Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.633
Revised: October 26, 2010
Accepted: November 2, 2010
Published online: February 7, 2011
AIM: To evaluate the natural history of human cytomegalovirus (HCMV) infection in a series of 28 ulcerative colitis patients in whom the search for HCMV was positive.
METHODS: A series of 85 patients with moderate-severe ulcerative colitis flare-up were evaluated for a HCMV search by performing a haematoxylin and eosin stain, immunohistochemical assay and nested polymerase chain reaction on rectal biopsies. Among 85 screened patients (19 of whom were steroid resistant/dependant), 28 were positive for HCMV; after remission the patients were followed up clinically and histologically.
RESULTS: Among the 22 patients with complete follow-up, in 8 (36%) patients HCMV-DNA persisted in the intestinal specimens. Among the HCMV positive patients, 4 (50%) experienced at least one moderate-severe flare-up of colitis without evidence of peripheral HCMV. Among the 14 HCMV negative patients, 3 with pouches developed pouchitis and 5 out of 11 (45%) experienced a colitis flare-up.
CONCLUSION: Our preliminary results suggest that HCMV may remain in the colon after an acute colitis flare-up despite remission; it seems that the virus is not responsible for the disease relapse.
- Citation: Criscuoli V, Rizzuto MR, Montalbano L, Gallo E, Cottone M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol 2011; 17(5): 633-638
- URL: https://www.wjgnet.com/1007-9327/full/v17/i5/633.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i5.633
The etiology of inflammatory bowel disease (IBD) is still unknown. The most current hypothesis is that IBD derives from an unsuitable and exaggerated immune response to normal mucosal resident bacterial microflora, likely induced by an external agent, in genetically predisposed individuals. Among the external agents, environmental factors unquestionably play a major role in the pathogenesis of IBD. Evidence of associations of bacterial factors derives from both human and animal studies. The virus takes an unclear part in the pathogenesis and disease development, but is probably involved.
In recent years several papers have described the link between viral infection and IBD onset, reactivation or steroid resistance[1-4].
No clear data is available on the natural history of HCMV infection superimposed on IBD. The following questions remain unanswered: (1) does the virus disappear after remission of acute colitis relapse? (2) does the persistence of the virus detected by sensitive assays increase the risk of relapse?
The aim of this study was an attempt to answer the questions raised above and to evaluate the natural history of human cytomegalovirus (HCMV) infection after a severe colitis exacerbation.
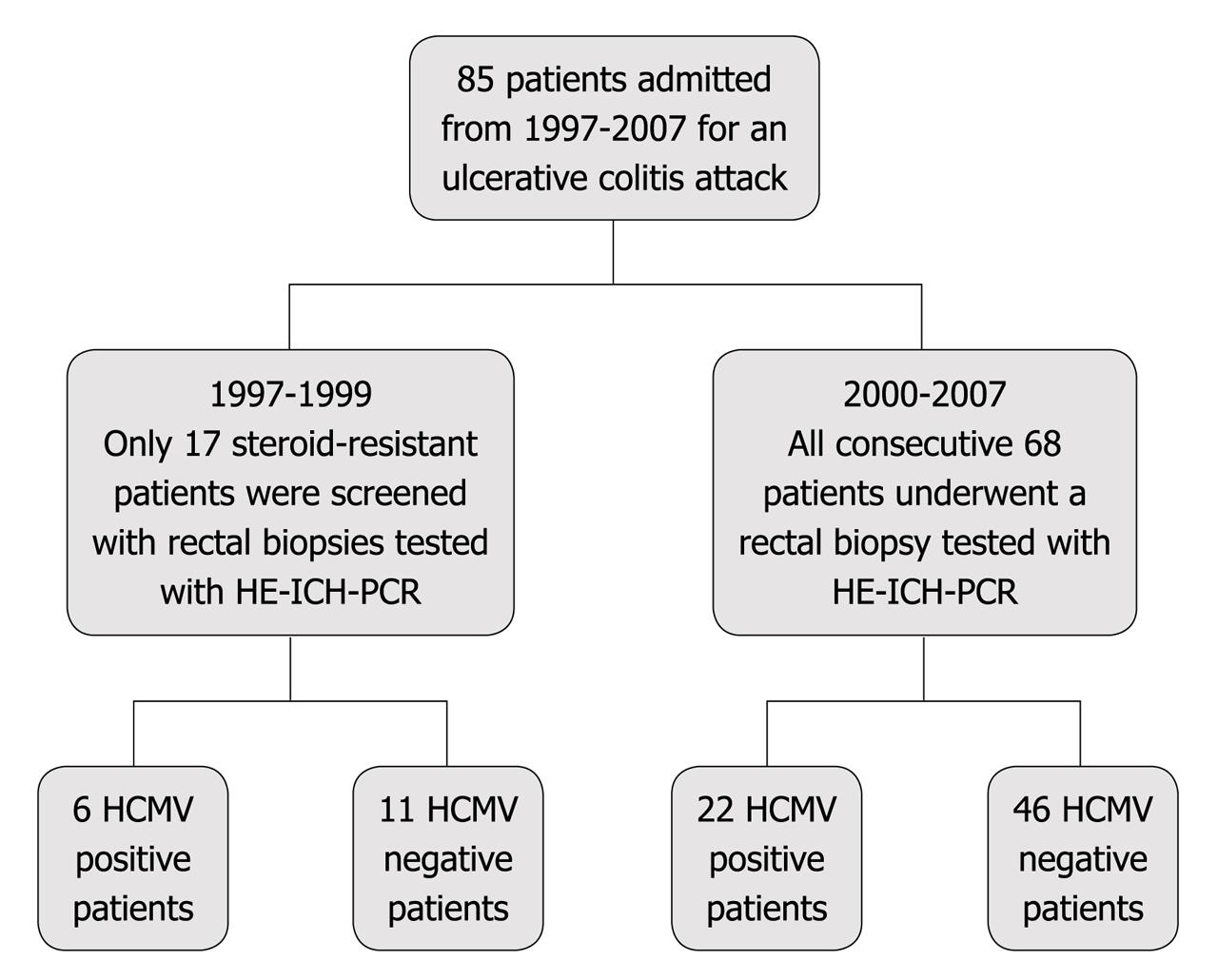
From 1997 to 2007, a prospective study was conducted on 85 patients with ulcerative colitis who were admitted to the Medicine department of V. Cervello Hospital in Palermo due to a severe colitis attack (according to the Truelove-Witts criteria) (Figure 1). All patients were treated with conventional and standardized corticosteroid treatment (1 mg/kg per day) and were endoscopically evaluated with a different approach: from 1997 to 1999 sigmoidoscopy was performed only in steroid resistant patients (17 patients) whereas from 2000 to 2007 sigmoidoscopy was performed in all patients (68 patients) at ward admission, taking systematically rectal biopsies. Therefore a total of 85 patients (Table 1) was investigated for HCMV infection with 3 different techniques.
| Patient characteristics | Total | HCMV+ | HCMV- |
| Sex (M/F) | 50/35 | 19/9 | 31/26 |
| Age at ward admission (yr) | |||
| ≥ 50 | 17 | 40 | |
| < 50 | 11 | 17 | |
| Disease extension | |||
| Left sided colitis | 39 | 11 (39) | 28 (49) |
| Subtotal and pancolitis | 46 | 17 (61) | 29 (51) |
| Previous azathioprine treatment | 21 | 11 (39) | 10 (17.5) |
| Previous biologic treatment | 9 | 2 (7) | 7 (12) |
| Active disease at sigmoidoscopy | |||
| Severe | 51 | 16 (57) | 35 (61) |
| Mild/moderate | 34 | 12 (43) | 22 (39) |
| Onset of disease (new diagnosis) | 5 | 4 (14) | 1 (2) |
| Reactivation (established disease) | 80 | 24 (86) | 56 (98) |
The rectal biopsies were immediately fixed with 10% buffered formalin for 2 h to obtain tissue fixation; afterwards the preparation was subjected to several processes (lavage, dehydration, clearing, paraffin impregnation and embedding) to prepare sections with a thickness of 3-4 μm.
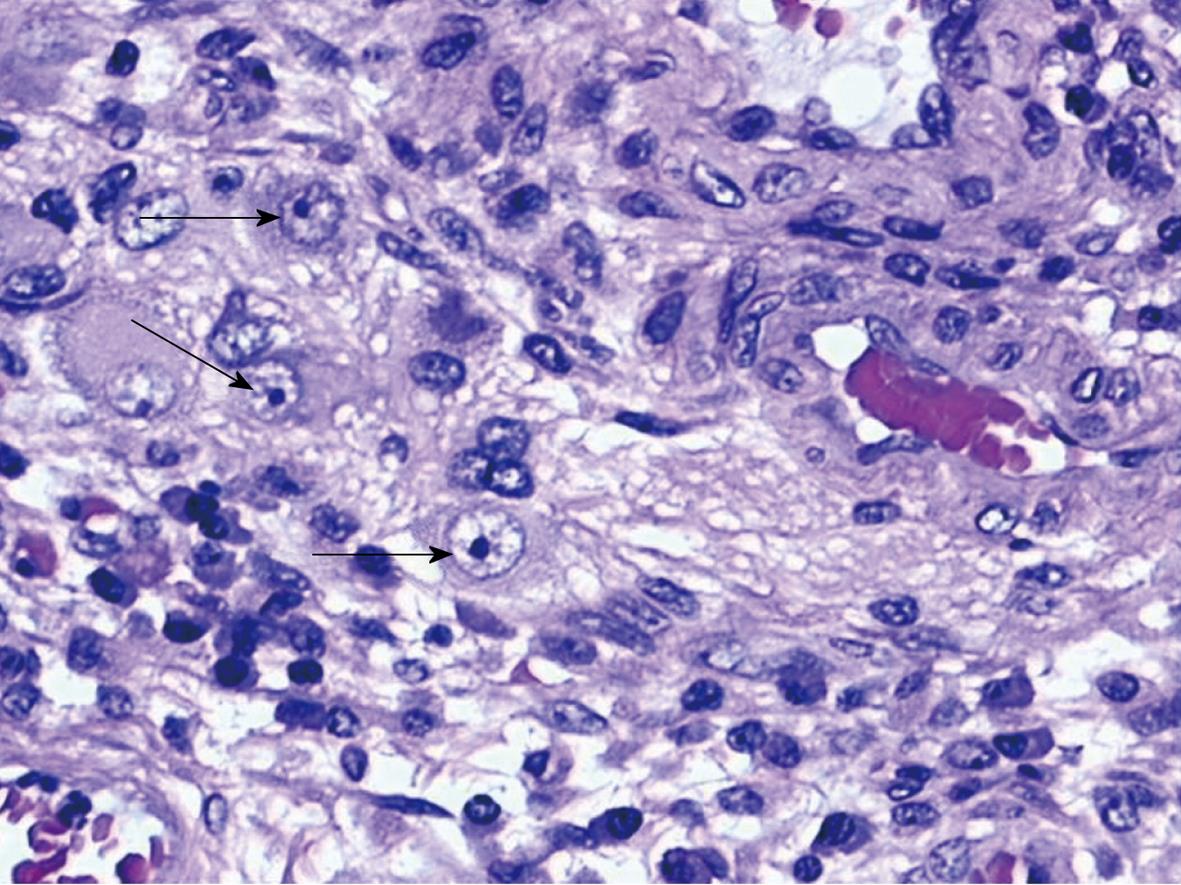
The histologic specimens were examined using the following techniques: (1) Light microscopy with hematoxylin and eosin (HE) stain in order to document the microscopic disease activity and allow the detection of cytomegalic cells, markers of infected viral cells. Cytomegalic cells, which are 2-or 4-fold larger (25-35 μm) than surrounding cells, contain a basophilic intranuclear inclusion (8-10 μm) eccentrically placed and sometimes surrounded by a clear halo giving it an “owl’s eye” appearance, and thickened nuclear membrane, frequently associated with smaller granular intracytoplasmatic inclusions. Intranuclear inclusions were observed in epithelial, endothelial, stromal and smooth muscle cells. A biopsy was regarded as positive by light microscopy for HCMV if a single cell showed intranuclear or cytoplasmic inclusions and cytomegalic characteristics (Figure 2); (2) Immunohistochemical (ICH) procedure for HCMV performed on a paraffin- embedded section with monoclonal mouse antibodies anti-Human CMV (clone BM204) and conjugated to a peroxidase-labeled amino acid polymer by peroxidase-antiperoxidase (PAP) method in order to detect viral proteins. Nuclear or cytoplasmic antigen was identified by the typical brown reaction product of the PAP method; and (3) Nested polymerase chain reaction (nPCR): (a) DNA extraction: DNA was extracted from 10 mm sections of paraffin wax embedded tissues. Five sections were cut with a standard microtome from every paraffin wax block and transferred into a 1.5 mL microtube. To prevent cross contamination between the samples, the microtome blade was washed with xylene and ethanol after sectioning of each block. DNA extraction was performed using a conventional method. The conventional method consisted of xylene/ethanol dewaxing followed by overnight proteinase k digestion in lysis buffer. The sample was heated at 95°C for 5 min to inactivate the proteinase K. We checked the quality of samples by PCR for the housekeeping gene b-globin (fragment of 268 bp); and (b) Detection of viral DNA: Nested PCR was used to detect the presence of viral DNA in colon tissues. Two pairs of primers annealed to the gB region of HCMV. Primers used for the first-round product and second-round PCRs are as follows (5' to 3'): first-round primer 1, GAGGACAACGAAATCCTGTTGGGCA; first-round primer 2, GTCGACGGTGGAGATACTGCTGAGG; second-round primer 3, ACCACCGCACTGAGGAATGTCAG, and second-round primer 4, TCAATCATGCGTTTGAAGAGGTA, to obtain a CMV fragment of 100 bp.
PCR reaction mixture contained 0.5 U Taq polymerase, 1 × PCR Buffer (50 mmol/L KCl and 10 mmol/L Tris-HCl (pH 8.4), 0.2 mmol/L of each dNTPs, 1.5 mmol/L MgCl2, 10 pmol of each primers and 100-200 ng of extracted DNA. The conditions for the first-round PCR were as follows: denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min followed by 5 min final extension at 72°C.
The conditions for second-round PCR were as follows: denaturation at 94°C for 5 min, followed by 25 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min followed by 5 min final extension at 72°C.
The PCR amplification products were run on 2% agarose gel and stained with ethidium bromide and visualized under ultraviolet light.
All patients were also tested for HCMV-pp65 antigenemia in peripheral leukocytes by CMV-CINAkit (Argene® Biosoft). The samples were considered positive for active HCMV infection using a cut-off value of 5 positive fluorescent nuclei/2 × 105 leukocytes[5]; between 1 to 4 positive cells the result was considered questionable and the blood sample was repeated after 48 h to value a possible increase of cell nuclei positivity. If we detected histopathological examination positive we considered the patient HCMV-infected but not surely with active replication; if both tests (histology and pp65 antigenemia) were positive we judged the patients candidates for antiviral treatment due to a probable active replication. However the last decision about antiviral treatment was related to the disease activity without significant improvement of the disease course after treatment for underlying disease.
Sigmoidoscopy was performed in patients reaching clinical remission in order to control endoscopic activity and the presence of HCMV.
The patients were followed clinically as outpatients quarterly to evaluate the remission time, the number of relapses, the immunosuppressive therapies and the need for surgery.
We define as remission a combination of clinical parameters (stool frequency ≤ 3 per day with no bleeding); the term relapse is used to define a flare of symptoms in a patient with established ulcerative colotis (UC) who is in clinical remission, either spontaneously or after medical or surgical treatment.
In the case of clinical relapse, sigmoidoscopy was performed with multiple biopsies and a search for HCMV in rectal biopsies by the 3 methods and peripheral pp65antigenemia was conducted.
Moreover the asymptomatic patients were examined by colonoscopy every year in order to follow up the natural history of HCMV infection regarding histologic persistence, to understand more clearly the possible role of “bystander” or promoter to the disease relapse.
Oral and written informed consent was obtained from each patient before any procedure.
The rate of surgery and clinical relapse rates in the 2 groups of patients (HCMV positive and negative) were compared using χ2 test.
The median clinical and endoscopic follow-up was 40 mo.
Among the 85 patients evaluated for HCMV infection from 1997 to 2007, in 28 (12 women and 16 men) the intestinal biopsy resulted positive by routine HE/ICH staining and nPCR assay according to the standard methods previously described. The overall prevalence data was 33% (Table 2). There was no association between HCMV and active immunosuppressive or biologic treatment. Ten patients among a total of 28 also displayed positive pp65 antigenemia (35%). Out of 10 positive patients for both histology and pp65 antigenemia, 7 received antiviral treatment (5 with ganciclovir and 2 with foscarnet) because of steroid resistance and clinical disease worsening, achieving remission in 5 patients. In these 5 treated patients HCMV disappeared at the first endoscopic control. Two patients were operated on because of a rapid clinical disease worsening: 1 patient died due to toxic megacolon. Among the 3 patients not treated with antiviral therapy for a contraindication, 1 achieved remission with therapy for underlying disease, 2 patients were operated on for intractable disease and 1 of these 2 patients died due to post-operative complications.
| HCMV+ (HE-ICH/PCR) | HCMV- | |
| Total patients | 28 | 57 |
| Steroid dependent/resistant (%) | 68 | 19 |
| Medical remission | 22 (78) | 51 (89) |
| Surgical remission | 6 (21) | 6 (10.5) |
| Death | 3 (10) | 0 |
| Persistence of HCMV DNA+ at follow-up | 8 (28.5) | 0 |
Among the 18 patients who were histologically positive but pp65 antigenemia negative, 2 were operated on for intractable disease and 1 died; 16 improved with conventional therapy. Among the overall number of 28 patients who tested positive for HCMV, 6 underwent total colectomy (21%), 2 of them treated with antiviral treatment. Among the 57 patients who were negative for HCMV detection, 6 patients were operated on (10.5%).
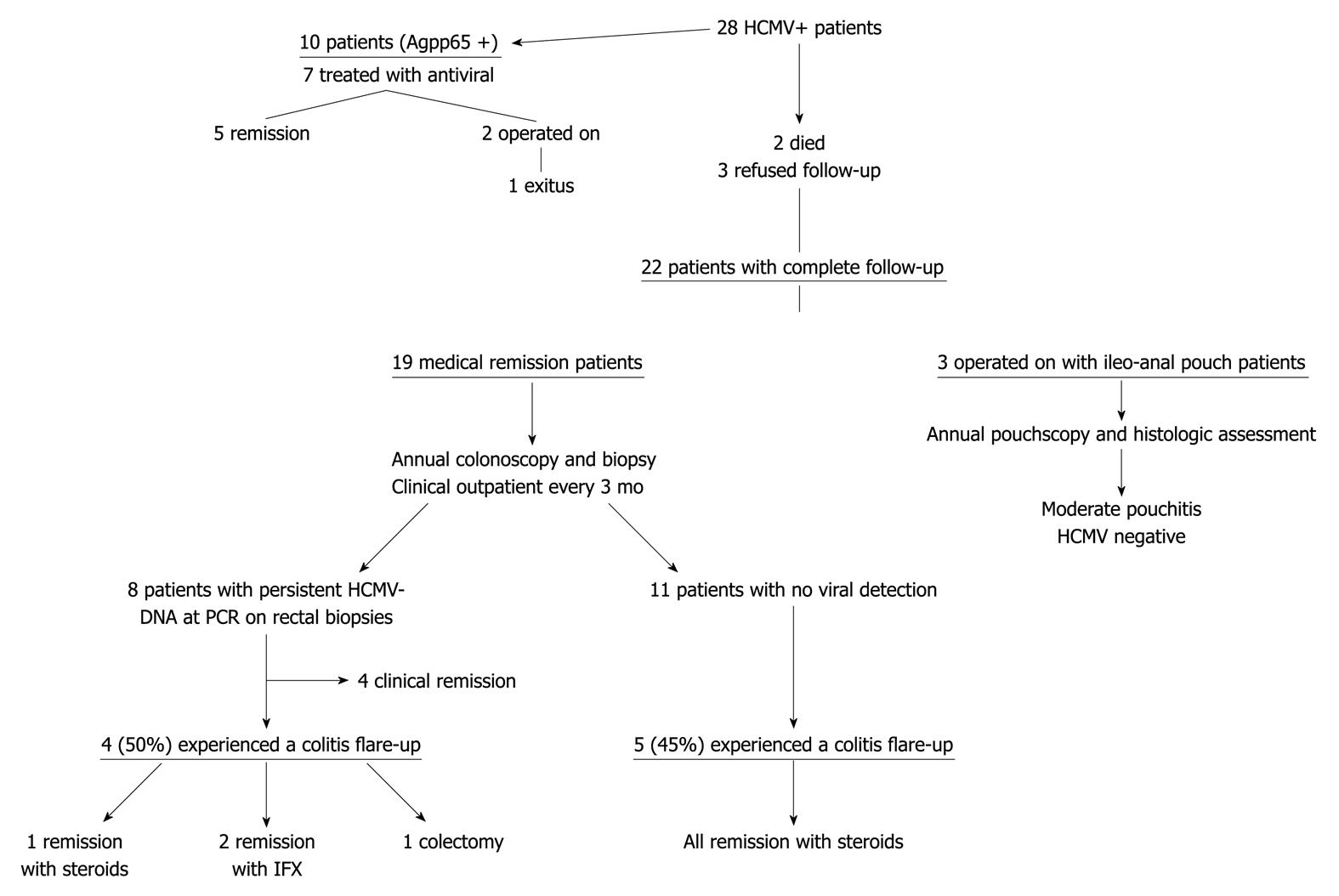
Among the 25 surviving patients, 22 remained on follow-up (19 on medical remission and 3 with ileoanal pouch anastomosis) because 3 refused to undertake regular clinical and colonoscopic controls.
The 3 patients operated on underwent endoscopic and histological assessment of the ileoanal pouch during follow-up, showing a moderate grade of pouchitis according to PDAI 1 year after surgery, without detection of HCMV in the pouch.
Eighteen patients achieved clinical remission with medical treatment and 1 patient became steroid-dependent; all were followed up for an average period of 46 mo, all were clinically and endoscopically evaluated in accordance with the methods described above.
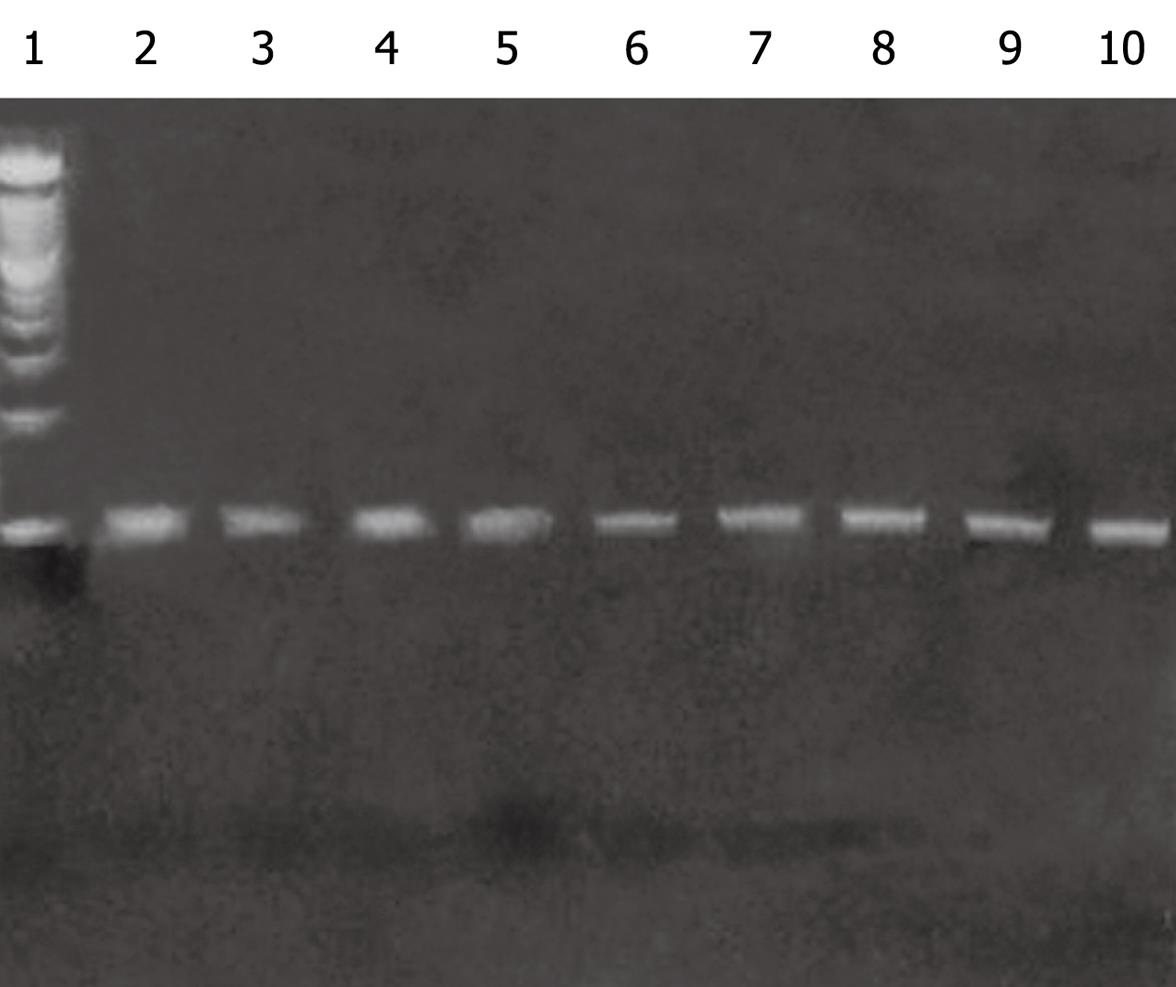
During the routine endoscopic and biopsy controls 11/19 patients were negative for HCMV detection both by traditional histology with HE and ICH in the intestinal biopsies and by nPCR assay, whereas 8 were positive for HCMV viral DNA detection by nPCR (Figure 3) and negative on light microscopy (HE and ICH) and on pp65 antigenemia.
Among the 8 patients in which positivity for HCMV-DNA persisted in the intestinal biopsies, 3 were treated with antiviral therapy at first detection of HCMV. Four experienced (50%) an early-moderate colitis flare up (within 3 mo) during the follow-up without detection of pp65 antigenemia in the peripheral leukocytes (Figure 4). A patient achieved remission with conventional steroid treatment, 2 patients became steroid-dependent and were treated with anti-TNF therapy, both achieving remission. The fourth patient, who was steroid-dependent, intolerant to azathioprine and with a contraindication to anti-TNF therapy, underwent a total colectomy due to severity of disease. None were treated with antiviral treatment due to absence of positive antigenemia (Table 3). Among the 11 HCMV negative patients 5 (45%) experienced a reactivation of colitis and achieved remission with steroid treatment.
| Patient No. | Age (yr)/sex | Immunomodulators at HCMV diagnosis | Disease relapse | Outcome |
| 1 | 42/F | Aza | No | Remission with 5-asa |
| 2 | 71/M | No | Yes | Remission with IFX |
| 3 | 58/M | Aza | Yes | Procto-colectomy |
| 4 | 74/M | No | Yes | Remission with IFX |
| 5 | 65/F | No | No | Remission with 5-asa |
| 6 | 65/M | No | Yes | Remission with steroid |
| 7 | 45/M | Aza | No | Leukapheresis-IFX |
| 8 | 28/M | No | No | Remission with Aza |
After remission, 8 patients (among 22) were treated with azathioprine (in 5 of them HCMV-DNA was present when the treatment was started) and 14 patients were treated with mesalazine (3 HCMV-DNA positive).
The χ2 test comparing surgical intervention among positive HCMV (21%) and negative HCMV (10.5%) patients was not statistically significant (P = 0.17, odds ratio 2.32).
The χ2 test comparing the relapse rate among HCMV positive (50%) and negative (45%) patients was not statistically significant (P = 0.36).
Our study was an attempt to answer two recurrent questions about the role of HCMV in IBD. The questions are about HCMV disappearance after remission of acute colitis relapse: we have demonstrated that HCMV persists in the colon after recovery of a colitis flare with a HCMV co-infection in a series of UC patients and remains detectable by sensitive methods such as nPCR without histological/ICH virus detection in a minority of patients. From this small series the persistence of virus in the colon of UC patients does not favour disease relapse because the virus persists in a latent state. The absence of pp65 antigenemia during the follow-up relapse and the response to the conventional corticosteroid treatment do not suggest a remarkable role of HCMV in the re-activation. Furthermore, it is unlikely that immunosuppressive treatment favours relapse of UC when HCMV persists in the colon.
To establish a connection between HCMV and UC more reports are needed. The interaction with the immune system plays a key role in the pathogenesis of HCMV disease, and the main determinant is an immunological impairment. As mentioned above, even most primary infections in humans are asymptomatic. No trace of the infection is observable except for seroconversion. In recent years, however, the disease has become more common probably due to the widespread use of immunosuppressants in oncology, in transplantation medicine and in chronic disease[6].
What happens in intestinal tissue after persistence of HCMV-DNA is not known; maybe the colonic epithelial cells harbour the latent virus that became detectable using the highly sensitive PCR assay. The reactivation of virus from latency may depend on a complex interplay of biological factors with the host that, above all in patients with UC, are not clearly understood. Experimental studies suggest that latent CMV infection in the mouse may modulate mucosal immunity altering the susceptibility to gut microbiota without viral reactivation[7].
Dimitroulia et al[8] demonstrated that HCMV is frequently detected in IBD patients, showing that the virus genome was detected in intestinal tissue by polymerase chain reaction in 32.9% of the total IBD patients, while the HCMV genome in the blood was detected in 27.1% of these patients. Matsuoka et al[9] showed that HCMV is frequently reactivated in a series of active UC seropositive patients, but that reactivation has little effect on the clinical course and that most of the colitis reactivation with positive HCMV responds to conventional immunosuppressive therapies.
Kou et al[10] demonstrated that the detected copy number of HCMV-DNA by PCR method is higher in the inflamed colonic tissue than in non inflamed colonic tissue in patients with UC refractory to immunosuppressive therapy. The author strongly supported the hypothesis that HCMV infection is involved in exacerbation of patients with IBD and the early detection of genome in intestinal tissue is important for an eventual change to the therapeutic approach.
We show that patients with HCMV infection were more frequently operated on than those without superimposed HCMV (even though not statistically significant) and this may suggest that the virus is a marker of risk of surgical treatment. This observation is an agreement with Cooper et al[11] that showed in 1977 that HCMV infection might be responsible for acute toxic dilatation with increased colonic resection rate.
In patients who have undergone proctocolectomy we did not detect HCMV in the follow-up biopsies of the ileal pouch. Casadesus et al[12] detected HCMV in a small series of patients with UC who underwent proctocolectomy and hypothesize that the virus may play an etiological role in pouchitis; other case reports[13,14] demonstrate that HCMV is a rare but possible cause of refractory pouchitis to be considered for antiviral therapy.
In conclusion, our preliminary results suggest that HCMV may remain in the colon despite remission, probably activating a delicate balance with the host immune system in order to avoid its elimination, but the effects are not clear. The variability of genotypes may give an answer to the virulence and cell tropism so that we might be able to understand the different behaviour related to different host conditions.
A multicentre study including a more numerous population (almost 500 patients) with a longer follow-up is warranted to define whether the virus infection causes a disease complication or its presence does not alter the course of disease.
A limitation of study is that the assessment is based upon very few patients but it is very difficult to find a large population; possibly a multicentre study would solve this problem.
We performed the nPCR assay that detects only the presence of viral DNA in colon tissues. Maybe a quantitative assay that can distinguish between active and latent disease would give some useful information to correlate the replication of HCMV with bowel disease activity.
Cytomegalovirus (CMV) infection has been described as a cause of relapse of inflammatory bowel disease (IBD), in particular in ulcerative colotis patients, especially those receiving high-dose corticosteroid therapy. No clear data is available on the natural history of human CMV (HCMV) superimposed on IBD.
The paper aimed to answer two questions concerning HCMV infection in ulcerative colitis patients.
This paper looks at the role of long-term HCMV persistence in patients with moderate to severe ulcerative colitis together with the likelihood this brings of relapse in patients that both cleared and did not clear the infection.
This is one of the first studies investigating the persistence of HCMV in colonic tissue of ulcerative patients after an acute flare up of disease.
Nested polymerase chain reaction is a molecular assay that detects HCMV DNA using specific primers that amplify the gB region of HCMV. HCMV-pp65 antigenemia is an assay for polymorphonuclear leukocytes.
I think there are some useful observations in the study, but some clarifications are needed.
Peer reviewer: Dr. Douglas K Rex, MD, FACP, FACG, FASGE, Department of Medicine, Indiana University School of Medicine, 550 N. University Blvd., Indianapolis, IN 46202, United States
S- Editor Sun H L- Editor O’Neill M E- Editor Zheng XM
| 1. | Berk T, Gordon SJ, Choi HY, Cooper HS. Cytomegalovirus infection of the colon: a possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355-360. [Cited in This Article: ] |
| 2. | Orvar K, Murray J, Carmen G, Conklin J. Cytomegalovirus infection associated with onset of inflammatory bowel disease. Dig Dis Sci. 1993;38:2307-2310. [Cited in This Article: ] |
| 3. | Loftus EV Jr, Alexander GL, Carpenter HA. Cytomegalovirus as an exacerbating factor in ulcerative colitis. J Clin Gastroenterol. 1994;19:306-309. [Cited in This Article: ] |
| 4. | Criscuoli V, Casà A, Orlando A, Pecoraro G, Oliva L, Traina M, Rizzo A, Cottone M. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36:818-820. [Cited in This Article: ] |
| 5. | Torrús D, Portilla J, Hernández-Aguado I, Boix V, Plazas J, Gimeno A, Torromé M, Llopis C, Valls V, Sánchez-Payá J. Usefulness of pp65 antigenemia for the early diagnosis of cytomegalovirus disease in patients with AIDS. Eur J Clin Microbiol Infect Dis. 1999;18:630-635. [Cited in This Article: ] |
| 6. | Baroco AL, Oldfield EC. Gastrointestinal cytomegalovirus disease in the immunocompromised patient. Curr Gastroenterol Rep. 2008;10:409-416. [Cited in This Article: ] |
| 7. | Onyeagocha C, Hossain MS, Kumar A, Jones RM, Roback J, Gewirtz AT. Latent cytomegalovirus infection exacerbates experimental colitis. Am J Pathol. 2009;175:2034-2042. [Cited in This Article: ] |
| 8. | Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:879-884. [Cited in This Article: ] |
| 9. | Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331-337. [Cited in This Article: ] |
| 10. | Kou T, Nakase H, Tamaki H, Kudo T, Nishio A, Chiba T. Cytomegalovirus infection in patients with ulcerative colitis diagnosed by quantitative real-time PCR analysis. Dig Dis Sci. 2006;51:1052-1055. [Cited in This Article: ] |
| 11. | Cooper HS, Raffensperger EC, Jonas L, Fitts WT Jr. Cytomegalovirus inclusions in patients with ulcerative colitis and toxic dilation requiring colonic resection. Gastroenterology. 1977;72:1253-1256. [Cited in This Article: ] |
| 12. | Casadesus D, Tani T, Wakai T, Maruyama S, Iiai T, Okamoto H, Hatakeyama K. Possible role of human cytomegalovirus in pouchitis after proctocolectomy with ileal pouch-anal anastomosis in patients with ulcerative colitis. World J Gastroenterol. 2007;13:1085-1089. [Cited in This Article: ] |
| 13. | Muñoz-Juarez M, Pemberton JH, Sandborn WJ, Tremaine WJ, Dozois RR. Misdiagnosis of specific cytomegalovirus infection of the ileoanal pouch as refractory idiopathic chronic pouchitis: report of two cases. Dis Colon Rectum. 1999;42:117-120. [Cited in This Article: ] |
| 14. | Moonka D, Furth EE, MacDermott RP, Lichtenstein GR. Pouchitis associated with primary cytomegalovirus infection. Am J Gastroenterol. 1998;93:264-266. [Cited in This Article: ] |