Published online Jul 7, 2011. doi: 10.3748/wjg.v17.i25.3027
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: July 7, 2011
AIM: To determine hepatitis C virus (HCV) seroprevalence and its genotypes, and to identify the factors associated with HCV infection.
METHODS: This cross-sectional study, conducted in two prisons (one male and one female) in the State of Sergipe, Brazil, comprised 422 subjects. All of the prisoners underwent a rapid test for the detection of HCV antibodies. Patients with a positive result were tested for anti-HCV by enzyme linked immunosorbent assay and for HCV RNA by qualitative polymerase chain reaction (PCR). The virus genotype was defined in every serum sample that presented positive for PCR-HCV. In order to determine the factors independently associated with positive serology for HCV, multivariate logistic regression was used.
RESULTS: HCV seroprevalence was 3.1%. Of the 13 subjects with positive anti-HCV, 11 had viremia confirmed by PCR. Of these, 90.9% had genotype 1. A total of 43 (10.2%) were injecting drug users, and HCV seroprevalence in this subgroup was 20.6%. The variable most strongly associated with positive serology for HCV was use of injecting drugs [odds ratio (OR), 23.3; 95% confidence interval (CI), 6.0-90.8]. Age over 30 years (OR, 5.5; 95%CI, 1.1-29.2), history of syphilis (OR, 9.8; 95%CI, 1.7-55.2) and history of household contact with HCV positive individual (OR, 14.1; 95%CI, 2.3-85.4) were also independently associated with HCV infection.
CONCLUSION: Most of the HCV transmissions result from parenteral exposure. However, there is evidence to suggest a role for sex and household contact with an infected subject in virus transmission.
- Citation: Santos BFO, Santana NO, Franca AVC. Prevalence, genotypes and factors associated with HCV infection among prisoners in Northeastern Brazil. World J Gastroenterol 2011; 17(25): 3027-3034
- URL: https://www.wjgnet.com/1007-9327/full/v17/i25/3027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i25.3027
Hepatitis C virus (HCV) is one of the main causes not only of chronic viral hepatitis, but also of cirrhosis and end-stage liver disease in the world[1]. Hepatitis C, given its treatment costs, high morbidity and mortality, generates a significant burden in healthcare systems. According to the World Health Organization, there are over 170 million people with chronic hepatitis C and approximately 3 to 4 million new cases each year[2].
HCV seroprevalence in the general population has wide geographical variation. Studies performed in Brazil have shown an anti-HCV prevalence of 5.9% in the Amazon region, 0.9% in the State of Rio de Janeiro and 0.34% in the State of Santa Catarina[3].
There are six HCV genotypes, with several subtypes. For each genotype, there is a different pattern of treatment response and, consequently, a distinct therapeutic approach[4,5]. There is a wide geographical variation when it comes to genotype distribution, so that genotypes 1, 2 and 3 are more frequent in Europe, the USA and Japan; genotype 4 in central Africa, Egypt and the Middle East; genotype 5 in South Africa; and genotype 6 in Asia. Brazil in general has a higher prevalence of genotype 1, followed by genotype 3[6].
Most HCV transmissions are due to parenteral exposure. It has been estimated that HCV is 10 times more infectious than HIV, per unit of blood, requiring less exposure to reach high prevalence[1]. Other routes have been described, such as sexual and vertical transmission, but these are less common than the parenteral one. Risk factors already proposed include use of injecting drugs (ID), tattoos, occupational blood exposure and hemodialysis[4,5]. It is important to highlight that HCV prevalence is higher in certain groups, such as prisoners[4]. Although these subjects represent only 0.8% of the American population, approximately 39% of the cases of chronic HCV infection have a history of imprisonment[7]. There are several international studies which determined hepatitis C prevalence in prisons, but studies in Latin America are scarce.
The following factors are related to higher prevalence of HCV in prisoners: duration of incarceration, use of ID, adverse socioeconomic situation and poor health care. Therefore, there is a potential public health issue, since the prison system works as a concentrator of hepatitis C subjects and a dissemination center of this infection. Risk behavior may precede imprisonment and continue afterwards[8,9].
A large number of HCV carriers are asymptomatic and remain undiagnosed for a long time, resulting in further complications, such as liver cirrhosis, liver failure and hepatocellular carcinoma. These asymptomatic patients also represent a natural reservoir of the disease, and a source of dissemination[2].
Prisons in the State of Sergipe, Brazil, do not currently screen for HCV and there are no statistics concerning HCV status of the prisoners incidentally diagnosed. Given the regional variation of HCV prevalence among prisoners, the lack of data in Sergipe and its importance in order to implement effective strategies to prevent HCV transmission, we conducted a study of the prevalence of HCV infection among prisoners, as well as HCV genotypes in viremic subjects, and factors associated with positive serology for HCV.
This was a cross-sectional study performed in two prisons (one male and one female) in the State of Sergipe, Brazil. The study was conducted in the male prison in September 2009 and in the female one in February 2010. Subjects eligible for this study included all prisoners who agreed and signed the consent form.
Structured and individual interviews were privately conducted. Before the interview, it was explained that any collected information would be kept confidential. Subjects’ names were not collected. Each questionnaire received a code number, in order to allow further connection to its respective blood sample, and was formed by closed questions, including sociodemographic characteristics and risk behaviors, such as the ones involving drug use and sexual practices, before and during the imprisonment.
After the interviews, the subjects underwent a rapid test for the detection of HCV antibodies (kit HCV Rapid Test Bioeasy). Peripheral blood from those with a positive result in the rapid test was collected by a finger prick with a single use lancet. Then, six blood spots (two to confirm serology and four for molecular biology) were blotted onto high-quality filter paper (Schleicher & Scheull 903). For each circle, approximately three drops of blood were used. Afterwards, filter paper was left to dry at room temperature for 30 min or until the blood spot was completely dry. The material was kept in aluminum envelopes, along with a bag containing silica gel, and posted to Genoma Center. Only patients with positive anti-HCV had their results confirmed by qualitative polymerase chain reaction (PCR). Those with positive qualitative PCR had the HCV genotype determined.
Continuous variables are reported as mean ± SD, and analyzed using the Mann-Whitney U test. Categorical variables are presented as percentages and analyzed using Chi-square (χ2) or Fisher’s exact tests. P < 0.05 were considered to be statistically significant. In order to identify parameters independently associated with positive serology for anti-HCV, a logistic regression model was determined. Variables with P < 0.1 in univariate analysis were included in the multivariate analysis. Before indicating which variables would be inserted in the initial model, multicollinearity issues were solved. Backward selection of variables was performed, with entry and retention set at a significance level of 0.05. The discrimination capability of the final model was evaluated through the area under the ROC curve (AUC), and the goodness-of-fit of the logistic model was verified by the Hosmer-Lemeshow test (P > 0.05). Variables that continued in the model were tested for possible interaction among them. Each interaction (between two variables) was individually tested and then added to the final model if they showed statistical significance. Statistical analyses were performed using the Statistical Package for the Social Sciences, version 17 (Chicago, IL, USA).
This study was approved by the Research Ethics Committee of the Federal University of Sergipe in April 2009 (N° CAAE 0038.0.107.000-09). Prison authorities did not have access to any questionnaires or blood samples.
Of 382 men, 303 (79%) agreed to participate in the research, and of 137 women, 119 (87%) participated. All included subjects underwent the rapid test for the detection of antibodies to HCV, but one of the subjects with a positive result for this test did not provide a blood sample for qualitative PCR. HCV seroprevalence was 3.1%. From the 13 subjects with positive anti-HCV, eleven had confirmed viremia by PCR. Of these, 10 (90.9%) had genotype 1 (Table 1).
The mean age of the subjects was 32.7 (± 8.8) years, and the most frequent age group was 25-30 years (28.4%). A total of 303 (72%) were men (recruited in the State Penitentiary of Areia Branca); and 119 (28%) were women (recruited in the Female Penitentiary of Sergipe). Seventy-two (60.5%) women and 39 (12.8%) men had drug dealing or drug use as the reason for imprisonment. Many of the subjects were multiracial (38%) and single (39.6%). More than half of the population declared themselves as Catholics (56.4%) (Table 2).
| n (total = 422) | % | |
| Gender | ||
| Male | 303 | 71.8 |
| Female | 119 | 28.2 |
| Age (yr) | ||
| Mean (SD) | 32.7 (8.8) | 21.1 |
| ≤ 25 | 89 | 28.4 |
| 25-30 | 120 | 19.4 |
| 30-35 | 82 | 14.5 |
| 35-40 | 61 | 16.6 |
| > 40 | 70 | |
| Education level | ||
| Uneducated | 45 | 10.7 |
| Less than high school | 312 | 73.9 |
| High school or more | 65 | 15.4 |
| Religion | ||
| Catholic | 238 | 56.4 |
| Protestant | 86 | 20.4 |
| Other | 98 | 23.2 |
| Race | ||
| White | 141 | 33.5 |
| Black | 106 | 25.2 |
| Multiracial | 160 | 38 |
| Other | 14 | 3.3 |
| Marital status | ||
| Single | 167 | 39.6 |
| Married | 117 | 27.7 |
| Widowed | 15 | 3.6 |
| Stable union | 111 | 26.3 |
| Divorced | 12 | 2.8 |
| HCV seropositivity | 13 | 3.1 |
A total of 150 (35.5%) subjects reported previous sexually transmitted disease (STD), of which gonorrhea was the most frequently declared. Two hundred and forty-seven (58.5%) participants affirmed that they had paid or been paid for sex, and 109 (25.8%) rarely or never used condoms. Regarding drug use, 311 (73.7%) subjects had used illegal drugs, while 10.2% stated that they had used ID. Among ID users (IDU) (n = 43), 32.6% shared needles and syringes or other injecting equipment, 7% started injecting in prison and 11.6% continued injecting at the time of the interview. HCV seroprevalence among IDU was 20.6% (Table 3).
| n (total = 43) | % | |
| Drug | ||
| Cocaine | 21 | 48.8 |
| Heroine | 2 | 4.7 |
| Benzydamine | 12 | 27.9 |
| Other | 11 | 25.6 |
| Duration of use (mean in years) | 5.05 (5.7) | |
| Use in the last 2 mo | 5 | 11.6 |
| Started to use during imprisonment | 3 | 7 |
| Uses inside the prison | 5 | 11.6 |
| Needle sharing | 14 | 32.6 |
| Anti-HCV (+) | 9 | 20.9 |
As shown in Tables 4 and 5, we studied the association between serologic HCV status and sexual practices, drug use, sociodemographic and behavioral characteristics. Positive serology for HCV was significantly associated with the following characteristics: previous imprisonment; household contact with a HCV carrier; history of tattooing, though there was no significance considering only tattooing inside prison; previous syphilis; and use of illegal drugs, including inhaled cocaine, marijuana and ID. However, use of crack was not associated with HCV infection. Moreover, those with positive anti-HCV presented significantly higher mean age, higher mean CAGE score and higher mean duration of use of inhaled cocaine, marijuana and ID. There was no significant association between HCV and marital status (data not shown), gender, ethnicity, religion and sexual orientation. Rarely or never having used a condom, and a history of paying or being paid for sex were not associated with a higher HCV seroprevalence. An association was not observed between STD and HCV, except for syphilis. Regarding partner characteristics, the only variable that was significantly associated with HCV was a positive anti-HCV partner. Both groups (positive and negative anti-HCV) had similar mean duration of imprisonment, mean number of sexual partners in the last year, mean age at the time of the first sexual intercourse, mean family income and average years of education.
| Variable | HCV (-) | HCV (+) | P-value |
| Gender (male) | 295 (72.1%) | 8 (61.5%) | 0.531 |
| Mean age (yrs) | 32.56 (8.8) | 36.77 (6.4) | 0.019 |
| Ethnicity (white) | 137 (33.6%) | 4 (30.8%) | 0.833 |
| Christian religion | 314 (76.8%) | 10 (76.9%) | 0.99 |
| Family income (R$) | 777 (1205) | 684 (472) | 0.923 |
| Years of schooling (mean) | 6.5 (5.4) | 6.4 (2.9) | 0.565 |
| Mean incarcerated time (mo) | 43.5 (40) | 30.6 (30) | 0.253 |
| Previous imprisonment | 139 (34.0%) | 11 (84.6%) | < 0.001 |
| History of alcohol use | 298 (73.0%) | 10 (73.9.0%) | 0.756 |
| CAGE (mean) | 1.00 (1.2) | 1.69 (1.2) | 0.033 |
| Household contact with HCV carrier | 17 (4.2%) | 4 (30.8%) | 0.002 |
| History of tattooing | 244 (59.7%) | 13 (100%) | 0.003 |
| Tattooing inside prison | 110 (27.0%) | 6 (46.2%) | 0.202 |
| History of piercing | 39 (9.5%) | - | 0.62 |
| Previous blood transfusion | 39 (9.5%) | - | 0.62 |
| Previously shared razors, toothbrushes, nail trimmers or scissors | 241 (58.9%) | 8 (61.5%) | 0.85 |
| Getting wounded by a sharp weapon in a struggle | 129 (31.5%) | 3 (23.1%) | 0.762 |
| Total | 409 (96.9%) | 13 (3.1%) |
| Variable | HCV (-) | HCV (+) | P-value |
| Never or rarely used condom | 103 (25.3%) | 6 (46.2%) | 0.109 |
| Sexual orientation (Heterosexual) | 315 (77.2%) | 13 (100%) | 0.081 |
| Number of partners in the last year (mean) | 2.44 (8.1) | 2.54 (5.3) | 0.594 |
| Age at first sexual intercourse (mean in years) | 14.54 (2.3) | 15.00 (1.7) | 0.456 |
| Sexually transmitted diseases | 144 (35.2%) | 6 (46.2%) | 0.557 |
| History of genital herpes | 6 (1.5%) | 1 (7.7%) | 0.198 |
| History of syphilis | 17 (4.2%) | 3 (23.1%) | 0.019 |
| History of gonorrhea | 109 (26.7%) | 2 (15.4%) | 0.528 |
| Partner | |||
| HCV (+) | 4 (1.0%) | 2 (15.4%) | 0.012 |
| Illegal drug user | 223 (54.5%) | 10 (76.9%) | 0.11 |
| Injecting drug user | 24 (5.9%) | 1 (7.7%) | 0.553 |
| Previous imprisonment | 124 (30.3%) | 5 (38.5%) | 0.548 |
| Ever paid or been paid for sex | 242 (59.2%) | 5 (38.5%) | 0.136 |
| History of illegal drug use | 298 (72.9%) | 13 (100%) | 0.025 |
| Inhaled cocaine | 194 (47.4%) | 11 (84.6%) | 0.008 |
| Duration of inhaled cocaine (mean in mo) | 107.6 (273.1) | 86.1 (80.3) | 0.006 |
| Marijuana | 277 (67.7%) | 13 (100%) | 0.012 |
| Duration of marijuana use (mean in mo) | 129.5 (220.5) | 198.5 (101.4) | 0.002 |
| Crack | 129 (31.5%) | 5 (38.5%) | 0.561 |
| Duration of crack use (mean in mo) | 77.9 (254.1) | 162.0 (371.9) | 0.369 |
| History of injecting drug use | 34 (8.3%) | 9 (69.2%) | < 0.001 |
| Duration of injecting drug use (mean in mo) | 26.1 (148.0) | 261.2 (445.7) | < 0.001 |
| Use of injecting drugs inside prison | 5 (1.2%) | - | 0.855 |
| Ever shared needles and syringes or other injecting equipment | 11 (2.7%) | 3 (23.1%) | 0.007 |
| Total | 409 (96.9%) | 13 (3.1%) |
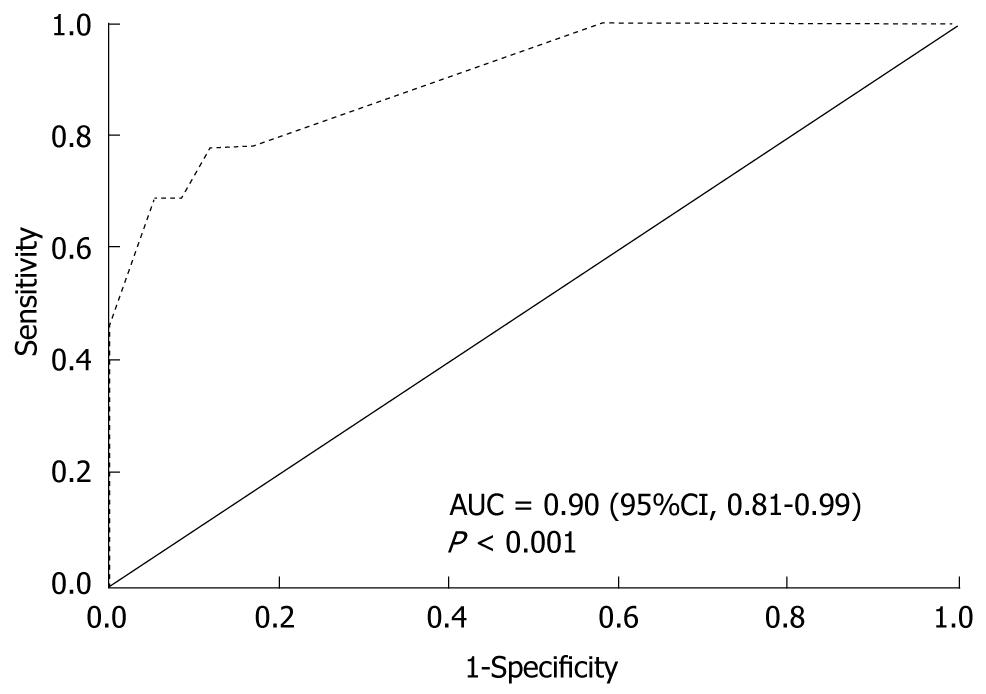
Multivariate logistic regression was performed using HCV status as the dependent variable. Among variables that presented collinearity issues (data not shown), the ones with greater clinical impact were chosen. Continuous variables were turned into dichotomous ones, using the receiver operating characteristic (ROC) curve to choose the cut-off point that presented the best discrimination capability. Table 6 shows the variables independently associated with positive serology for HCV, and the strongest association verified was between positive serology for HCV and use of ID (OR, 23.3; 95% confidence interval (CI), 6.0-90.8). The chance of presenting with positive anti-HCV was 14 times higher among subjects that had lived with an HCV carrier compared with those without this history, even after adjusting for other variables, such as use of ID. Age over 30 years and previous syphilis were also independently associated with positive serology for HCV. None of the tested interactions was statistically significant (data not shown). The Hosmer-Lemeshow test had P = 0.420, so the final model was considered to be adequate. Moreover, the area under the ROC curve was 0.90 (95%CI, 0.81-0.99; P < 0.001), and the discrimination capability of the final model was considered to be good (Figure 1).
| Characteristic | Crude odds ratio | Adjusted odds ratio | 95%CI | P-value |
| Injecting drug user | 24.8 | 23.3 | 6.0-90.8 | 0.0000 |
| History of household contact with HCV carrier | 10.2 | 14.1 | 2.3-85.4 | 0.004 |
| Previous syphilis | 6.9 | 9.8 | 1.7-55.2 | 0.009 |
| Age > 30 yr | 5.6 | 5.5 | 1.1-29.2 | 0.043 |
To our knowledge, this is the first study to determine HCV seroprevalence, and factors associated with this infection in inmates in Northeastern Brazil. HCV seroprevalence observed in this research was 3.1%, which is higher than that found in the general population (1.14%)[4]. Nevertheless, this percentage is below expectation, especially if we consider the following aspects: the prevalence among prisoners described in other regions of Brazil or even in other countries; the absence of damage control programs in the evaluated prisons; and higher prevalence is expected in an already imprisoned population[10]. In Brazil, the studies conducted by Guimarães et al[5], Burattini et al[8] and Coelho et al[11] verified positive serology for HCV in 41%, 34% and 9% of inmates, respectively. Catalan-Soares et al[12], in a study involving 63 prisoners, observed lower HCV seroprevalence (6.3%), but it was still twice that found in the present study. Moreover, Strazza et al[9], in a study in a Brazilian female prison, found an HCV seroprevalence of 16.2%. In the USA, in an incarcerated population, 16%-41% presented serological evidence of HCV infection, and approximately 12%-35% had chronic hepatitis C[7]. Experiences in Spain, England and France reported a prevalence of 48%, 30% and 30%, respectively[13].
It is important to point out that, depending on the studied region, even inside one country, HCV seroprevalence presents a wide variation. HCV seroprevalence seems to increase along with the proportion of IDU. Vescio et al[10], in a meta-analysis, concluded that the most important source of heterogeneity among studies is the different proportion of IDU in each population. In addition, according to the same study, HCV seroprevalence among IDU also has an important influence on this heterogeneity. In our research, 10.2% of the inmates declared that they had already used ID. This proportion varies from 3% to 69% throughout the world[10]. Perhaps the explanation for the proportion of IDU not being as high as expected in our population is linked to the low percentage of imprisonments motivated by drug dealing or drug use, and also to other social and cultural characteristics not assessed in the present investigation.
It has been reported that HCV-RNA may be detected in 40%-90% of subjects with positive anti-HCV[2]. In our population, there was a high proportion of positive HCV-RNA-84.6% of the inmates with positive anti-HCV-that is, subjects capable of infecting others. This information corroborates the hypothesis that prisoners are important carriers of HCV and a potential source of transmission[7,13], especially when many of them will return to society.
Genotyping of hepatitis C provides not only epidemiological data, but also information from the perspective of the therapeutic response. Treatment offers better results for genotypes 2 and 3[14]. Genotyping was performed in all 11 cases in which HCV-RNA was detected. We only identified genotypes 1 and 3, and genotype 1 was the most frequent (90.9%). Other studies also showed genotype 1 as the most frequent[14-18], but always with a higher frequency of genotype 3 when compared with that in the present study.
There was no significant difference in HCV seroprevalence with respect to gender. Previous results in the medical literature are conflicting, in spite of one meta-analysis demonstrating a discreet predominance of positive anti-HCV among women[10]. However, this meta-analysis did not consider confounding variables that could be responsible for such an association. One proposed confounding factor is the higher proportion of women incarcerated for drug dealing or drug use[19]. Regarding ethnicity, previous studies showed that Caucasians had a higher chance of presenting positive for anti-HCV[20,21], but race did not influence the serological status in our population.
Age over 30 years was independently associated with positive serology for HCV, which was also observed in other studies[11,16,20]. This finding may be explained by a higher risk of exposure to HCV over the years. Guimarães et al[5] found a different result, in which younger subjects had a higher chance of infection, but this finding might represent a local peculiarity.
Self-reported use of drugs, including ID, has been shown to be both valid and reliable[22]. Despite statistical significance in univariate analysis, inhaled cocaine did not remain in the final model. In the medical literature, HCV transmission through sharing materials used for inhaling cocaine remain controversial[23]. Use of ID remained in the final model and it was the factor most strongly associated with positive serology for HCV. This finding is consistent with those of other studies[9-11,20,24-28] and supports the effectiveness of HCV parenteral transmission. Use of ID during imprisonment has been reported by 3%-28% of inmates[7]. In the present study, only a minority of IDU (11.6%) referred to injecting inside prison. As previously mentioned[10], difficulty in obtaining equipment for use of ID can lead to sharing, making HCV transmission easier. In our population, 32.6% declared that they shared needles, so a needle exchange program might be effective.
It has been demonstrated that HCV seroprevalence was three times higher in prisoners who had tattoos, when compared to those who did not[10]. In spite of observing an association between tattoos and HCV in univariate analysis, we did not identify an independent effect of this variable in HCV seroprevalence. In accordance with Hellard et al[29], in this study tattoos were strongly associated with use of drugs, presenting multicollinearity issues in multivariate analysis. However, it is important to point out that tattooing inside prison was not associated with positive anti-HCV, unlike previous findings[29]. Therefore, this might not be an important route of transmission in the studied population.
Other proposed routes of transmission do not seem to be relevant in the studied population. All of the subjects with positive anti-HCV denied a history of blood transfusion. Sharing personal care items and a history of getting wounded by a sharp weapon in a struggle occurred equally in prisoners in both groups-positive and negative anti-HCV.
It has been suggested that previous imprisonment would be associated with HCV infection[5]. Despite its significance in univariate analysis, this variable was not independently associated with a positive serology for HCV. Subjects with previous imprisonment, when compared to those without this background, had a higher proportion of IDU, and for this reason would present higher HCV seroprevalence. For IDU, imprisonment is a fairly common event, due to the illegality of their behavior or to crimes committed because of the high cost of drugs on the black market[30].
Sexual transmission of HCV is controversial[10]. Some authors consider this route of transmission ineffective[31,32], which is corroborated by the fact that use of condoms did not seem to protect the studied prisoners from HCV infection. An association between HCV and syphilis has been described[5,33,34] and we observed that a history of previous syphilis was independently associated with positive serology for HCV, even after adjustment for ID use and other confounding factors. Syphilis may be a marker of sexual promiscuity, but variables that evaluate this aspect, such as number of partners in the last year, other previous STD and having already paid or been paid for sex, were not associated with HCV infection. We suggest that HCV is not associated with STD in general, but with genital ulcers, inherent in syphilitic infection. As previously suggested[35], blood containing HCV would penetrate more effectively through injured genital skin. Other studies corroborate the hypothesis of genital ulcers influencing HCV transmission[23,35]. Therefore, in spite of not being the main route, sexual transmission seems to have a role in this population.
Some studies[25,35] stated that homosexuality would lead to a higher chance of HCV infection. This association was not confirmed in our study. All the subjects with positive anti-HCV denied homosexual practices. This finding demonstrates that perhaps the association found in other studies might be related to risk behaviors, instead of homosexuality itself, which is in accordance with Fox et al[26] and Mahfoud et al[15].
A previous partner infected by HCV and household contact with an HCV carrier were associated with positive serology for HCV. However, only household contact with an infected subject was retained in the final model. One possible explanation would be that, although both groups referred equally to sharing personal care items, household contact may lead to blood to blood contact by common use of such objects sporadically or in an unobserved manner.
Most HCV infections are acquired before imprisonment[10,36], but transmission inside prisons has been reported[22,37], which justifies implementation of prevention programs, especially in populations with a high proportion of susceptible subjects, such as the one in this study.
Our study has some limitations. This research included populations from two institutions, which makes external validity difficult for other populations in the world or even in other institutions in Northeastern Brazil. Some prisoners may not have answered some questions correctly, especially the ones concerning STD, chronological aspects and with legal implications, such as the use of ID inside prison. Strengths of the study include: a short period of data collection, showing the real HCV prevalence at that moment; interviews were conducted before test results were available, minimal ascertainment bias; and high sensitivity of the rapid test used, which avoids underestimation of HCV seroprevalence. It has been described that this test has both sensitivity and specificity close to 100%[38,39].
The data shown corroborate the hypothesis that, in the studied prisoners, parenteral HCV transmission is the main route. However, there is evidence to suggest a role for sex and household contact in HCV transmission. This study demonstrated low HCV seroprevalence, with a high proportion of subjects having genotype 1. The large number of susceptible individuals in the studied population, the poor response of genotype 1 to antiviral treatment and the progress of chronic infection make prevention programs more important. It has been shown that treatment is cost-effective[40], even in an imprisoned population[41]. Entering the prison system could be an opportunity to treat and break the transmission cycle. In addition, treatment adherence and side effects could be closely monitored.
Data on hepatitis C in Brazil are still scarce, so more epidemiological studies are necessary in order to guide and monitor prevention programs. We defend the offer of anti-HCV tests for those with a higher chance of infection, such as those with a previous history of syphilis, those aged over 30 years, IDU, or those who had lived with an HCV carrier, to improve the positive predictive value of the tests. This active research should be guided, if possible, by local studies. Even the ones not eligible for treatment may reduce transmission and progress to end-stage liver disease after receiving counseling.
Most studies have shown that hepatitis C virus (HCV) prevalence is higher in certain groups, such as prisoners. Duration of incarceration, use of injecting drugs, adverse socioeconomic situation and poor health care are related to higher prevalence of HCV in this population.
There is a wide regional variation of HCV prevalence among prisoners and studies that aimed to determine HCV prevalence in prisoners in Latin America are scarce. Most HCV transmission results from parenteral exposure, but other routes have been described. Sexual transmission is still controversial. There is a potential public health issue, since the prison system works as a concentrator of hepatitis C and a dissemination center of this infection. Many HCV carriers are asymptomatic and represent a natural reservoir of the disease, and a source of dissemination
The data shown corroborate the hypothesis that parenteral transmission is the main route. There is evidence to suggest the role of sexual and household contact in HCV transmission. Household contact may lead to blood to blood contact, by common use of personal objects sporadically or in an unobserved manner. This study also demonstrated a low HCV seroprevalence, probably due to the low proportion of injecting drug users.
Since this study describes HCV prevalence in a regional prison, it may allow the development of strategies to guide and monitor prevention programs. Household contact with an infected subject must not be neglected, and, in the future, may be a risk factor to be considered in routine evaluation.
The obtained results show that most of the HCV transmissions are due to parenteral exposure and that transmission through sex and household contact with an infected subject play an important role. The paper is well written and the results appear to be well described and critically discussed (also in consideration of other studies).
Peer reviewer: Fernando Goglia, Professor, Dipartimento di Scienze Biologiche, Università del Sannio, Benevento, Via Port’Arsa 11, 82100 Benevento, Italy
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:352-358. [Cited in This Article: ] |
| 2. | Rantala M, van de Laar MJ. Surveillance and epidemiology of hepatitis B and C in Europe - a review. Euro Surveill. 2008;13:21. [Cited in This Article: ] |
| 3. | Andrade AF, Oliveira-Silva M, Silva SG, Motta IJ, Bonvicino CR. Seroprevalence of hepatitis B and C virus markers among blood donors in Rio de Janeiro, Brazil, 1998-2005. Mem Inst Oswaldo Cruz. 2006;101:673-676. [Cited in This Article: ] |
| 4. | Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41-46. [Cited in This Article: ] |
| 5. | Guimarães T, Granato CF, Varella D, Ferraz ML, Castelo A, Kallás EG. High prevalence of hepatitis C infection in a Brazilian prison: identification of risk factors for infection. Braz J Infect Dis. 2001;5:111-118. [Cited in This Article: ] |
| 6. | Perone C, Del Castillo DM, Pereira GL, Carvalho Nde O, Januário JN, Teixeira R. [High prevalence of genotype 1 in individuals with hepatitis C in Belo Horizonte, MG]. Rev Soc Bras Med Trop. 2008;41:238-242. [Cited in This Article: ] |
| 7. | Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS. 2005;19 Suppl 3:S41-S46. [Cited in This Article: ] |
| 8. | Burattini M, Massad E, Rozman M, Azevedo R, Carvalho H. Correlation between HIV and HCV in Brazilian prisoners: evidence for parenteral transmission inside prison. Rev Saude Publica. 2000;34:431-436. [Cited in This Article: ] |
| 9. | Strazza L, Massad E, Azevedo RS, Carvalho HB. [Behavior associated with HIV and HCV infection in female prison inmates in São Paulo, Brazil]. Cad Saude Publica. 2007;23:197-205. [Cited in This Article: ] |
| 10. | Vescio MF, Longo B, Babudieri S, Starnini G, Carbonara S, Rezza G, Monarca R. Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis. J Epidemiol Community Health. 2008;62:305-313. [Cited in This Article: ] |
| 11. | Coelho HC, de Oliveira SA, Miguel JC, Oliveira Mde L, Figueiredo JF, Perdoná GC, Passos AD. Predictive markers for hepatitis C virus infection among Brazilian inmates. Rev Soc Bras Med Trop. 2009;42:369-372. [Cited in This Article: ] |
| 12. | Catalan-Soares BC, Almeida RT, Carneiro-Proietti AB. Prevalence of HIV-1/2, HTLV-I/II, hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and Trypanosoma cruzi among prison inmates at Manhuaçu, Minas Gerais State, Brazil. Rev Soc Bras Med Trop. 2000;33:27-30. [Cited in This Article: ] |
| 13. | Sabbatani S, Giuliani R, Manfredi R. Combined pegylated interferon and ribavirin for the management of chronic hepatitis C in a prison setting. Braz J Infect Dis. 2006;10:274-278. [Cited in This Article: ] |
| 14. | Silva MB, Andrade TM, Silva LK, Rodart IF, Lopes GB, Carmo TM, Zarife MA, Dourado I, Reis MG. Prevalence and genotypes of hepatitis C virus among injecting drug users from Salvador-BA, Brazil. Mem Inst Oswaldo Cruz. 2010;105:299-303. [Cited in This Article: ] |
| 15. | Mahfoud Z, Kassak K, Kreidieh K, Shamra S, Ramia S. Prevalence of antibodies to human immunodeficiency virus (HIV), hepatitis B and hepatitis C and risk factors in prisoners in Lebanon. J Infect Dev Ctries. 2010;4:144-149. [Cited in This Article: ] |
| 16. | Lopes CL, Teles SA, Espírito-Santo MP, Lampe E, Rodrigues FP, Motta-Castro AR, Marinho TA, Reis NR, Silva AM, Martins RM. Prevalence, risk factors and genotypes of hepatitis C virus infection among drug users, Central-Western Brazil. Rev Saude Publica. 2009;43 Suppl 1:43-50. [Cited in This Article: ] |
| 17. | Meyer MF, Wedemeyer H, Monazahian M, Dreesman J, Manns MP, Lehmann M. Prevalence of hepatitis C in a German prison for young men in relation to country of birth. Epidemiol Infect. 2007;135:274-280. [Cited in This Article: ] |
| 18. | Campiotto S, Pinho JR, Carrilho FJ, Da Silva LC, Souto FJ, Spinelli V, Pereira LM, Coelho HS, Silva AO, Fonseca JC. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res. 2005;38:41-49. [Cited in This Article: ] |
| 19. | Miller ER, Bi P, Ryan P. The prevalence of HCV antibody in South Australian prisoners. J Infect. 2006;53:125-130. [Cited in This Article: ] |
| 20. | Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25-37. [Cited in This Article: ] |
| 21. | Baillargeon J, Black SA, Leach CT, Jenson H, Pulvino J, Bradshaw P, Murray O. The infectious disease profile of Texas prison inmates. Prev Med. 2004;38:607-612. [Cited in This Article: ] |
| 22. | Butler T, Kariminia A, Levy M, Kaldor J. Prisoners are at risk for hepatitis C transmission. Eur J Epidemiol. 2004;19:1119-1122. [Cited in This Article: ] |
| 23. | Rauch A, Rickenbach M, Weber R, Hirschel B, Tarr PE, Bucher HC, Vernazza P, Bernasconi E, Zinkernagel AS, Evison J. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395-402. [Cited in This Article: ] |
| 24. | Mohtasham Amiri Z, Rezvani M, Jafari Shakib R, Jafari Shakib A. Prevalence of hepatitis C virus infection and risk factors of drug using prisoners in Guilan province. East Mediterr Health J. 2007;13:250-256. [Cited in This Article: ] |
| 25. | Adjei AA, Armah HB, Gbagbo F, Ampofo WK, Quaye IK, Hesse IF, Mensah G. Correlates of hepatitis C virus infection among incarcerated Ghanaians: a national multicentre study. J Med Microbiol. 2007;56:391-397. [Cited in This Article: ] |
| 26. | Fox RK, Currie SL, Evans J, Wright TL, Tobler L, Phelps B, Busch MP, Page-Shafer KA. Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005;41:177-186. [Cited in This Article: ] |
| 27. | Gates JA, Post JJ, Kaldor JM, Pan Y, Haber PS, Lloyd AR, Dolan KA. Risk factors for hepatitis C infection and perception of antibody status among male prison inmates in the Hepatitis C Incidence and Transmission in Prisons Study cohort, Australia. J Urban Health. 2004;81:448-452. [Cited in This Article: ] |
| 28. | Massad E, Rozman M, Azevedo RS, Silveira AS, Takey K, Yamamoto YI, Strazza L, Ferreira MM, Burattini MN, Burattini MN. Seroprevalence of HIV, HCV and syphilis in Brazilian prisoners: preponderance of parenteral transmission. Eur J Epidemiol. 1999;15:439-445. [Cited in This Article: ] |
| 29. | Hellard ME, Aitken CK, Hocking JS. Tattooing in prisons--not such a pretty picture. Am J Infect Control. 2007;35:477-480. [Cited in This Article: ] |
| 30. | Jürgens R, Ball A, Verster A. Interventions to reduce HIV transmission related to injecting drug use in prison. Lancet Infect Dis. 2009;9:57-66. [Cited in This Article: ] |
| 31. | Zocratto KB, Caiaffa WT, Proietti FA, Carneiro-Proietti AB, Mingoti SA, Ribeiro GJ. HCV and HIV infection and co-infection: injecting drug use and sexual behavior, AjUDE-Brasil I Project. Cad Saude Publica. 2006;22:839-848. [Cited in This Article: ] |
| 32. | Alary M, Joly JR, Vincelette J, Lavoie R, Turmel B, Remis RS. Lack of evidence of sexual transmission of hepatitis C virus in a prospective cohort study of men who have sex with men. Am J Public Health. 2005;95:502-505. [Cited in This Article: ] |
| 33. | Adjei AA, Armah HB, Gbagbo F, Ampofo WK, Boamah I, Adu-Gyamfi C, Asare I, Hesse IF, Mensah G. Correlates of HIV, HBV, HCV and syphilis infections among prison inmates and officers in Ghana: A national multicenter study. BMC Infect Dis. 2008;8:33. [Cited in This Article: ] |
| 34. | Miranda AE, Vargas PM, St Louis ME, Viana MC. Sexually transmitted diseases among female prisoners in Brazil: prevalence and risk factors. Sex Transm Dis. 2000;27:491-495. [Cited in This Article: ] |
| 35. | Marx MA, Murugavel KG, Tarwater PM, SriKrishnan AK, Thomas DL, Solomon S, Celentano DD. Association of hepatitis C virus infection with sexual exposure in southern India. Clin Infect Dis. 2003;37:514-520. [Cited in This Article: ] |
| 36. | Alizadeh AH, Alavian SM, Jafari K, Yazdi N. Prevalence of hepatitis C virus infection and its related risk factors in drug abuser prisoners in Hamedan--Iran. World J Gastroenterol. 2005;11:4085-4089. [Cited in This Article: ] |
| 37. | O'Sullivan BG, Levy MH, Dolan KA, Post JJ, Barton SG, Dwyer DE, Kaldor JM, Grulich AE. Hepatitis C transmission and HIV post-exposure prophylaxis after needle- and syringe-sharing in Australian prisons. Med J Aust. 2003;178:546-549. [Cited in This Article: ] |
| 38. | Owusu-Ofori S, Temple J, Sarkodie F, Anokwa M, Candotti D, Allain JP. Predonation screening of blood donors with rapid tests: implementation and efficacy of a novel approach to blood safety in resource-poor settings. Transfusion. 2005;45:133-140. [Cited in This Article: ] |
| 39. | Montebugnoli L, Borea G, Miniero R, Sprovieri G. A rapid test for the visual detection of anti-hepatitis C virus antibodies in whole blood. Clin Chim Acta. 1999;288:91-96. [Cited in This Article: ] |
| 40. | Macalino GE, Hou JC, Kumar MS, Taylor LE, Sumantera IG, Rich JD. Hepatitis C infection and incarcerated populations. Int J Drug Policy. 2004;15:103-114. [Cited in This Article: ] |
| 41. | Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48:1387-1395. [Cited in This Article: ] |