destruction, and/or crypt fusion and crypt abscess. Microvascular alterations with fluorescein leaks into the crypt lumen (therefore making the lumen brighter than the surrounding epithelium) were observed (Figure 2A and B).
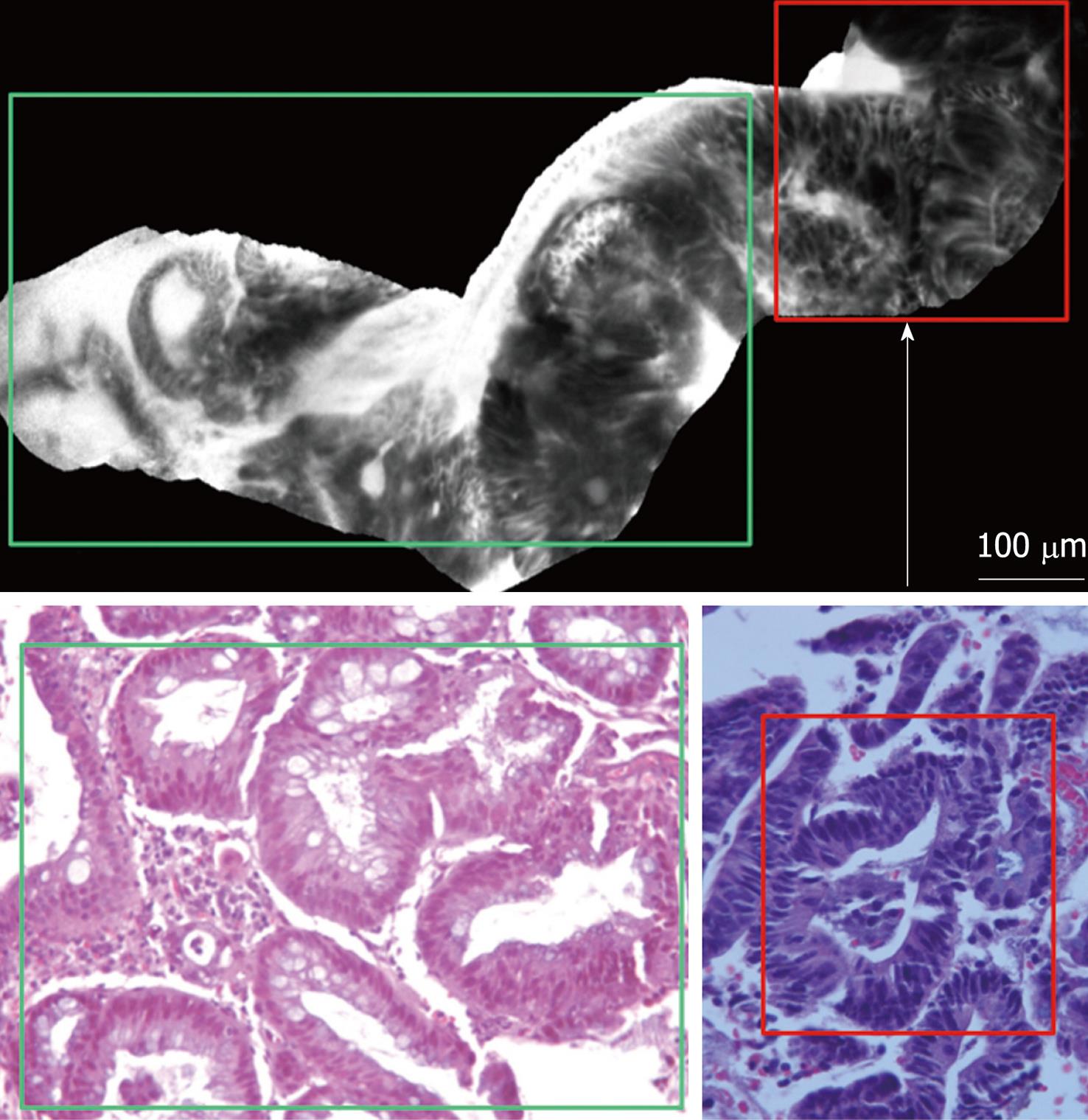
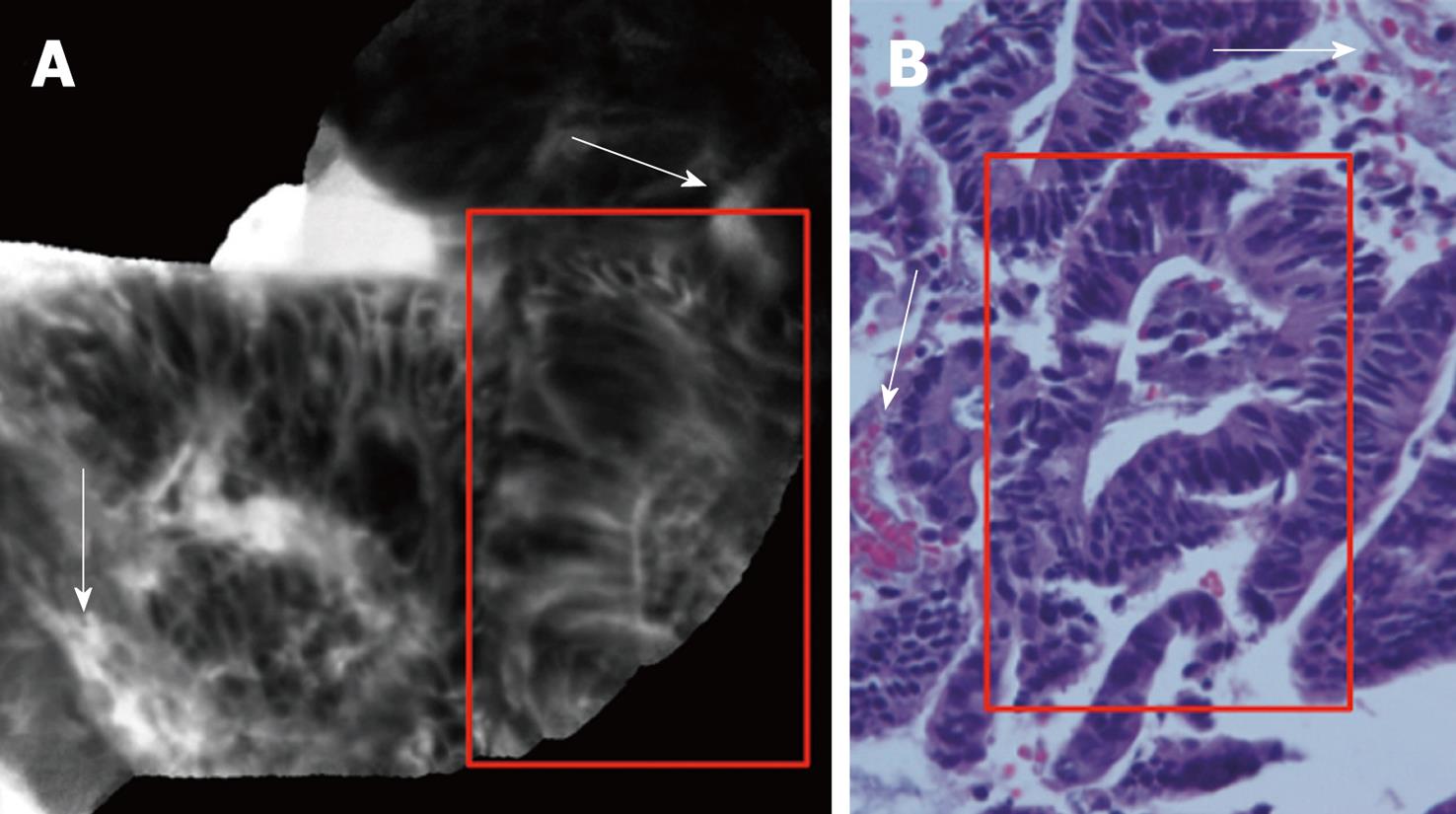
DALM was characterized by “dark” cells, with mucin depletion and goblet cell/crypt density attenuation; the architectural pattern was irregular, as well as the epithelial thickness, with villiform structures and “dark” epithelial border. The blood vessels were dilatated and irregularly-branching, with poor orientation to adjunct tissue, and fluorescein extravasation (Figure 3A and B).
pCLE imaging of colorectal mucosa adjacent to lesion (1 to 2 cm around DALM) showed the switch from the inflamed mucosa, to the neoplastic mucosa as evidenced by DALM (Figure 4).
CLE is a new technology that has enabled endoscopists to collect real-time in vivo histological images or “virtual biopsies” of the gastrointestinal mucosa during endoscopy.
CLE can be performed currently with 2 devices: one integrated into an endoscope (Pentax, Japan, herein termed eCLE) and one as a stand-alone probe (herein termed pCLE) capable of passage through the accessory channel of most endoscopes (Cellvizio, Mauna Kea Technologies, Paris, France)[7-9]. There are no data, at present, comparing pCLE with eCLE to demonstrate the superiority of any one system. pCLE has several advantages and disadvantages compared with eCLE. Advantages include the greater versatility of pCLE probes, which can be used in conjunction with virtually any endoscope (high-resolution endoscopes, NBI, cholangioscope, etc.), ad hoc usage (such as when a lesion is detected with a normal endoscope) and acquisition at video frame rate of 12 frames/s. allowing in vivo imaging of capillary flow. Disadvantages include a slightly lower resolution (approximately 1 μm compared with 0.7 μm for eCLE) and smaller field of view (240-600 μm).
Recently, the use of eCLE in the diagnosis of CUC was reported. Watanabe et al[5] and Li et al[6] reported on real-time inflammation activity assessment by CLE. The inflammation activity assessment includes crypt architecture, cellular infiltration, and vessel architecture. These studies evidenced that images taken with the CLE provided information that was equivalent to conventional histology, differentiating between active and non-active CUC patients during ongoing endoscopy.
Hurlstone et al[10] assessed the clinical applicability and predictive power of the CLE for the in vivo differentiation of ALM and DALM in CUC. The study evidenced that ALM and DALM can be differentiated with a high overall accuracy, enabling the safe selection of patients suitable for endoluminal resection vs immediate referral for surgery.
To the best of our knowledge, this is the first report that addressed the application of pCLE for the in vivo characterization of colonic mucosa and DALM in a patient with CUC during ongoing videocolonoscopy.
Our study showed that the pCLE system permits high-quality cellular, subsurface vascular and stromal imaging in vivo, with an excellent correlation between CLE and standard histopathologic examination for both inflammation and dysplasia in ulcerative colitis.
Post-acquisition specifically-developed software (“mosaicing”, Mauna Kea Technologies) was used to reconstitute the dynamic high-resolution pictures into a larger static image. By the use of mosaicing, the image area could be increased 2- to 4-fold, and image definition could be further enhanced to allow finer detail visualization. As a result of the large static image comprising of many single pictures of a video sequence, evaluation of the examination is easier and more efficient. Thereby, these features lead to an excellent correlation between CLE and standard histopathologic examination (Figures 1-4).
The main aspect of inflamed mucosa consisted of dilation of crypt openings, enlarged spaces between crypt, and microvascular alterations with fluorescein leaks into the crypt lumen (therefore making the lumen brighter than the surrounding epithelium); DALM showed typical neoplastic features of Mainz CLE criteria for prediction of intraepithelial neoplasia[11] (Figure 4).
In conclusion, this is the first study to address the novel applicability of pCLE for the in vivo characterization of mucosal inflammation and dysplasia in CUC. With appropriate training and careful patient selection, CLE imaging may become a suitable imaging modality in patients with CUC. The ability to target biopsies to areas suggestive of dysplasia in vivo allows rapid, highly accurate diagnosis “on table”, reducing inappropriate, non-significant histopathology.
Peer reviewer: Dr. Sanchoy Sarkar, Department of Gastroenterology, Royal Liverpool University Hospital, Prescot Street, Liverpool, L78XP, United Kingdom
S- Editor Sun H L- Editor Rutherford A E- Editor Zheng XM
| 1. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [Cited in This Article: ] |
| 2. | Leidenius M, Kellokumpu I, Husa A, Riihelä M, Sipponen P. Dysplasia and carcinoma in longstanding ulcerative colitis: an endoscopic and histological surveillance programme. Gut. 1991;32:1521-1525. [Cited in This Article: ] |
| 3. | Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800-806. [Cited in This Article: ] |
| 4. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [Cited in This Article: ] |
| 5. | Watanabe O, Ando T, Maeda O, Hasegawa M, Ishikawa D, Ishiguro K, Ohmiya N, Niwa Y, Goto H. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S286-S290. [Cited in This Article: ] |
| 6. | Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391-1396. [Cited in This Article: ] |
| 7. | De Palma GD. Confocal laser endomicroscopy in the "in vivo" histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770-5775. [Cited in This Article: ] |
| 8. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715-731. [Cited in This Article: ] |
| 9. | Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275-1283. [Cited in This Article: ] |
| 10. | Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235-1241. [Cited in This Article: ] |
| 11. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [Cited in This Article: ] |