Published online Oct 14, 2011. doi: 10.3748/wjg.v17.i38.4298
Revised: June 9, 2011
Accepted: June 16, 2011
Published online: October 14, 2011
AIM: To investigate the apoptotic activities of casticin in hepatocellular carcinoma (HCC) cells and its molecular mechanisms.
METHODS: PLC/PRF/5 and Hep G2 cell lines were cultured in vitro and the inhibitory effect of casticin on the growth of cells was detected by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolim bromide (MTT) assay. The apoptotic cell death was examined using the cell apoptosis enzyme linked immunosorbent assay (ELISA) detection kit, flow cytometry (FCM) after propidium iodide (PI) staining and DNA agarose gel electrophoresis. The caspase activities were measured using ELISA. Reactive oxygen species (ROS) production was evaluated by FCM after dichlorodihydrofluorescein diacetate (DCFH-DA) probe labeling. Intracellular glutathione (GSH) content was measured using a glutathione assay kit. The expression of death receptor (DR)4 and DR5 proteins was analyzed by Western blotting and FCM.
RESULTS: Casticin significantly inhibited the growth of human HCC (PLC/PRF/5 and Hep G2) cells in a dose-dependent manner (P < 0.05). Casticin increased the percentage of the sub-G1 population in HCC cells in a concentration-dependent manner. The potency of casticin to PLC/PRF/5 cells was higher than that of 5-flurouracil (26.8% ± 4.8% vs 17.4% ± 5.1%) at 10 μmol/L for 24 h. Casticin increased the levels of Histone/DNA fragmentation and the levels of active caspase-3, -8 and -9 in a concentration-dependent manner (P < 0.05). Treatment with 30 μmol/L casticin for 24 h resulted in the formation of a DNA ladder. Casticin reduced the GSH content (P < 0.05), but did not affect the level of intracellular ROS in PLC/PRF/5 and Hep G2 cells. The thiol antioxidants, acetylcysteine (NAC) and GSH restored GSH content and attenuated casticin-induced apoptosis. In contrast, the nonthiol antioxidants, butylated hydroxyanisole and mannitol failed to do so. In the HCC cells treated with casticin for 24 h, DR5 protein level was increased. The expression of DR5 protein induced by casticin was inhibited by NAC. Pretreatment with DR5/Fc chimera protein, a blocking antibody, effectively attenuated the induction of apoptosis by casticin.
CONCLUSION: Casticin-induced apoptosis of HCC cells is involved in GSH depletion and DR5 upregulation.
- Citation: Yang J, Yang Y, Tian L, Sheng XF, Liu F, Cao JG. Casticin-induced apoptosis involves death receptor 5 upregulation in hepatocellular carcinoma cells. World J Gastroenterol 2011; 17(38): 4298-4307
- URL: https://www.wjgnet.com/1007-9327/full/v17/i38/4298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i38.4298
Hepatocellular carcinoma (HCC) is currently the fifth most common malignant neoplasm in the world[1], causing over 600 000 deaths each year[2]. HCC is prevalent in Asia and Africa and its incidence has steadily increased in European and American populations[3,4]. The majority of patients with HCC die within one year after the diagnosis was established. Unfortunately, HCC is often diagnosed at its late stage when potentially curative therapies are least effective. The 5-year relative survival rate is only 7%[5]. Patients with surgically resectable localized HCC have a better prognosis, but their 5-year survival rate is only 15%-39%[6], thus, new therapeutic agents for this malignant disease are urgently needed.
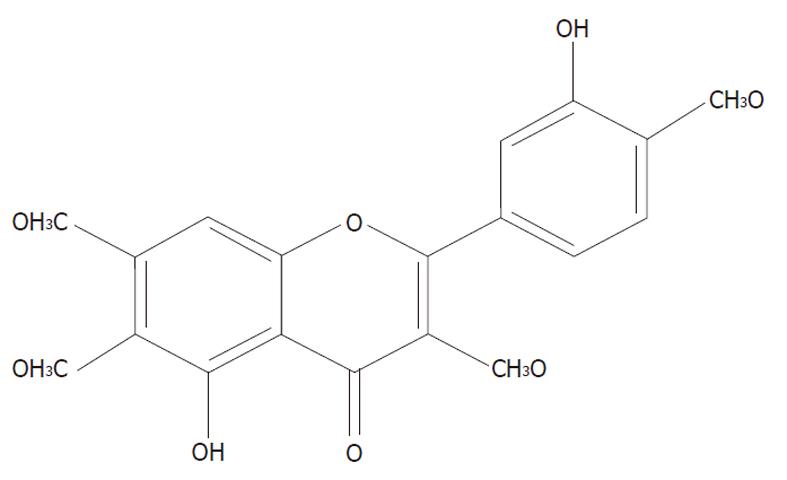
Casticin is one of the main components from Fructus Viticis (Manjingzi in Chinese name), a traditional Chinese medicine prepared from the fruit of Vitex trifolia L. (family Verbenaceae) that is also used as an anti-inflammatory agent and for the treatment of certain cancers in China[7]. Its chemical structure is shown in Figure 1. Casticin has been shown to inhibit lymphocyte proliferation in vitro[8] and has an anti-inflammatory effect in vivo[9]. In recent years, many studies have demonstrated its anti-carcinogenic activity in breast cancer[10], lung cancer and colon cancer[11]. Casticin was also reported to inhibit the growth of human myelogenous leukemia cells[12] and induce cell death of leukemia cells through induction of apoptosis or mitotic catastrophe[13]. However, the precise mechanisms underlying casticin inducing apoptosis of HCC cells are still unclear. In the present study, we investigated the effects and molecular mechanism of casticin on the apoptotic cell death of HCC cells in vitro. We found that casticin significantly induced apoptosis of HCC cells by glutathione (GSH) depletion and upregulation of DR5.
PLC/PRF/5 (p53 mutant) and Hep G2 (p53 wild type) human HCC cells were obtained from American Type Culture Collection (Rockville, MD, United States) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin 100 U/mL and streptomycin 100 μg/mL (Life Technologies, Inc., Shanghai, China) in an incubator containing 50 mL/L CO2 at 37 °C. Casticin was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China), and has a molecular weight of 374.3 kDa, appears as yellow crystals and has a purity of 98.0%. Casticin was prepared in dimethyl-sulfoxide (DMSO) as a 10 mmol/L stock solution and diluted in a medium to the indicated concentration before use. The followings were purchased from Hunan Clonetimes Biotech Co., Ltd. (Changsha, China): RPMI-1640 medium (Invitrogen, CA, United States), fetal bovine serum (Invitrogen), Cell Apoptosis enzyme linked immunosorbent assay (ELISA) Detection Kit (Roche), N-acetylcysteine (NAC; Sigma, MO, United States), glutathione (GSH; Sigma), propidium iodide [propidium iodide (PI); Sigma], ethidium bromide (EB; Sigma), N-(4-hydroxyphenyl) retinamide (4HPR; Sigma), butylated hydroxyanisole [butylated hydroxyanisole (BHA); Sigma], mannitol (Sigma), Glutathione Assay kit (Calbiochem, Darmstadt, Germany), Apoptotic DNA Ladder Detection Kit (Bodataike Company, Beijing, China), Caspase 3 Activity Detection Kit (Millipore, MA, United States), Caspase 8 Colorimetric Activity Assay Kit 25 (Millipore), Caspase 9 Colorimetric Activity Assay Kit (Millipore), zVAD-fmk (R and D Systems, MN, United States), zIETD-fmk (R and D Systems), zLEHD-fmk (R and D Systems), 5-fluorouracil (5-FU; Sigma), death receptor (DR)5/Fc chimera protein(R and D Systems), 2',7'-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes Inc., OR, United States), mouse anti-human DR5 and DR4 (Santa Cruz Biotechnology, CA, United States), Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Zymed Laboratories, CA, United States), mouse IgG1 immunoglobulin (Dako Cytomation, CA, United States).
Cells were seeded in a 96-well plate at a density of 0.5 × 104 cells/well and incubated for 24 h, followed by treatment with various concentrations of casticin or 5-fluorouracil for 24 h. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) colorimetric analysis was performed as described previously[14]. The IC50 value, i.e., 50% of the cell growth inhibition compared with (DMSO) control, was calculated by nonlinear regression analysis using GraphPad Prism software (San Diego, CA).
Cells were seeded at a density of 4 × 106 cells/mL in 100 mL culture flasks for 24 h and then treated with the medium containing various concentrations of casticin or 5-fluorouracil for the indicated time. Propidium iodide staining for DNA content analysis was performed as described previously[15].
The cell apoptosis ELISA detection kit was used to detect apoptosis in cells treated with casticin according to the manufacturer’s protocol. Briefly, cells were seeded in a 96-well plate at a density of 1 × 104 cells / well for 24 h, added with the medium containing various concentrations of casticin. After 24 h, we transferred the cytoplasm of the control and treatment group to the 96-well plate peridiumed by the streptavidin, incubated with the biotinylated histone antibody and peroxidase-tagged mouse anti-human DNA for 2h at room temperature. The absorbance at 405 nm was measured with EXL-800 type Enzyme-Linked Immunosorbent apparatus.
Cells were seeded at a density of 4 × 106 cells/mL in 100 mL culture flasks for 24 h and treated with medium containing various concentrations of casticin for 24 h. This assay was performed as described previously[15].
To evaluate caspase activity, cell lysates were prepared after their respective treatment with the testing agents. Assays were performed in 96-well plates by incubating 20 μg cell lysates in 100 μL reaction buffer (1% NP-40, 20 mmol/L Tris-HCl (pH 7.5), 137 mmol/L NaCl, 10% glycerol) containing a 5 μmol/L caspase-3 substrate Ac-DEVD-pNA or caspase-8 substrate Ac-IETD- pNA or caspase-9 substrate Ac-LEHD- pNA . Lysates were incubated at 37 °C for 2 h. Thereafter, the absorbance at 405 nm was measured with an enzyme-labeling instrument (ELX-800 type). In the caspase inhibitor assay, cells were pretreated with a caspase inhibitor (20 μmol/L zVAD-fmk or zIETD-fmk or zLEHD-fmk) for 1 h prior to the addition of casticin.
Intracellular reactive oxygen species (ROS) accumulation was measured by flow cytometry using the fluorescent probe DCFH-DA[15]. Cells were incubated with 10 μmol/L DCFH-DA for 30 min at 37 °C in dark. After incubation, the cells were washed with phosphate buffered saline (PBS) and analyzed within 30 min using FACScan (Becton Dickinson, San Jose, CA, United States) equipped with an air-cooled argon laser tuned to 488 nm. The specific fluorescence signals corresponding to DCFH-DA were collected with a 525-nm band pass filter. As a rule, 10 000 cells were counted in each determination.
Intracellular GSH contents were measured using a Glutathione Assay kit. In brief, 5 × 106 cells were homogenized in 5% metaphosphoric acid using a Teflon pestle (Racine, WI). Particulate matter was separated by centrifugation at 4000 ×g. The supernatant solution was used for GSH measurement according to the manufacturer’s instructions. The GSH content was expressed as nmol/106 cells.
Cells were cultured at an indicated concentration for 24 h, and then collected. Five hundred thousand cells for each receptor analysis were transferred to polystyrene tubes, washed twice with PBS and resuspended in PBS containing 0.5% bovine serum albumin (BSA) (Sigma). A specific monoclonal antibody to either DR5, DR4 or unspecific mouse IgG1 as isotype control was applied at 5 μg/mL. Cells were incubated for 20 min with gentle rocking at room temperature. Cells were washed twice in PBS, and secondary fluorescein isothiocyanate-conjugated polyclonal goat antibody to mouse IgG1 (1:200 in PBS containing 0.5% BSA) was added, followed by incubation protected from light for 30 min with gentle rocking at room temperature. Cells were then washed and resuspended in PBS containing 0.5% BSA. All analyses were carried out on FACScan using CellQuest software (Pharmingen BD Biosciences, CA, United States).
Total cell extracts were obtained as described previously[15]. Cell lysate containing 50 μg of protein was separated on a 10% SDS-polyacrylamide gel for electrophoresis and then blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, United States). Anti-DR5, -DR4 and -β-actin (1:1000 dilutions for each) were used as primary antibodies. Signals were detected using an ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ, United States). Images were scanned followed by densitometric analysis with Alphazmager 2200 software (Silk Scientific Inc., Utah, United States). The ratios of DR5 or DR4/β-actin were determined for the expression level of DR5 or DR4.
The database was set up with the SPSS 15.0 software package (SPSS Inc, Chicago, IL, United States) for analysis. Data were presented as mean ± SD. The means of multiple groups were compared with one-way analysis of variance (ANOVA), after the equal check of variance, and the two-two comparisons among the means were performed using the least-significant difference (LSD) method. Statistical comparison was also performed with two-tailed t test when appropriate. P < 0.05 was considered statistically significant.
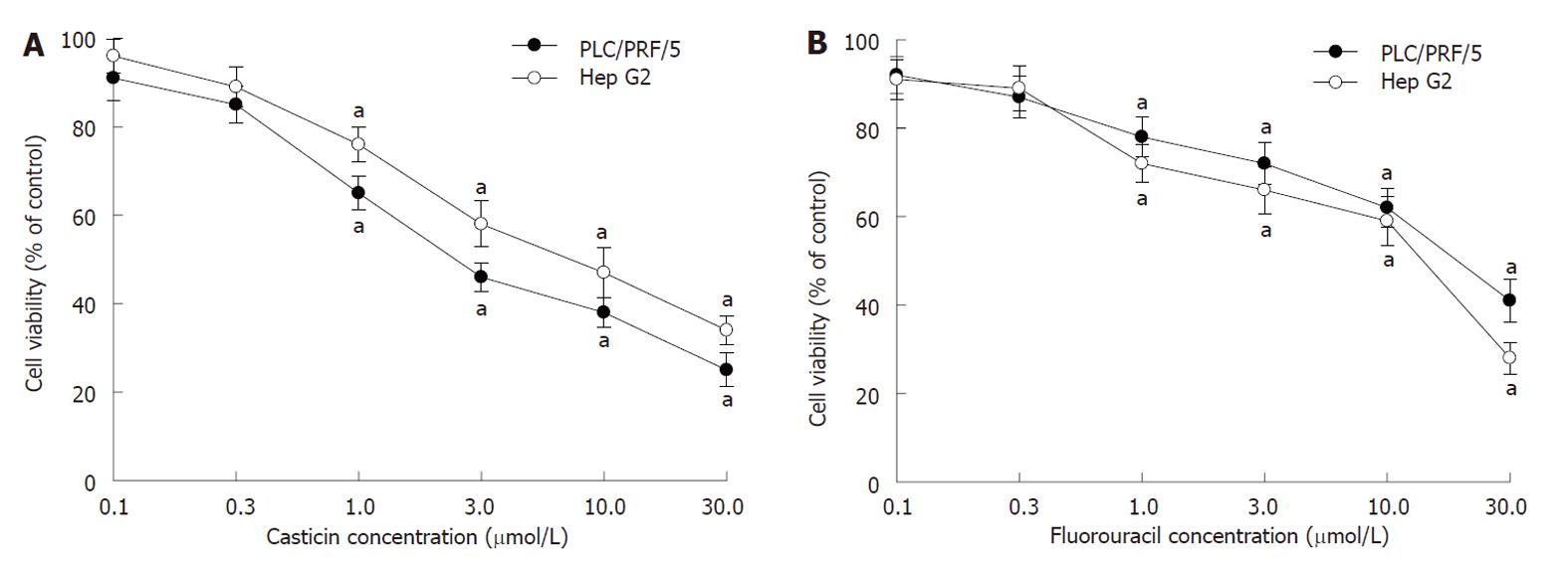
To characterize the effect of casticin on cell growth, two kinds of cell lines, including PLC/PRF/5 (p53 mutant) and Hep G2 (p53 wild type) cells, were treated with various concentrations of casticin for 24 h, and cell viability was assessed by MTT assay. Figure 2A shows that casticin significantly inhibited the growth of human HCC (PLC/PRF/5 and Hep G2) cells in a dose-dependent manner. When the IC50 for 24 h was 9.4 and 13.6 μmol/L, respectively, the potency of casticin to PLC/PRF/5 cells was stronger than that of 5-FU with an IC50 of 16.8 μmol/L (Figure 2B).
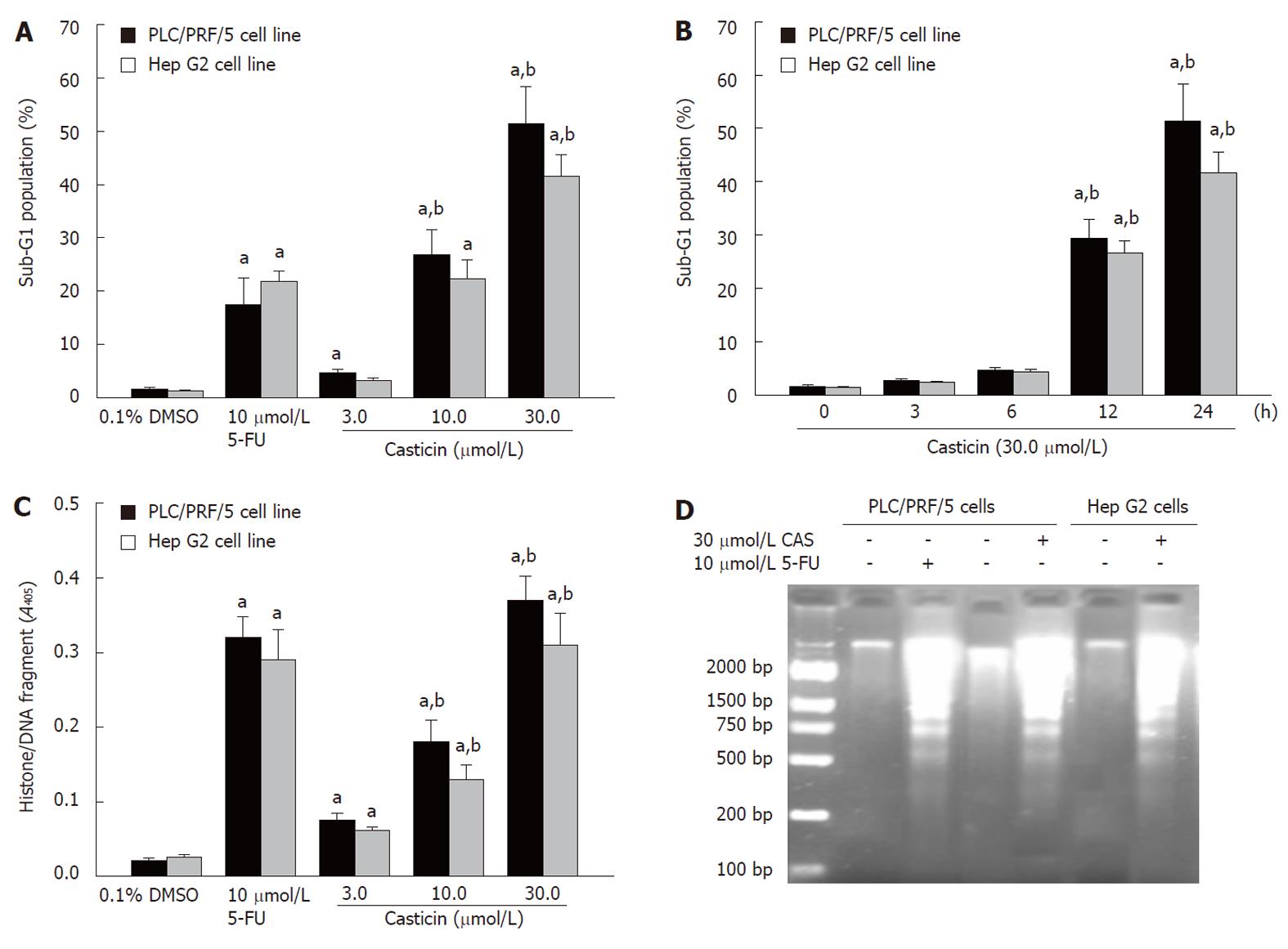
To investigate whether apoptosis was involved in cell growth inhibition by casticin, we detected apoptosis increase using flow cytometric analysis in hypodiploid cell populations. Figure 3A shows that casticin increased the percentage of the sub-G1 cell population in PLC/PRF/5 and Hep G2 cells in a concentration-dependent manner (P < 0.05). The potency of casticin to PLC/PRF/5 cells was higher than that of 5-flurouracil (26.8% ± 4.8% vs 17.4% ± 5.1%) at 10 μmol/L for 24 h. The sub-G1 population in PLC/PRF/5 and Hep G2 cells by casticin was increased at 12 h and peaked at 24 h (Figure 3B). Histone/DNA fragment of PLC/PRF/5 and Hep G2 cells, as measured by the cell apoptosis ELISA detection kit, was increased in a dose-dependent manner (P < 0.05) after treatment with casticin (Figure 3C). Furthermore, DNA fragmentation analysis by agarose gel electrophoresis showed a typical ladder pattern of internucleosomal DNA fragments in PLC/PRF/5 and Hep G2 cells treated with 30 µmol/L casticin for 24 h (Figure 3D). These results suggested that casticin inhibited HCC cell growth through a mechanism involving the induction of apoptosis.
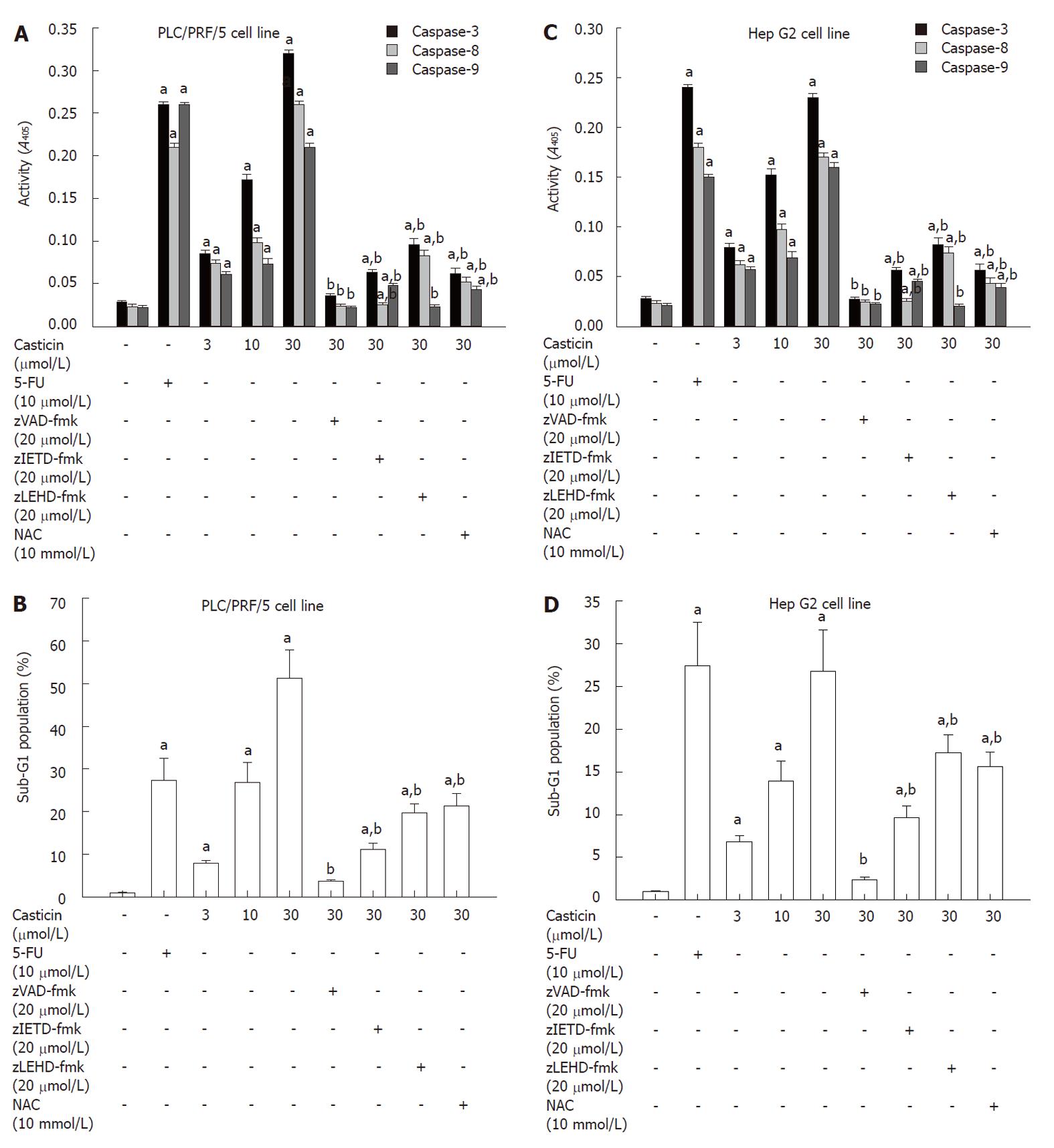
To determine the effectors active in casticin-induced apoptotic pathways, we examined whether caspases were actually activated during casticin-induced cell death of HCC cells. Figure 4A shows that treatment of PLC/PRF/5 cells with casticin for 24 h increased the levels of active caspase-3, -8 and -9 (P < 0.05) in a concentration-dependent manner (P < 0.05).
We further examined the role of caspases activated during apoptosis induced by casticin treatment using the pan-caspase inhibitor zVAD-fmk, the caspase-8 inhibitor zIETD-fmk and the caspase-9 inhibitor zLEHD-fmk. Figure 4B shows that zVAD-fmk abrogated apoptosis induced by casticin and zIETD-fmk and zLEHD-fmk attenuated casticin-induced apoptosis. The similar findings were observed in the Hep G2 cell line (Figure 4C and D). These data indicate that casticin-induced apoptosis was essentially dependent on the activation of caspase-3, -8 and -9.
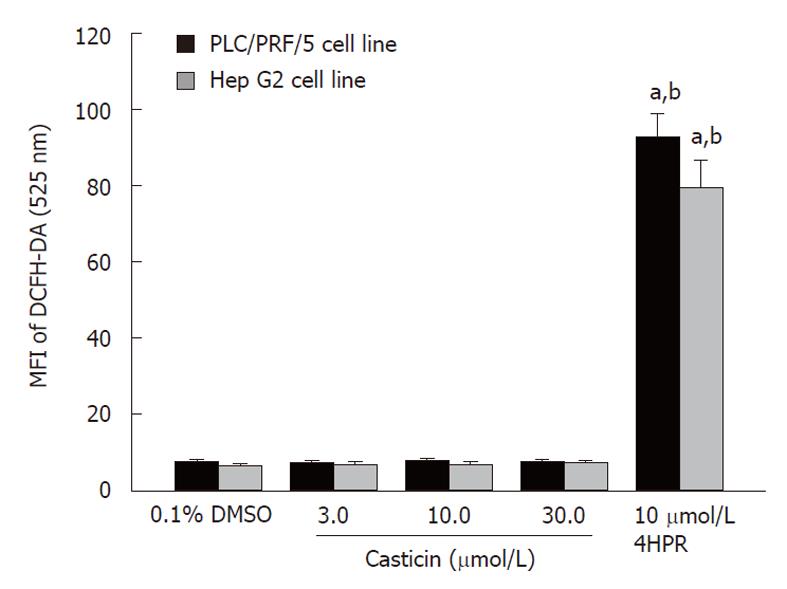
Because NAC, a antioxidant, could attenuate induction of apoptosis by casticin (Figure 4B and D), it was plausible to speculate that casticin-induced apoptosis by promoting intracellular ROS generation. Thus, we examined whether casticin promoted ROS generation in HCC cells. Unexpectedly, we failed to detect any increase of intracellular ROS generation in PLC/PRF/5 and Hep G2 cells treated with casticin, at concentrations ranging from 3.0 to 30.0 μmol/L. 4HPR, an agent known to promote ROS generation in cancer cells[16,17], did increase the ROS generation in HCC cell lines (Figure 5).
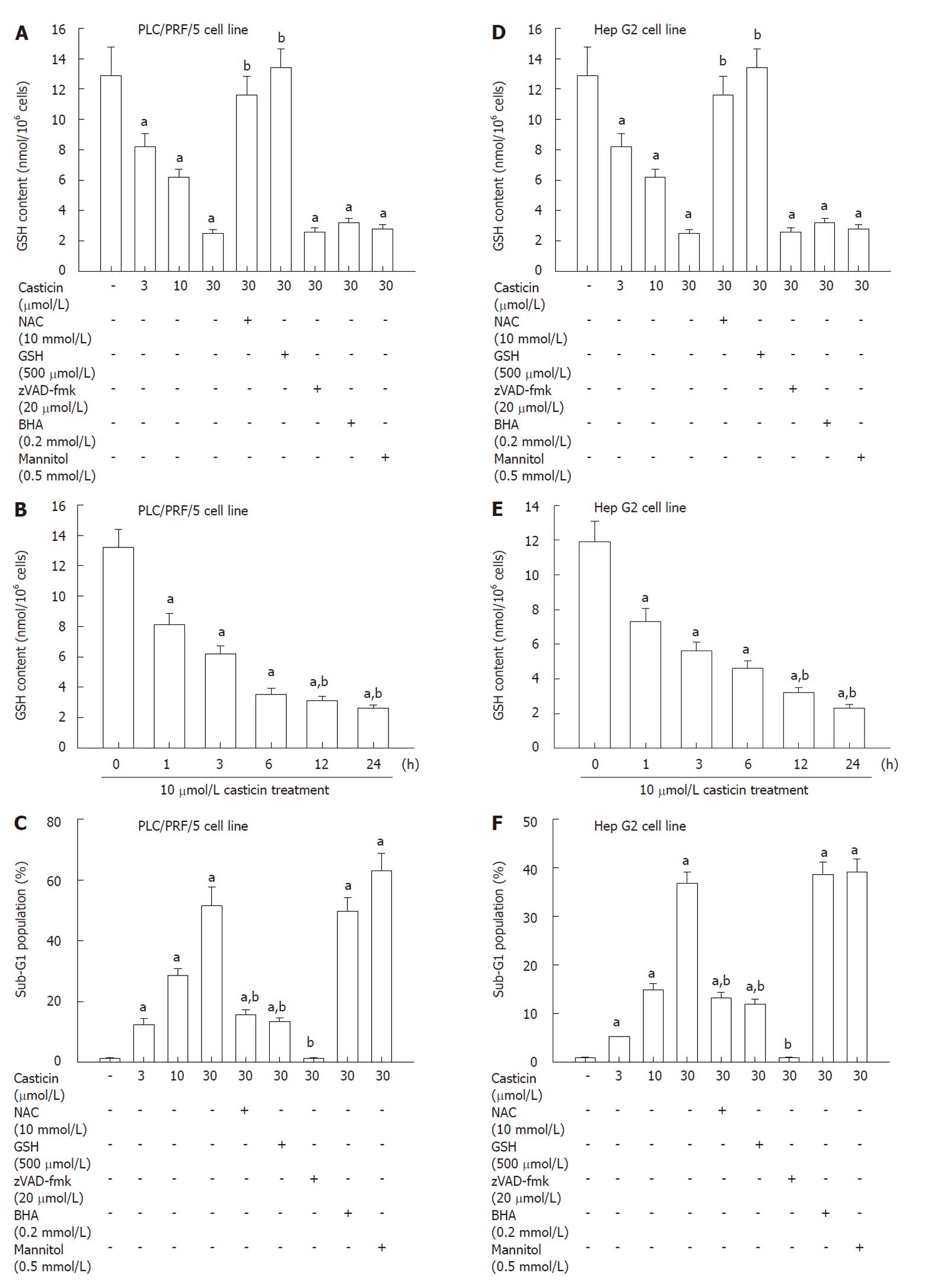
Earlier studies demonstrated that flavonoid toxicity was strictly dependent on the intracellular GSH content[18,19]. We measured the intracellular GSH in cells treated with casticin. In PLC/PRF/5 cells treated with casticin (3.0, 10.0 and 30.0 μmol/L) for 24 h, the intracellular GSH content significantly was decreased in a concentration-dependent manner (Figure 6A). In addition, time-course studies indicated that casticin caused a progressive decrease in GSH content from 1 h onwards (Figure 6B). Importantly, the pan-caspase inhibitor z-VAD-fmk, which successfully prevented apoptosis execution, failed to prevent GSH decrease (Figures 4A and B, 6A and C). This excludes the possibility that GSH depletion in casticin-treated cells could be a trivial, secondary consequence of cell death.
To shed light on the mechanisms accounting for GSH depletion as well as on the relationship between GSH depletion and apoptosis induction, we determined the effects of thiol antioxidants including NAC and GSH, and nonthiol antioxidants including butylated hydroxyanisole (BHA) and mannitol on GSH content and apoptosis by casticin treatment. Figure 6A and C show that thiol antioxidants, NAC and GSH, restored GSH content and attenuated casticin-induced apoptosis. In contrast, nonthiol antioxidants, BHA and mannitol, failed to do so (Figure 6A and C). The similar findings were observed in Hep G2 cell line (Figure 6D-F). The results suggest that casticin-induced apoptosis of HCC cells is at least partially dependent on GSH depletion.
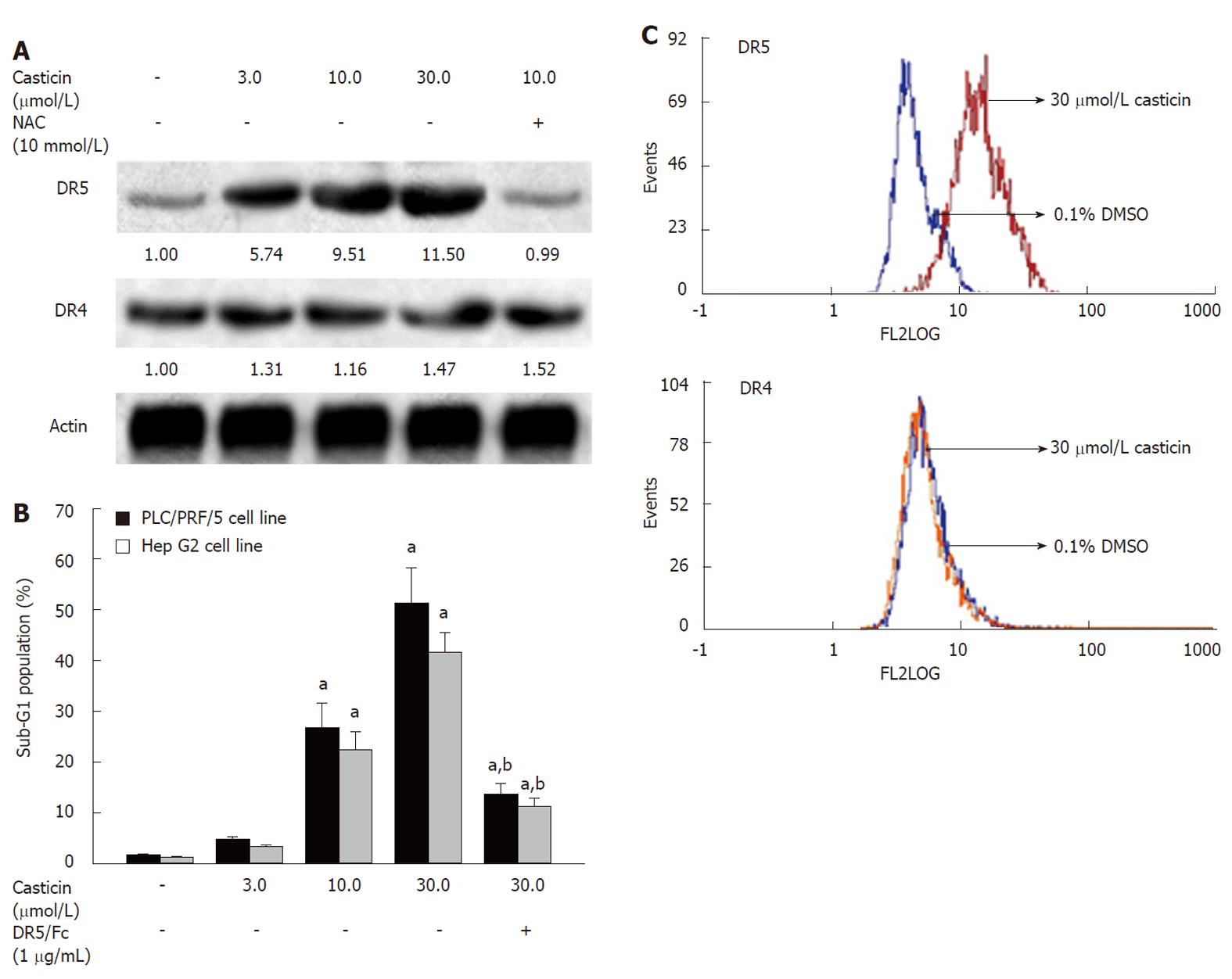
Because casticin induced caspase-8 activation (Figure 4A), we wondered whether casticin induced DR5 upregulation. Therefore, we compared the effects of casticin on the expression of DR4 and DR5 using Western blotting. In PLC/PRF/5 cell line, casticin increased the expression of DR5 at protein levels, but not affected the expression of DR4 protein; however, it failed to do so in the presence of NAC (Figure 7A). In addition, flow cytometry (FCM) analysis showed that casticin increased DR5 protein levels on the surface of Hep G2 cells by casticin treatment, but DR4 expression levels were not obviously altered (Figure 7 C).
To further define the role of DR5 upregulation in casticin-induced apoptotic cell death, we examined the effects of DR5/Fc chimera protein, a blocking antibody on induction of apoptosis by casticin. Figure 7B shows that the pretreatment with 1 μg/mL DR5/Fc chimera protein effectively attenuated induction of apoptosis by casticin in PLC/PRF/5 and Hep G2 cells. Our findings suggest that casticin-induced apoptosis of HCC cells is involved in upregulation of DR5.
The polymethoxyflavone from Fructus Viticis, casticin is a potent novel molecule with a wide range of actions, many of which are potentially useful for cancer prevention or treatment[20-23]. In the present study, we investigated the effects of casticin on the cell growth using two kinds of human HCC (PLC/PRF/5 with p53 mutant and HepG2 with p53 wild type) cell lines. We have demonstrated that casticin was a potent agent in inhibiting the growth of human HCC cells. The cancer suppressor p53 is an important factor that affects the cell response to drug effects on growth inhibition and apoptosis induction[24,25]. The majority of evidence supports the notion that cells with wild-type p53 exhibit increased sensitivity to radiation or chemotherapeutic agents, whereas cells lacking wild-type p53 expression still undergo apoptosis but need a relatively high dose of radiation or chemotherapeutic drugs[24,25]. The study by Haïdara et al[10] demonstrated the apoptotic effect of casticin in p53 mutant breast cancer cell lines. Our results indicated that casticin inhibited cell growth and induced apoptosis regardless of p53 status in human HCC cells. Therefore, we conclude that casticin-induced apoptosis in human cancer cells is p53-independent.
Apoptotic cells presented some common characteristics. In the stage of apoptosis, activation of a cascade of various caspases, was followed by DNA fragmentation, nuclear fragmentation, the appearance of apoptotic bodies and cellular shrinkage. Hence, caspase-3 activation, sub-G1 cell population, Histon/DNA fragment and DNA ladder were regarded as the characters specific for apoptosis. Our present study showed activation of caspase-3, increase of the percentage of sub-G1 population and Histone/DNA fragment and presentation of DNA ladder in casticin-treated HCC cells, demonstrating that casticin inhibited HCC cell growth through induction of apoptosis.
We also demonstrated that casticin induced apoptosis by depleting intracellular GSH content, rather than by promoting ROS generation, in HCC cells. Our findings are supported by the following lines of evidence: (1) casticin did not promote intracellular ROS generation although it decreased intracellular GSH content; and (2) Thiol-containing antioxidants rather than nonthiol antioxidants reduced casticin-induced apoptosis; this suppression correlated with their ability to prevent a casticin-induced decrease of GSH content. NAC, an aminothiol and synthetic precursor of intracellular cysteine and GSH, functions through either its antioxidative/radical scavenging properties as an antioxidant or its thiol-disulfide exchange activity as a reductant[26,27]. We found that only thiol containing antioxidants with reducing activity, including NAC and GSH, rather than nonthiol antioxidants, including BHA and mannitol, suppressed casticin-induced apoptosis. Therefore, it is likely that NAC and GSH inhibit casticin-induced apoptosis via their reducing activity. It appears that our findings have clinical implications regarding the rational use of casticin in combination with other agents for cancer prevention and/or treatment. The polymethoxyflavone should not be used in combination with the agents with reducing activity, such as NAC, in order to avoid the potential contradictory interaction.
The activation of caspases plays an important role in apoptosis triggered by various proapoptotic signals[28,29]. It is generally recognized that there are two major apoptotic pathways: one involves death signals transduced through death receptors, and the other relies on a signal from the mitochondria[22,28]. Both pathways are involved in an ordered activation of a set of caspases, which in turn cleave cellular substrates leading to the morphological and biochemical changes of apoptosis. The activation of caspase-8 and caspase-9 has been documented to play central roles in mediating apoptosis signaled by death receptors and by mitochondria, respectively[15,28]. However, caspase-8 can activate the caspase-9-mediated apoptotic pathway via activating or cleaving the Bid protein[28,29]. We found that casticin activated caspase-8 and -9. The presence of the caspase-8 inhibitor z-IETD-fmk and the caspase-9 inhibitor zLEHD-fmk attenuated the apoptosis induced by casticin, indicating that caspase-8 and -9 activation were required for the apoptosis induced by casticin in PLC/PRF/5 cells. Moreover, casticin upregulated DR5 expression, and DR5/Fc chimera protein reduced casticin–induced apoptosis, which shows that DR5 upregulation is required for casticin–induced apoptosis. Therefore, our results, for the first time, highlight a novel simultaneous both death receptor and mitochondria-mediated mechanism by casticin-induced apoptosis in HCC cells. The study by Zou et al[30] demonstrated that the synthetic triterpenoid methyl-2-cyano-3,12-dioxoolean-1,9-dien-28-oate induced upregulation of DR5 expression by activation of the CCAAT/enhancer binding protein homologous protein (CHOP) via intracellular GSH depletion-induced the endoplasmic reticulum stress. Quercetin, a flavonoid compound decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug, arsenic trioxide, in human leukemia cell lines[31]. However, why and how the intracellular GSH depletion induces DR5 expression upregulation need to be further investigated.
In summary, our studies have demonstrated that the polymethoxyflavone from Fructus viticis, casticin is a potent apoptosis-inducing agent in human HCC cells, which acts through depleting intracellular GSH content and upregulating DR5, and subsequent activation of caspase-3, -8 and -9. Moreover, casticin inhibited the growth of HCC cells independent of p53 status. For this reason, we suggest that casticin may be a good candidate for additional evaluation as a cancer therapeutic agent for human HCC as well as other types of cancer.
Hepatocellular carcinoma (HCC) is currently the fifth most common malignant neoplasm in the world, leading to over 600 000 deaths each year. HCC is prevalent in Asia and Africa and its incidence has steadily increased in European and American populations. New therapeutic agents for this malignant disease are urgently needed.
Casticin is one of the main components from Fructus Viticis (Manjingzi in Chinese name), a traditional Chinese medicine prepared from the fruit of Vitex trifolia L. (family Verbenaceae) that is also used as an anti-inflammatory agent and for the treatment of certain cancers in China. In recent years, many studies have demonstrated its anti-carcinogenic activity in breast cancer, lung cancer and colon cancer. However, the precise mechanisms underlying casticin-induced apoptosis of HCC cells is still not clear.
In this study, the effects and molecular mechanism of casticin on apoptotic cell death of HCC cells in vitro were studied. Casticin significantly induced apoptosis of HCC cells by glutathione (GSH) depletion and upregulation of reactive oxygen species .
Casticin is a potent apoptosis-inducing agent in human HCC cells, which acts through depleting intracellular GSH content and upregulating DR5, and subsequent activation of caspase-3, -8 and -9. Moreover, casticin inhibited the growth of HCC cells independent of p53 status. The authors suggested that casticin may be a good candidate for additional evaluation as a cancer therapeutic agent for human HCC as well as other types of cancer.
Casticin is one of the main components from Fructus Viticis (Manjingzi in Chinese name), a traditional Chinese medicine prepared from the fruit of Vitex trifolia L. (family Verbenaceae).
In this manuscript, the authors demonstrate that the treatment with casticin, main components from Fructus Viticis, induced apoptosis through the depletion of glutathione DR5 up-regulation in HCC cells. A series of experiments were well planned and well performed, and the manuscript is well written. The findings are important to those with closely related research interest.
Peer reviewer: Kotaro Miyake, MD, PhD, Department of Surgery, Institute of Health Biosciences, The University of Tokushima Graduate School, 3-18-15 Kuramoto, Tokushima 770-8503, Japan
S- Editor Lv S L- Editor Ma JY E- Editor Zhang DN
| 1. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [PubMed] [Cited in This Article: ] |
| 2. | Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142-1143. [PubMed] [Cited in This Article: ] |
| 4. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2221] [Cited by in F6Publishing: 2124] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] [Cited in This Article: ] |
| 6. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. [PubMed] [Cited in This Article: ] |
| 7. | The State Pharmacopoeia Commission of China. Pharmacopoeia of the Peoples Republic of China. Beijing: Chemical Industry Press 2000; 185-189. [Cited in This Article: ] |
| 8. | You KM, Son KH, Chang HW, Kang SS, Kim HP. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines in vitro. Planta Med. 1998;64:546-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Lin S, Zhang H, Han T, Wu JZ, Rahman K, Qin LP. In vivo effect of casticin on acute inflammation. Zhong Xi Yi Jie He Xue Bao. 2007;5:573-576. [PubMed] [Cited in This Article: ] |
| 10. | Haïdara K, Zamir L, Shi QW, Batist G. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006;242:180-190. [PubMed] [Cited in This Article: ] |
| 11. | Kobayakawa J, Sato-Nishimori F, Moriyasu M, Matsukawa Y. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.). Cancer Lett. 2004;208:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Ko WG, Kang TH, Lee SJ, Kim NY, Kim YC, Sohn DH, Lee BH. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem Toxicol. 2000;38:861-865. [PubMed] [Cited in This Article: ] |
| 13. | Shen JK, Du HP, Yang M, Wang YG, Jin J. Casticin induces leukemic cell death through apoptosis and mitotic catastrophe. Ann Hematol. 2009;88:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Cao JG, Peng SP, Sun L, Li H, Wang L, Deng HW. Vascular basement membrane-derived multifunctional peptide, a novel inhibitor of angiogenesis and tumor growth. Acta Biochim Biophys Sin (Shanghai). 2006;38:514-521. [PubMed] [Cited in This Article: ] |
| 15. | Yang XH, Zheng X, Cao JG, Xiang HL, Liu F, Lv Y. 8-Bromo-7-methoxychrysin-induced apoptosis of hepatocellular carcinoma cells involves ROS and JNK. World J Gastroenterol. 2010;16:3385-3393. [PubMed] [Cited in This Article: ] |
| 16. | Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, Heyman RA, Teng M, Chandraratna RA, Shudo K. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931-4939. [PubMed] [Cited in This Article: ] |
| 17. | Sun SY, Yue P, Lotan R. Induction of apoptosis by N-(4-hydroxyphenyl)retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors, and apoptosis-related genes in human prostate carcinoma cells. Mol Pharmacol. 1999;55:403-410. [PubMed] [Cited in This Article: ] |
| 18. | Kachadourian R, Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med. 2006;41:65-76. [PubMed] [Cited in This Article: ] |
| 19. | Kachadourian R, Leitner HM, Day BJ. Selected flavonoids potentiate the toxicity of cisplatin in human lung adenocarcinoma cells: a role for glutathione depletion. Int J Oncol. 2007;31:161-168. [PubMed] [Cited in This Article: ] |
| 20. | Ono M, Yanaka T, Yamamoto M, Ito Y, Nohara T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J Nat Prod. 2002;65:537-541. [PubMed] [Cited in This Article: ] |
| 21. | Díaz F, Chávez D, Lee D, Mi Q, Chai HB, Tan GT, Kardono LB, Riswan S, Fairchild CR, Wild R. Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo. J Nat Prod. 2003;66:865-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Li WX, Cui CB, Cai B, Wang HY, Yao XS. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J Asian Nat Prod Res. 2005;7:615-626. [PubMed] [Cited in This Article: ] |
| 23. | Csupor-Löffler B, Hajdú Z, Zupkó I, Réthy B, Falkay G, Forgo P, Hohmann J. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother Res. 2009;23:672-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | O'Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285-4300. [PubMed] [Cited in This Article: ] |
| 25. | Pirollo KF, Bouker KB, Chang EH. Does p53 status influence tumor response to anticancer therapies? Anticancer Drugs. 2000;11:419-432. [PubMed] [Cited in This Article: ] |
| 26. | Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205-227. [PubMed] [Cited in This Article: ] |
| 27. | Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591-40598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | He Q, Huang Y, Sheikh MS. Proteasome inhibitor MG132 upregulates death receptor 5 and cooperates with Apo2L/TRAIL to induce apoptosis in Bax-proficient and -deficient cells. Oncogene. 2004;23:2554-2558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Zou W, Yue P, Khuri FR, Sun SY. Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res. 2008;68:7484-7492. [PubMed] [Cited in This Article: ] |
| 31. | Ramos AM, Aller P. Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochem Pharmacol. 2008;75:1912-1923. [PubMed] [Cited in This Article: ] |