Published online Jul 28, 2011. doi: 10.3748/wjg.v17.i28.3286
Revised: September 2, 2010
Accepted: September 9, 2010
Published online: July 28, 2011
Fistula-in-ano is a difficult problem that physicians have struggled with for centuries. Appropriate treatment is based on 3 central tenets: (1) control of sepsis; (2) closure of the fistula; and (3) maintenance of continence. Treatment options continue to evolve - as a result, it is important to review old and new options on a regular basis to ensure that our patients are provided with up to date information and options. This paper will briefly cover some of the traditional approaches that have been used as well as some newer promising procedures.
- Citation: Bleier JI, Moloo H. Current management of cryptoglandular fistula-in-ano. World J Gastroenterol 2011; 17(28): 3286-3291
- URL: https://www.wjgnet.com/1007-9327/full/v17/i28/3286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i28.3286
Fistula-in-ano is a difficult problem that physicians have struggled with since the time of Hippocrates. The ideal treatment is based on 3 central tenets: (1) Control of sepsis; (2) closure of the fistula; and (3) maintenance of continence. Treatment options have continued to evolve - as a result, it is important to review old and new options on a regular basis to ensure that our patients are provided with up to date information and options. The incidence of fistula-in-ano is 9 per 100 000 (compared to 6 per 100 000 for Crohn’s disease and 8 per 100 000 for ulcerative colitis)[1], and is therefore a problem commonly encountered. This paper will briefly cover some of the approaches that have traditionally been used as well as some newer promising procedures.
The reader should be aware that despite the long standing history of fistula-in-ano and the multiple approaches that are utilized, there is a paucity of high quality data to guide decision making. A recent Cochrane review concluded among other things that there was a “crying need for well powered, well conducted randomized controlled trials” and that recurrence and rates of incontinence were the most important factors when considering repair[2]. We agree with this assessment, and base our management on the best available data. However, this illustrates the need for continual review of appropriate management techniques.
One of the most clinically useful classification systems for perianal fistulas (by the American Gastroenterological Association) divides them into simple and complex - this classification facilitates operative decision making[3]. Simple fistulas are low - i.e. they involve a small (or sometimes none) portion of the sphincter complex. These fistulas include superficial, low intersphincteric or low trans-sphincteric fistulae. In addition, communication between the anal canal and skin is only via one tract and is not associated with inflammatory bowel disease, radiation or involving any other organs.
Conversely, complex fistulas are anatomically higher: they involve significant portions of the sphincter musculature, may have multiple tracts, involve other organs (i.e. vagina) and may be associated with radiation or inflammatory bowel disease. Recurrent fistulas are usually included in this category as well.
The management of superficial fistulae is fairly straightforward. Superficial or simple fistulas that, by definition, do not traverse any (or an insignificant portion) of the sphincter musculature should be treated with simple fistulotomy. This is a time-honored, effective approach with a success rate that approaches 100%, with little or no effect on continence[4]. Lay-open fistulotomy is easy, and management of these wounds is straightforward. These procedures involve unroofing of the fistula tract and curettage of the epithelialized lining. Conservative wound care with sitz baths and analgesics postoperatively is usually all that is needed. There is some evidence to suggest that healing is quicker with marsupialization of the tract[5].
Intersphincteric fistulae arise from cryptoglandular infections that remain contained between the internal and external sphincters. Partial division of the internal sphincter alone is standard treatment for other benign anorectal disease such as anal fissure, and is similarly safe. This technique involves cutting a portion (usually to a maximum of 30%) of the internal sphincter only, and yet maintains excellent preservation of continence. From this we extrapolate that division of the internal sphincter along the length of an intersphincteric fistula is similarly safe, and with limited changes in continence, is also an acceptable form of treatment of intersphincteric fistula. If an intersphincteric fistula involves a significant portion of the internal sphincter (over 30%) then thought should be given to a sphincter sparing-type procedure - incontinence can result if too much internal sphincter muscle is divided.
Fistulae that traverse a significant portion of both sphincter muscles are termed trans-sphincteric and are part of the group of complex fistulae. Lay-open fistulotomy along these tracts are effective at fistula closure for the same pathophysiologic reasons as simple fistulae but, based on the amount of musculature involved, may result in significant changes in continence, violating one of the basic principles of appropriate management. Thus, this is no longer considered an acceptable approach.
Initial management of complex or trans-sphincteric fistulae begins with control of the septic focus. Initially this may involve drainage of anorectal abscess, and if the fistula tract can be identified, a draining seton should be placed. This is a foreign piece of material inserted through the fistula tract which functions to maintain the fistula tract in an open state, preventing a closed space infection, and allowing for drainage and sepsis resolution. This is a safe, and usually simple, method for control of the basic pathophysiologic insult that creates the fistula. Appropriate complex fistula management almost always indicates initial seton placement. By allowing for resolution of sepsis, and establishment of a well-formed tract, the clinician is offered the luxury of time and the ability to characterize the anatomy of the fistula, which underpins effective subsequent management.
The draining seton is usually a silastic or similarly biologically inert, low profile material that does not incite an inflammatory reaction. We typically use a vessel loop. The fistula tract is identified, and traversed with a fistula probe if possible. A suture is then attached and pulled through. The other end is then tied to the vessel loop and then pulled through. The vessel loop is tied to itself with a permanent suture in a loose fashion. In addition, the external fistula opening is usually widened and debrided of chronic granulation tissue. In this manner, almost any fistula, with its associated local sepsis is temporized, and infection and inflammation can clear. Appropriate anorectal hygiene in the form of sitz baths or showering is used until the infection clears. This creates a stable situation in which a fistula can be maintained indefinitely. Once sepsis and inflammation have cleared, the presence of the seton in an uninfected tract allows for accurate delineation of the fistula anatomy, either via careful clinical exam, ultrasound or radiologic study. If there is any question as to the possibility of a significant trans-sphincteric component at the time of initial operation, a seton should be placed, and minimal added morbidity will be incurred.
One option for subsequent management of a trans-sphincteric fistula is the use of a cutting seton. The principle that underlies this is the hypothesis that slow division of the muscle allows for fibrosis and scarring and that overall integrity of the sphincter complex is maintained. The technique involves sequential tightening of the seton through the fistula tract by way of serial placement of additional suture material on the seton and/or by asking the patient to pull on the seton at a regular frequency (once every few days), or by tightening of the original seton. Many different types of setons can be used for this, including the initially placed silastic seton, or more commonly, the silastic seton is replaced with a silk suture which is narrower, and inflammation-inducing. This allows for faster cutting and induction of scarring. This procedure, however, has several disadvantages, despite a sound theoretical basis: It requires frequent office visits for tightening, sometimes weekly or bi-weekly. It is usually quite uncomfortable or painful for the patient, and usually results in the need for narcotic or non-steroidal analgesia. Most importantly, there is substantial literature which demonstrates that despite the scarring through a maintained sphincter complex, continence can be negatively affected. This procedure must be approached with caution, especially in view of the sphincter sparing procedures, which are available. While reported success rates have been reported as similar to fistulotomy, changes in continence have been reported in greater than 60% of patients[6].
The advancement flap is a technique which is designed to address the pathophysiology of the fistula in a sphincter-sparing approach by closing the internal opening, thus depriving the fistula of it’s source of sepsis, and allowing the defunctionalized tract to heal by secondary intention.
The endoanal advancement approach involves advancing a healthy sleeve of rectal wall over the debrided internal opening, and suturing the flap over and distal to the internal opening. This is based on a broad pedicled flap dissected from the healthy proximal rectum. This procedure is technically more difficult and is often plagued by difficult exposure, especially on posteriorly located fistulae within the rectal hollow. Fistulas with higher internal openings are often quite difficult to reach as well. Additionally, this procedure involves the creation of a large defect in previously undamaged rectum, and runs the risk of devascularization and loss of a much larger portion of rectal wall. Failure or ischemia of these flaps may result in the creation of a much larger defect than existed previously. In addition, dissection in a scarred or chronically inflamed plane can place the sphincter at risk. Success rates for this approach vary widely through the literature, and range from 0 to 63%[7,8].
Cutaneously based flaps are advanced from the anodermal skin over the internal opening, and are based on pedicled flap principles as well. These flaps avoid placing otherwise healthy rectum at risk, but also have their own associated morbidities. These similarly require extensive experience with maintaining viability of skin-based flaps, and also run the risk of injuring the sphincter. Additionally, advancement of anodermal skin into the anal canal may result in chronic irritation and seepage, pathophysiologically akin to the ectropion, which may be the result of over-extensive hemorrhoidectomy. Data are sparse regarding the optimal flap approach. Mucosa-only flaps may minimize the risk to unaffected rectum, but a randomized trial comparing partial thickness advancement to mucosa alone demonstrated improved efficacy in fistula closure with thicker flaps. A retrospective study by the Cleveland Clinic, Florida found only a 33% recurrence rate for flaps used in non-inflammatory bowel disease (IBD) patients. Zimmerman[9] reported on his group’s success with anodermal advancement flaps and found that when used as initial therapy, this method had a greater than 75% success rate, with maintained continence in over 80%. Importantly Mitalas[10] found that repeat approaches using endoanal flaps still had a significant success rate, with 67% of patients having long-term success.
Fistulectomy is a less commonly performed technique for trans-sphincteric fistulae. It is based on the principle that removal of the chronic, epithelialized tract will allow healing by secondary intention of healthier tissue. Typically, the fistula tract is cored out over a probe or seton, leaving healthy peri-rectal fat only. Dissection is typically carried out from the external opening up to the external sphincter. This is a difficult and potentially morbid technique which may leave large tissue defects and may involve injury to the sphincter complex. Success rates are similar to fistulotomy, and subsequent incontinence rates have been shown to be as high as 15%[11]. Malik’s meta-analysis found only 2 studies comparing fistulectomy to fistulotomy, and found no significant difference between the two[5].
Fibrin glue injection was the first exciting modern development in sphincter-sparing approaches to complex fistulae. The technique is based on the injection of a liquid fibrin matrix through any fistula tract which would facilitate healthy tissue ingrowth and fistula closure. The major advantage is the extremely benign nature of the approach. It requires no dissection or risk to the sphincter musculature regardless of the anatomy or complexity of the fistula tract, and has potential applications in IBD as well. However, despite early enthusiasm, long-term results have been quite disappointing with success rates as low as 16%[12-16]. Few randomized trials document fibrin glue efficacy; only one small trial comparing it to conservative management showed improved outcomes with glue therapy. Draining setons were used, resulting in an unsurprisingly low 13% (since no fistulae were deliberately closed) cure rate, while 43% were cured with fibrin glue. A more recent review reported on a recurrence rate ranging from 10%-78%[17]. Newer approaches have modified the use of fibrin glue with the addition of adipose-derived stem cells, and this approach shows promise: in one study 71% of patients with the enhanced approach healed their fistulae, compared to 16% with fibrin glue alone.
Currently, we do not advocate this approach as a primary therapy given its lack of demonstrated success. Currently, it is most often used as an adjunct measure when combined with other methods such as advancement flap, but has demonstrated little success in the literature[13]. Nevertheless, it remains a very safe technique with minimal downside other than expense and time, and may be considered prior to more invasive options when other methods have failed.
The anal fistula plug (AFP) is a simple repair that does not involve an extensive dissection and therefore is a very attractive approach. Essentially, the plug is pulled through the fistula tract and secured in place at the internal opening (the wider portion of the plug is at the internal opening) and trimmed to the skin at the external opening with the external opening left open to drain.
Initial reports documented a very high success rate, with the initial descriptions by Ellis and Johnson documenting close to 80% success[18]. This was further supported by a study from Case Western documenting an 83% success rate[19], however, with more studies the overall success rate has been found to be lower with some studies reporting a 20% success rate[18,20,21]. Jacob and Keighley’s recent meta-analysis found success rates ranging from 35%-85%[2].
Thus far, it does not appear to be associated with any major complications and can be regarded as a sphincter-sparing approach. No impact on continence has been found. Wang compared the use of the fistula plug to endoanal flap advancement; both “sphincter-sparing” approaches, and found that the flap approach enjoyed an improved long-term closure rate[22]. These results were echoed by a study from the University of Minnesota[23]. Currently, we are conducting a randomized prospective trial comparing the plug to the ligation of the intersphincteric fistula tract (LIFT) procedure (see below).
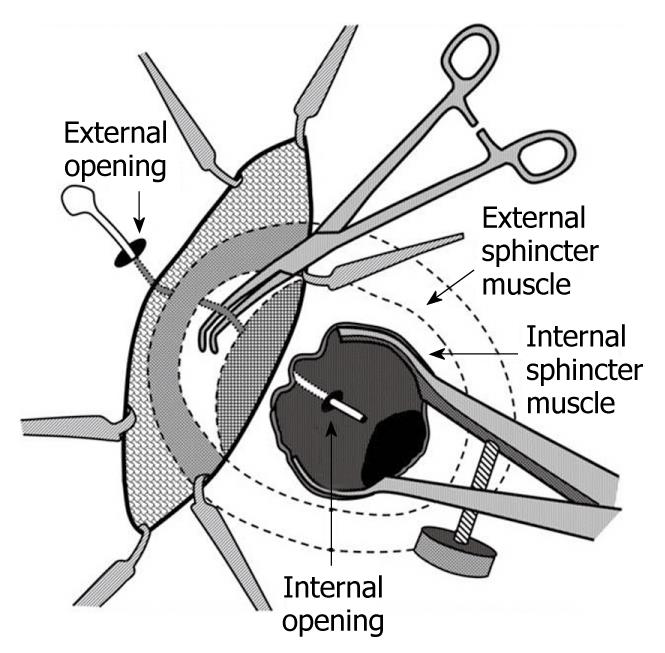
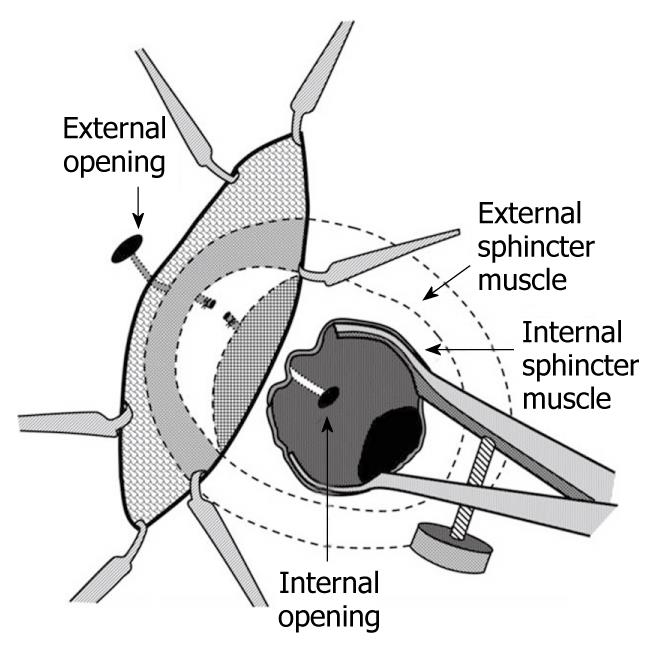
In 2007, Rojanasakul described the LIFT procedure, in which the fistula tract is identified between the internal and external sphincters (intersphincteric space) and subsequently divided and ligated. His group initially reported a 94% success rate with no impact on continence (Figure 1)[24]. The procedure is appealing as it appears to be a “sphincter-sparing” technique and is a relatively simple operation to perform. The first step is to identify the intersphincteric groove. Once the skin is incised in this area, a combination of blunt and sharp dissection is used to identify the fistula tract - a task made easier if a draining seton has been left in place for at least 6 wk (Figure 1). Once the tract is identified, it is ligated on both sides and divided (Figure 2).
There have been further reports from North America and Malaysia - these have shown lower success rates of 57% and 77%, respectively[25,26]. However, compared to other procedures this still offers a comparable success rate. Other advantages of the procedure are the low cost and the fact that that even if it does not work, other approaches can still be utilized. The long-term success of this technique remains to be determined, as well as waiting to see if these success rates can be duplicated by other centers. Other studies are underway examining the efficacy of the LIFT and there is also a randomized controlled trial comparing the LIFT to the AFP.
Recently, a modification of the LIFT procedure has been described. After the fistula tract is identified and divided, a biologic mesh is placed in the intersphincteric space to act as a barrier to re-fistulization. A video presented at the 2010 American Society of Colon and Rectal Surgeons meeting demonstrated this technique with promising results in a single surgeon series. This procedure entails a significant dissection of a large portion of the intersphincteric space, all the way up to the levator musculature, and the placement of a large piece of biologic mesh. Given the complexity and magnitude of this method, it may not be appropriate as first-line therapy.
First, is the fistula simple or complex? This will be elucidated by history, physical and appropriate use of imaging.
If it is a simple fistula, a primary fistulotomy with marsupialization of the wound is likely the best option as the addition of marsupialization may lead to faster wound healing.
In a complex fistula, treatment should be individualized. However, the authors advocate sphincter-sparing approaches first (after a draining seton has been placed for 6-12 wk). Based on evidence and simplicity of approach, the authors generally begin with either a fistula plug or LIFT procedure (and since we had clinical equipoise concerning these two repairs we are involved in a randomized controlled trial comparing the two; there is a PLUG trial also underway comparing the plug to advancement flap). We do not use the advancement flap as our first option because it is a more extensive operation and the “downside” of failure is bigger. An important point regarding the use of LIFT is the fact that it appears to “burn no bridges”; if a LIFT procedure fails, it sometimes results in an intersphincteric fistula, which then may more safely be treated with a primary fistulotomy, and there have been no reported issues in continence after this procedure. Alternatively, the failure may still result in a trans-sphincteric fistula (and when the plug fails it always results in the same type of fistula that was originally treated). Prior to attempting definitive management again, a draining seton is usually replaced, remaining in place for 6-12 wk. We usually try a LIFT or plug again but if this fails, we then move on to other options. Further imaging after failures can also be helpful to ensure that additional tracts have not been missed.
Crohn’s disease: Thirty percent of Crohn’s patients will experience perianal disease, including fistula-in-ano. Fistulae in Crohn’s patients usually have complex tracts and are often multiple and arborizing, making the treatment challenging. Collaboration between surgeon and gastroenterologist is critical with this condition as medical treatment with immunomodulators (i.e. Remicade and Humira) have demonstrated significant success in perianal fistula closure.
Like other cryptoglandular fistulae, these fistulas can have an acute and chronic phase - in the acute phase they can be associated with local infection and prior to initiating medical treatment, this focus of sepsis usually needs to be dealt with. An examination under anesthesia with drainage of the septic source, as well as placement of a draining seton (or setons) is appropriately employed. With the draining seton in place, it is rare to develop a recurrent abscess and medical therapy can be initiated. Chronic inflammation and proctitis usually define the chronic phase of this disease. Communication between surgeon and gastroenterologist should occur to determine optimal initial medical management, and subsequently, to decide if there is a role for removing the seton when the Crohn’s disease seems more quiescent. Further options can be considered as discussed below.
If there is a well-established, chronically draining fistula without associated abscess at initial presentation, treatment can be individualized, but in cases where there is still occasional purulent drainage, placement of a draining seton is encouraged to help resolve any residual infection. Primary fistulotomies should be avoided in Crohn’s patients as these are usually complex tracts involving significant portions of the sphincter, and impairment of sphincter function, especially in patients who are prone to looser bowel movements, can exacerbate continence issues. In addition, in the presence of multiple fistulas, the cumulative effect of several (even if they are superficial) fistulotomies can cause significant sphincter dysfunction. Treatment of a Crohn’s fistula depends on what technique the surgeon is most comfortable with, but conservative, sphincter-sparing approaches are the most appropriate. Options include mucosal advancement flaps, AFPs, the LIFT and possibly, the BioLIFT. Because of the potential for changes in continence that can be seen with a cutting seton, this approach is not one advocated by the authors. It is important to note that most approaches to repair are doomed to failure in the face of active proctitis, and thus all definitive treatment other than sepsis control need to be delayed until effective management of the disease process has been achieved.
Rectovaginal and rectourethral fistula: The vast majority of rectovaginal (RV) fistulas (> 80%) are the result of obstetrical trauma[27]. Other causes include inflammatory bowel disease, infection (from cryptoglandular origin, diverticulitis or Bartholin’s gland infection), radiation or neoplasm. RV fistulas are classified as “high” or “low”. Anovaginal fistulas are considered low fistulas and involve the sphincter mechanism. High fistulas are those that have their origin above the sphincter complex. The operative approach differs widely based on this anatomic classification. A careful history and physical examination is used to determine the underlying etiology. Further investigations such as colonoscopy, computed tomography scan, ultrasound, and pelvic magnetic resonance imaging can be useful to identify the etiology and anatomy. If obstetrical trauma is the suspected cause, an endoanal ultrasound can be used to assess if there is a sphincter defect along with manometry to document sphincter resting and squeeze pressures. Pudendal nerve latency testing can also be done to give the patient an idea of potential success rate if a sphincteroplasty is performed (if there appears to be nerve damage, a sphincteroplasty will have a lower success rate).
With a RV fistula and concomitant sphincter injury from obstetrical trauma, a sphincteroplasty is the best option. Although the technique may be beyond the scope of this review, this involves a perineal approach and has good short-term success but longer term functional outcome begins to drop off in 3-5 years.
Low RV fistulas that are not associated with a sphincter defect are classified as complex perianal fistulas as discussed in the Introduction. Options that can be used include advancement flaps (both rectal and vaginal), LIFT procedure, BioLIFT, and AFP. In some cases where the fistula is large or after the failure of multiple previous attempts at local closure, the use of pedicled muscle flaps, such as the bulbocavernosus (Martius) or gracilis flap may be required, usually accompanied by temporary gastrointestinal (GI) tract diversion.
High RV fistulas such as those that result from diverticulitis are managed by a transabdominal approach, usually requiring proctectomy or colectomy. Continuity via a coloanal anastomosis can be restored depending on the clinical scenario.
Rectourethral (RU) fistula is a rare complication usually seen after intervention in the male genitourinary tract. Appropriate management and maximizing success in treating RU fistulas relies on knowing the etiology and prior history of the patient. RU fistula most commonly arises as a complication after radical prostatectomy. Iatrogenic injuries to the rectum or local sepsis after anastomotic dehiscence are the most common causes. Rarely, minimally symptomatic patients may be observed, or an initial attempt at local repair with flap techniques may be employed. The use of an indwelling catheter is critical to healing. Although success rates are low, there is little downside to such an attempt in this highly-selected population. In cases where there are significant symptoms, and there is no history of pelvic radiation or IBD, the most appropriate first step in management is GI diversion. Up to one third of RU fistulas may heal with diversion alone[28]. Local flap repairs may then be employed if spontaneous healing does not occur. Success rates of local advancement flaps are improved if the patient is diverted.
If initial attempts at closure fail, the defect is very large, or if the patient has had prior pelvic irradiation, local closure techniques are doomed to failure. Repairs using local pedicle muscle flaps (i.e. gracilis or dartos flaps) are usually required for successful closure. Once closure of the fistula is documented (usually via contrast study) the diverting stoma can be closed.
Perianal fistulas present a common but challenging problem because of the involvement of the sphincter complex. Complex fistula treatment must always take in to account the need to spare sphincter function. Various treatments exist which indicates that there is no universally successful solution. Sphincter-sparing options continue to evolve and continued review of new techniques is important prior to proceeding with procedures that may impair continence; it is important that clinicians stay abreast of these changes so patients can be given the opportunity to access sphincter-sparing options.
Peer reviewer: AM El-Tawil, MSc, MRCS, PhD, Department of Surgery, University Hospital of Birmingham, East Corridor, Ground Floor, Birmingham, B15 2TH, United Kingdom; Benjamin Perakath, Professor, Dr., Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India
S- Editor Sun H L- Editor Webster JR E- Editor Zheng XM
| 1. | Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73:219-224. [Cited in This Article: ] |
| 2. | Jacob TJ, Perakath B, Keighley MR. Surgical intervention for anorectal fistula. Cochrane Database Syst Rev. 2010;CD006319. [Cited in This Article: ] |
| 3. | Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508-1530. [Cited in This Article: ] |
| 4. | Rizzo JA, Naig AL, Johnson EK. Anorectal abscess and fistula-in-ano: evidence-based management. Surg Clin North Am. 2010;90:45-68, Table of Contents. [Cited in This Article: ] |
| 5. | Malik AI, Nelson RL. Surgical management of anal fistulae: a systematic review. Colorectal Dis. 2008;10:420-430. [Cited in This Article: ] |
| 6. | García-Aguilar J, Belmonte C, Wong DW, Goldberg SM, Madoff RD. Cutting seton versus two-stage seton fistulotomy in the surgical management of high anal fistula. Br J Surg. 1998;85:243-245. [Cited in This Article: ] |
| 7. | Ortíz H, Marzo J. Endorectal flap advancement repair and fistulectomy for high trans-sphincteric and suprasphincteric fistulas. Br J Surg. 2000;87:1680-1683. [Cited in This Article: ] |
| 8. | van der Hagen SJ, Baeten CG, Soeters PB, van Gemert WG. Long-term outcome following mucosal advancement flap for high perianal fistulas and fistulotomy for low perianal fistulas: recurrent perianal fistulas: failure of treatment or recurrent patient disease? Int J Colorectal Dis. 2006;21:784-790. [Cited in This Article: ] |
| 9. | Zimmerman DD, Briel JW, Schouten WR. Endoanal advancement flap repair for complex anorectal fistulas. Am J Surg. 2001;181:576-577. [Cited in This Article: ] |
| 10. | Mitalas LE, Gosselink MP, Zimmerman DD, Schouten WR. Repeat transanal advancement flap repair: impact on the overall healing rate of high transsphincteric fistulas and on fecal continence. Dis Colon Rectum. 2007;50:1508-1511. [Cited in This Article: ] |
| 11. | Schouten WR, van Vroonhoven TJ. Treatment of anorectal abscess with or without primary fistulectomy. Results of a prospective randomized trial. Dis Colon Rectum. 1991;34:60-63. [Cited in This Article: ] |
| 12. | Buchanan GN, Bartram CI, Phillips RK, Gould SW, Halligan S, Rockall TA, Sibbons P, Cohen RG. Efficacy of fibrin sealant in the management of complex anal fistula: a prospective trial. Dis Colon Rectum. 2003;46:1167-1174. [Cited in This Article: ] |
| 13. | Ellis CN, Clark S. Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum. 2006;49:1736-1740. [Cited in This Article: ] |
| 14. | Sentovich SM. Fibrin glue for anal fistulas: long-term results. Dis Colon Rectum. 2003;46:498-502. [Cited in This Article: ] |
| 15. | Sentovich SM. Fibrin glue for all anal fistulas. J Gastrointest Surg. 2001;5:158-161. [Cited in This Article: ] |
| 16. | Gisbertz SS, Sosef MN, Festen S, Gerhards MF. Treatment of fistulas in ano with fibrin glue. Dig Surg. 2005;22:91-94. [Cited in This Article: ] |
| 17. | Swinscoe MT, Ventakasubramaniam AK, Jayne DG. Fibrin glue for fistula-in-ano: the evidence reviewed. Tech Coloproctol. 2005;9:89-94. [Cited in This Article: ] |
| 18. | Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49:371-376. [Cited in This Article: ] |
| 19. | Champagne BJ, O’Connor LM, Ferguson M, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49:1817-1821. [Cited in This Article: ] |
| 20. | Christoforidis D, Etzioni DA, Goldberg SM, Madoff RD, Mellgren A. Treatment of complex anal fistulas with the collagen fistula plug. Dis Colon Rectum. 2008;51:1482-1487. [Cited in This Article: ] |
| 21. | Lawes DA, Efron JE, Abbas M, Heppell J, Young-Fadok TM. Early experience with the bioabsorbable anal fistula plug. World J Surg. 2008;32:1157-1159. [Cited in This Article: ] |
| 22. | Wang JY, Garcia-Aguilar J, Sternberg JA, Abel ME, Varma MG. Treatment of transsphincteric anal fistulas: are fistula plugs an acceptable alternative? Dis Colon Rectum. 2009;52:692-697. [Cited in This Article: ] |
| 23. | Christoforidis D, Pieh MC, Madoff RD, Mellgren AF. Treatment of transsphincteric anal fistulas by endorectal advancement flap or collagen fistula plug: a comparative study. Dis Colon Rectum. 2009;52:18-22. [Cited in This Article: ] |
| 24. | Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90:581-586. [Cited in This Article: ] |
| 25. | Bleier JI, Moloo H, Goldberg SM. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53:43-46. [Cited in This Article: ] |
| 26. | Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53:39-42. [Cited in This Article: ] |
| 27. | Venkatesh KS, Ramanujam PS, Larson DM, Haywood MA. Anorectal complications of vaginal delivery. Dis Colon Rectum. 1989;32:1039-1041. [Cited in This Article: ] |