Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.2019
Revised: December 28, 2010
Accepted: January 4, 2011
Published online: April 21, 2011
AIM: To elucidate the molecular and cellular features responsible for the increase of regulatory T cells (Tregs) in gastric cancer.
METHODS: The frequencies of CD4+Foxp3+ Tregs and the level of transforming growth factor-β1 (TGF-β1) were analyzed from 56 patients with gastric cancer by flow cytometry and enzyme-linked immunosorbent assay respectively. Foxp3 gene expression was analyzed by real-time polymerase chain reaction. The gastric cancer microenvironment was modeled by establishing the co-culture of gastric cancer cell line, MGC-803, with sorting CD4+ T cells. The normal gastric mucosa cell line, GES-1, was used as the control. The production of TGF-β1 was detected in supernatant of MGC and GES-1. The carboxyfluorescein diacetatesuccinimidyl ester (CFSE) dilution assay was performed to evaluate the proliferation characteristics of induced Tregs. Neutralizing anti-TGF-β1 antibody was added to the co-culture system for neutralization experiments.
RESULTS: The level of serum TGF-β1 in gastric cancer patients (15.1 ± 5.5 ng/mL) was significantly higher than that of the gender- and age-matched healthy controls (10.3 ± 3.4 ng/mL) (P < 0.05). Furthermore, the higher TGF-β1 level correlated with the increased population of CD4+Foxp3+ Tregs in advanced gastric cancer (r = 0.576, P < 0.05). A significant higher frequency of CD4+Foxp3+ Tregs was observed in PBMCs cultured with the supernatant of MGC than GES-1 (10.6% ± 0.6% vs 8.7% ± 0.7%, P < 0.05). Moreover, using the purified CD4+CD25- T cells, we confirmed that the increased Tregs were mainly induced from the conversation of CD4+CD25- naive T cells, and induced Tregs were functional and able to suppress the proliferation of effector T cells. Finally, we demonstrated that gastric cancer cells induced the increased CD4+Foxp3+ Tregs via producing TGF-β1. Gastric cancer cells upregulated the production of TGF-β1 and blockade of TGF-β1 partly abrogated Tregs phenotype.
CONCLUSION: Gastric cancer cell can induce Tregs development via producing TGF-β1, by which the existence of cross-talk between the tumor and immune cells might regulate anti-tumor immune responses.
- Citation: Yuan XL, Chen L, Zhang TT, Ma YH, Zhou YL, Zhao Y, Wang WW, Dong P, Yu L, Zhang YY, Shen LS. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-β1. World J Gastroenterol 2011; 17(15): 2019-2027
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/2019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.2019
Gastric cancer (GC) is a common fatal malignancy from cancer worldwide[1,2]. Although the incidence of GC is declining in most developed countries, it remains one of the most common causes of cancer-related death in many Asian countries, such as China, Japan, and Korea[3,4]. Certain tumors, including GC, have developed the capacity to escape immune surveillance or to inhibit immune functions. Recently, emerging evidence suggests that CD4+ regulatory T cells (Tregs) play an important role in tumor escape from immunological control by suppressing the activation and proliferation of T cells, B cells, and natural killer (NK) cells[5,6].
Our recent results have showed that the existence of Tregs maintained immune tolerance in gastric tumor microenvironments[7]. In addition, we found increased expression of Foxp3 protein per cell in tumor-infiltrating Tregs and Tregs can mediate immune suppression via COX-2 production[8]. Interestingly, our and others data showed that after patients received curative resection for GC, the increased proportion of Tregs was significantly restored to normal levels[7,9,10]. These results strongly suggest that gastric cancer-related factors induce and/or expand the accumulation of Tregs. However, the detailed mechanism underlying the induction of Tregs during GC progress remains undefined.
Transforming growth factor-β1 (TGF-β1), as well as other mediators such as prostaglandin E2 and H-ferritin, has been reported to induce Treg cells[11]. In vitro, studies have shown that TGF-β1 can impose a regulatory phenotype on CD4+CD25- T cells through the induction of Foxp3 expression[12,13]. In contrast, other studies have shown that the development and functional capacity of CD4+CD25+ Tregs is normal in TGF-β1 deficient mice[14], questioning a role for TGF-β1 in mediating Treg development and function. Over the past few years, significant progress has been made in defining the cellular and molecular basis for these protumorigenic effects of TGF-β1 within tumor microenvironment[15]. The mechanism of TGF-β1 function in gastric cancer is believed to be mediated primarily by increasing the deposition of extracellular matrix and immunosuppression. However, the underlying mechanism of TGF-β1 responsible for regulating gastric cancer immunosuppression has not been fully elucidated yet.
In this study, we examined the serum level of TGF-β1 in gastric cancer patients and analyzed the correlation of TGF-β1 with the prevalence of Tregs. We confirmed that serum level of TGF-β1 was elevated in GC and correlated with increased CD4+Foxp3+ Treg cells. By the co-culture system in vitro, we evaluated the contribution of GC cell supernatant to CD4+ T cell dysfunction. Our results indicated that MGC supernatant can induce the increase of Tregs, which especially from the conversation of CD4+CD25- naive T cell and blockade of TGF-β1 production partly impaired the development of Tregs. These results suggested that the gastric cancer cells played a pivotal role in impairing the antitumor T cell response by induction of Tregs.
Fifty six patients with gastric cancer, who underwent surgery at Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine, China, were included in this study. Prior to the sample collection, appropriate permission was granted from the research ethical committee of Xinhua hospital, Shanghai Jiao Tong University School of Medicine. Peripheral bloods were collected from each patient and from 20 healthy volunteers as previously described[7]. Sera were frozen at -80°C immediately after centrifugation for later determination of concentrations of TGF-β1. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient. All patients were diagnosed by pathological analyses based on the UICC (International Union Against Cancer) criteria. At the time of sample collection, none of the patients had suffered other cancer, acute and chronic infections, autoimmune diseases, inflammatory diseases and none were receiving concomitant mediations. The laboratory characteristics of patients were as follow: WBC 4.2-10.6 × 109/L; RBC 3.9-5.7 × 1012/L; platelets 181-350 × 109/L; neutrophils 47.8%-73.9%; lymphocytes 15.2%-44.9%; monocytes 2.9%-10.2%. The clinicopathologic characteristics of the tumors are summarized in Table 1.
| Variables | n | TGF-β1 (ng/mL) | P | Tregs (%) | P |
| Gender | > 0.05 | > 0.05 | |||
| Male | 38 | 16.1 ± 6.8 | 8.0 ± 3.2 | ||
| Female | 18 | 14.0 ± 5.1 | 7.6 ± 2.5 | ||
| Age | > 0.05 | > 0.05 | |||
| < 55 | 25 | 14.1 ± 4.1 | 8.2 ± 4.1 | ||
| > 55 | 31 | 15.9 ± 4.6 | 7.7 ± 3.5 | ||
| TNM stage | < 0.05 | < 0.05 | |||
| Early stage (I/II) | 22 | 12.4 ± 5.0 | 6.3 ± 1.2 | ||
| Advanced stage (III/IV) | 34 | 18.1 ± 7.8 | 8.8 ± 2.4 | ||
| Histological type | > 0.05 | > 0.05 | |||
| Well and Moderately | 20 | 14.1 ± 4.9 | 7.0 ± 3.7 | ||
| Poor | 36 | 16.8 ± 7.9 | 8.6 ± 4.5 | ||
| Lymph node metastasis | < 0.05 | < 0.05 | |||
| Negative | 18 | 11.2 ± 5.2 | 6.5 ± 2.4 | ||
| Positive | 38 | 17.4 ± 7.2 | 8.6 ± 2.9 |
Human GC cell lines (MGC-803, SGC-7901) were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China), and normal gastric mucosa cell line (GES-1), derived from a human fetal gastric mucosa epithelium, was obtained from Beijing Institute for Cancer Research. The cells were routinely cultured in DMEM media (GIBCO, Invitrogen, USA) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco) in 5% CO2 at 37°C. MGC, SGC and GES cells were washed twice with PBS when they grew to 60%-80% confluence and then kept in serum-free culture medium for an additional 48 h. Supernatant was collected and debris was removed by centrifugation at 1500 g for 10 min, and then passed through a 0.45 mm filter (BD, USA). 100 μL supernatants were stored at -80°C for later determination of concentrations of TGF-β1. For co-culture assay, all the remaining supernatants were further concentrated 20-fold with a Microcon Ultracel YM-10 filter (Millipore, USA) according to the manufacturer's instructions. In the induction experiments, different volumes of supernatant protein concentrate from MGC or GES were added to sorted naive T cell culture system.
The cell supernatants of MGC, GES-1 and GC patients’ sera previously stored at -80°C were thawed, and measured for TGF-β1 concentration by enzyme-linked immunosorbent assay using human TGF-β1 immunoassay kit (R&D, USA) in triplicate following the manufacturer’s protocol. The minimum detectable dose of this assay is 30.0 pg/mL. The intra-assay coefficient of variation (CV) was 5.7% and the interassay CV was 10.6%.
Phenotype analysis of regulatory T cells (Tregs) and cell sorting were performed by BD FACS Aria flow cytometer (BD, USA) as previously described[8]. Briefly, the cells were labeled with CD3-PC7, CD127-PE, CD4-APC, and CD25 PerCP. Intracellular staining for Foxp3 was performed using Alexa Fluor® 488 anti-human Foxp3 Antibody and Foxp3 Fix/Perm Buffer Set (BioLegend, USA) following the manufacturer’s protocol. In the preliminary experiments, we found that CD25 expression was nonspecific and was higher in CD4+ T cells after coculturing with GC cells. Therefore, for consistency, the gating strategy for Tregs was based on the expression of CD4 and Foxp3. To analyze the prevalence of Treg cells, CD4+Foxp3+ Treg cells were evaluated after gating on CD3+CD4+ T cells and expressed as a percentage of the total CD4+ T cells. The FACS Aria was adjusted with Accudrop Fluorescent Beads (BD bioscience, USA) for optimum sorting conditions which allowed CD4+CD25+CD127low/- T cells and CD4+CD25-CD127+ T cells to be sorted. The purity of the isolated T cells was greater than 95%.
The purified CD4+CD25-CD127+ T cells (1 × 105) were cultured with conditioned medium described above in 96-well plates at 37°C and 5% CO2 in the presence or absence of soluble anti-human CD3 (10 μg/mL; eBioscience) plus anti-CD28 (10 μg/mL; eBioscience) and IL-2 (100 U/mL; Sigma, USA). After 72 h of cultivation, the proportion of CD4+Foxp3+ T cells was detected by FCM, and Foxp3 gene expression was analyzed by real-time PCR. For anti-TGF-β1 antibody neutralization experiments, neutralizing mouse anti-TGF-β1 antibody (500 μg/mL; Clone: 27235; R&D Systems, USA) and normal mouse IgG1 (500 μg/mL; Clone: 11711; R&D Systems, USA) were added to the culture medium with a final concentration of 0.1 μg/mL at the beginning of the culture.
Foxp3 mRNA expression was performed using the SYBR Premix Ex Taq™ (Takara) according to the manufacturer’s instructions. Amplification reactions were performed by primers specific for Foxp3 (forward, 5'-CAGCACATTCCCAGAGTTCCTC-3'; reverse, 5'-GCGTGTGAACCAGTGGTAGATC-3'). The relative quantity of the Foxp3 mRNA was normalized to the level of the internal control GAPDH mRNA level.
For the proliferation inhibition assay, the carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay was performed per standard technique. Briefly, the sorted CD4+CD25-CD127+ T cells were co-cultured with MGC-803, GES-1 or medium only for 2 d. As suppressor cells, equal numbers of cells were removed and placed in co-culture with CFSE-labeled CD4+CD25- T cells at a ratio of 1:1 in the presence of soluble 10 μg/mL mouse anti-human CD3 and 10 μg/mL mouse anti-human CD28 antibodies (eBioscience, USA). After 4 d, the cells were harvested and proliferation was measured by loss of CFSE dye with flow cytometry. Cell proliferation indices were calculated with Modfit software (Topsham, USA) based on the reduction of CFSE positive cells.
Data were expressed as mean ± SD. The statistical significance of the difference between the two means was assessed using Student’s t-test, and the one-way ANOVA with Tukey’s post test was performed for multiple comparisons. Correlation between variables was evaluated by Pearman’s rank correlation coefficients. All the statistical analyses were performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, USA) and a significant difference was considered as P < 0.05.
To determine whether serum TGF-β1 correlated with the clinicopathological findings, we summarized the mean values of TGF-β1 in patients with GC according to clinical variables as shown in Table 1. The mean level of serum TGF-β1 in GC patients (15.1 ± 5.5 ng/mL) was significantly higher than that of the gender- and age-matched healthy controls (10.3 ± 3.4 ng/mL) (P < 0.05), which was consistent with previous reports[16,17]. Furthermore, the serum TGF-β1 levels increased as GC stage progressed. Compared to those with early stage disease, patients with advanced stage disease had significantly elevated serum TGF-β1 (P < 0.05). As shown in Table 1, no significant differences in serum TGF-β1 levels were found in GC patients with different age, genders, and histological types (P > 0.05). However, the serum concentration of TGF-β1 was positively correlated with lymph node metastasis (P < 0.05). The results also showed that the population of CD4+Foxp3+ Tregs in the peripheral blood of advanced stage GC patients was significantly higher than that in healthy controls or early stage GC patients (P < 0.05) (Table 1).
The consistency of Tregs and serum TGF-β1 level in patients with GC encouraged us to perform a correlation study, and the results showed that the increased TGF-β1 was correlated with the Treg cells (r = 0.576, P < 0.05) in advanced stage patients, but not in early stage patients (r = 0.248, P < 0.05). The present results indirectly suggested the relationship of TGF-β1 and Tregs in gastric cancer.
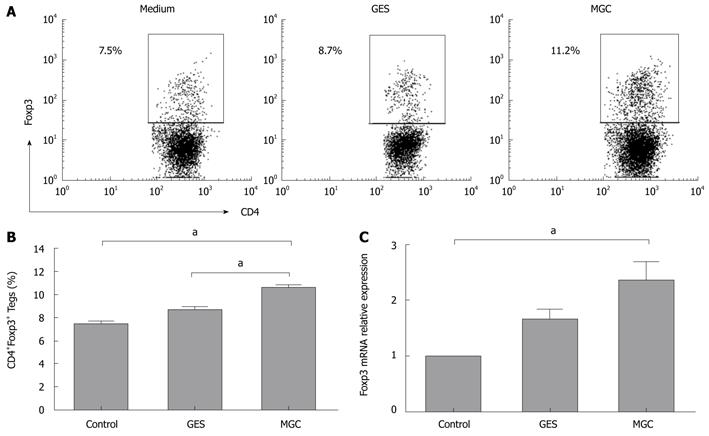
Based on the above results, we hypothesized that gastric cancer-derived stimulators may contribute to increased Tregs. To address this hypothesis, we established a co-culture system with human GC cells and PBMCs from healthy donors to model the gastric cancer microenvironment in vitro. After 3 d of culture, our data showed that a higher frequency of Tregs was observed in PBMCs cultured with the supernatant of MGC. However, the frequency of Tregs had almost no significant difference in PBMCs cultured with GES-1 cell supernatants and medium control (Figure 1A and B). When cocultured with MGC cell culture supernatant, Foxp3 mRNA expression level was higher than that with GES-1 and medium (Figure 1C).
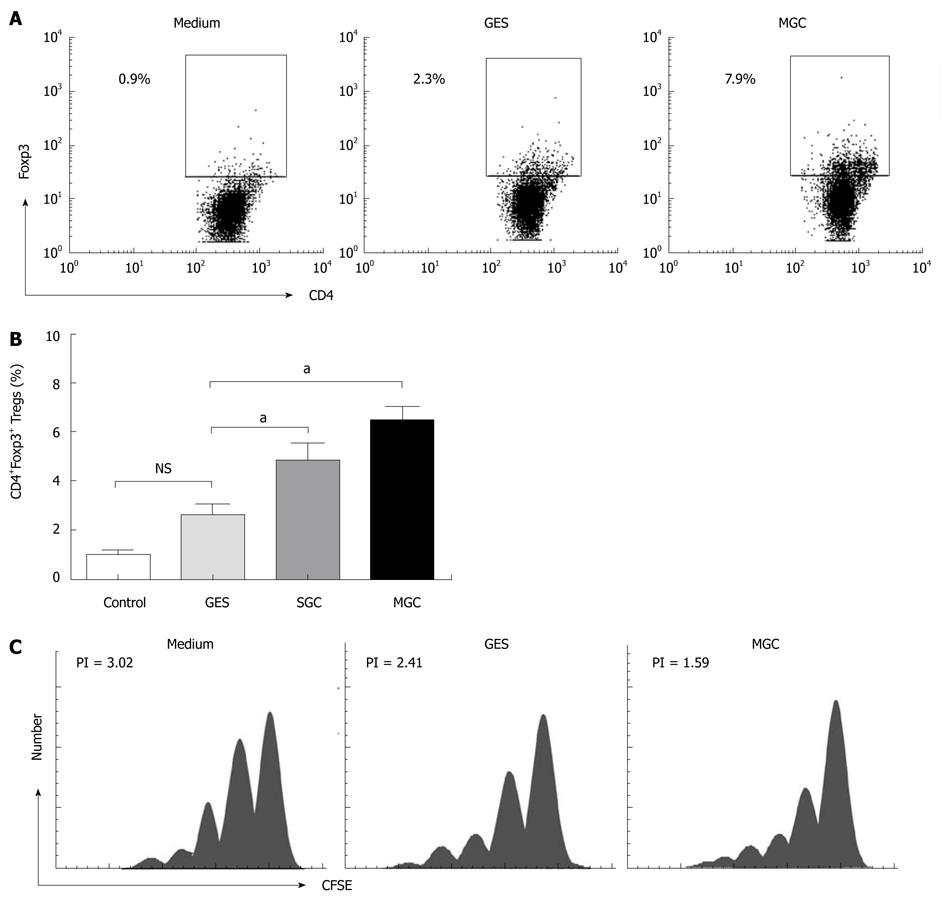
To investigate whether the supernatant of GC cell culture induced the increased Tregs from the conversion of natural CD4+CD25- T cells, we performed co-culture experiments with the sorted natural CD4+CD25- T cells using our previous method[7]. Because naive CD4+ T cells were more susceptible to the induction of Foxp3 by TGF-β1[18] and to minimize any potential contaminating CD25-Foxp3+ nTregs, we used the CD4+CD25+CD127low/- population for all of our coculture experiments. To ensure consistency, the same cell culture supernatants were used for conversion experiments. Our results showed that MGC cell supernatant can induce a higher population of CD4+Foxp3+ Tregs than GES-1 and medium (P < 0.05) (Figure 2A and B) and, the CD4+CD25- cells decreased respectively in coculture system. More interesting is that the induced Tregs correlated with the TGF-β1 level in different repeated experiments (r = 0.635, P < 0.05).
To further confirm that the supernatant of GC cell culture could increase Tregs population and Foxp3 expression, the cell culture supernatant from another GC cell strain, SGC-7901, was collected. As observed in the culture with supernatant of MGC cell culture, the supernatant of SGC cell cultures also increased Foxp3 expression in naive T cells (Figure 2B). Collectively, our results suggested that the GC supernatant can induce the increased Tregs, which was mainly because of the conversion of natural CD4+CD25- T cells.
In order to understand whether GC cell supernatant induced CD4+Foxp3+ Tregs can inhibit effector T cells, we analyzed the suppressive function of GC cell induced Tregs. To eliminate the influence of cell culture related factors, the induced co-cultured supernatants were removed after coculture with MGC, GES-1, or medium. Then the CFSE dilution assay was used to evaluate the proliferation characteristics of T cells. Compared with medium, a significant decrease in the proliferative response of responder CD4+CD25- T cells could be found with MGC after being cocultured (Figure 2C). These results demonstrated that GC cell induced Treg cells displayed the suppressive activity in vitro.
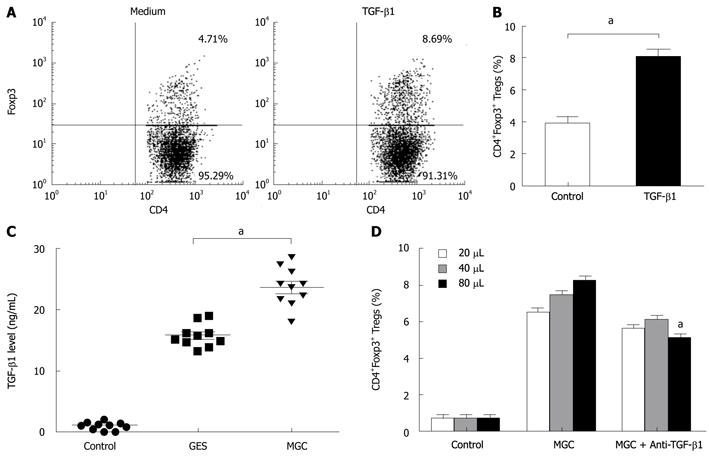
To further elucidate the possible mechanism of the conversion, we presumed that TGF-β1 from gastric cancer cells can serve as a key factor in the induction of Foxp3 expression of natural CD4+CD25- T cells. To address this possibility, we firstly performed the inducing experiments for TGF-β1. Consistent with other studies, compared with control Ab, TGF-β1 can induce an increase in Tregs (Figure 3A and B). MGC cells secreted significant higher TGF-β1 into supernatant than that GES-1 and medium (Figure 3C).
To test the role of TGF-β1 in GC cell mediated increasing Tregs, we compared the effects of neutralizing monoclonal antibody with activity against TGF-β1 and isotypic control antibody. To some extent, blocking TGF-β1 activity in MGC supernatant with anti-TGF-β1 mAb, instead of the isotypic control antibody, reduced the frequency of induced CD4+FOXP3+ T cells. But our results also showed that this blocking effect was not complete because the converted numbers of CD4+Foxp3+ T cells in MGC were still higher than that of the control (Figure 3D). Our data indicated that gastric cancer-derived TGF-β1 played a certain role in the conversion of natural CD4+CD25- T cells to CD4+Foxp3+ Treg cells.
In the current study, we showed that higher levels of TGF-β1 in gastric cancer patients have been correlated with the frequency of CD4+Foxp3+ regulatory T cell. Numerous studies have respectively reported an increased frequency of circulating Tregs and higher level of TGF-β1 during GC progression[7,9,16,19]. However, to date, there has been no report to directly demonstrate the relationship of higher TGF-β1 levels and increased frequency of Tregs in GC. Given that TGF-β1 is a key factor for Foxp3 expression maintenance, regulatory function, and homeostasis in peripheral CD4+CD25+ Treg cells[20], tumor-derived TGF-β1 may contribute to the development of Tregs during GC progression. Indeed, our work supports this possibility by demonstrating a mechanism of CD4+Foxp3+ Tregs development mediated through TGF-β1 production by GC cells.
TGF-β1 is a tumor suppressor growth factor, anti-inflammatory cytokine, and immunosuppressant. Therefore, the levels of TGF-β1 were different according to the carcinogenic process, the stage of carcinogenesis, and organ. It has been reported that high levels of TGF-β1 are produced by many types of tumors, including melanomas and cancers of the colon, stomach, liver, and prostate, as well as other malignancies[16,21,22]. Generally defective TGF-β1 signaling seems to be essential in the carcinogenic process, but the level of TGF-β1 is increased in advanced cases or some types of cancer. TGF-β1 levels were significantly increased in gastric cancer tissue compared with adjacent normal tissues[17]. In this study, our data confirmed the higher level of serum TGF-β1 in patients with gastric cancer. Furthermore, compared to early stage patients, elevated serum TGF-β1 was observed in patients with advanced stages. However, the role of TGF-β1 varied in different tumor stages, in which TGF-β1 seems to act as a tumor suppressor in early stages of tumorigenesis and during later stages of tumorigenesis, TGF-β can foster tumor progression, and metastasis[23,24]. Our results showed that the serum concentration of TGF-β1 was positively correlated with lymph node metastasis in GC. This study reinforced the role of TGF-β1 in promoting GC progression. An increase in the Treg population has been observed in both the periphery and tumor microenvironment in patients with cancer[25]. We find a positive correlation between TGF-β1 and Tregs in advanced stage GC patients. To our knowledge, this is the first report to show the correlation of TGF-β1 level with increased Treg cells in GC.
In this report, an in vitro co-culture system was used to understand the underlying mechanisms responsible for the upregulation of Tregs observed in our clinical cohorts. After co-culture with GC cell supernatants, an increased population of CD4+Foxp3+ T cells was found in PBMCs. Of note, upregulation of Foxp3 mRNA expression supported that Tregs increased in the culture system. This increase was observed using a different GC cell line, suggesting that the induction of Tregs is a feature common to GC cells. Multiple mechanisms have been involved in production of increased Treg cells in the tumor microenvironment including expansion, conversion and recruitment. Mizukami et al[26] found that CCL17 and CCL22 are related to the increased population of Foxp3+ Tregs in early GC[26]. Using the optimized conditions for sorting effector and Treg cells, we provided evidence that the conditioned medium obtained from GC supernatant was capable of inducing the conversion of CD4+CD25- T cells to CD4+Foxp3+ Tregs, which is different from the effects of chemokines on Treg infiltration in GC microenvironment. Moreover, the induced Tregs were functional and inhibited the proliferative response of CD4+CD25- effector T cells. Although a prior study showed that increased Treg frequency was derived from natural Treg self expansion by factors secreted by hepatocellular carcinoma cell[27], our data clearly demonstrated that the conversion of natural CD4+CD25- T cells may be an important pathway of Treg cell maintenance in GC. Of course, we cannot discount the important role of chemokines in inducing Tregs migration to the microenvironment of GC.
Tumor cell supernatants include a complex protein component. Based on the fact that GC cells could produce a higher level of TGF-β1 and TGF-β1 correlated with Tregs from our data, we questioned if gastric cancer derived TGF-β1 induced Treg increases in the coculture system. Although TGF-β1 can promote the generation of Tregs in vitro, it has been controversial whether TGF-β1 is involved in the generation or maintenance of Tregs under pathologic conditions, especially in tumor environments[28]. Some studies showed that tumor-derived factors such as TGF-β1 may contribute to CD4+CD25+ Treg cell expansion[29], but also enhance their suppressor ability[27]. In contrast to them, Zhao et al[30] recently found that neutralization of TGF-β1 did not affect Foxp3 expression in ovarian carcinoma cells. The role of TGF-β1 needs to be elucidated in GC. Our results demonstrated that a higher TGF-β1 level was found in GC cell supernatant and TGF-β1 can induce increased Treg cells. Surprisingly, although blocking TGF-β1 could decrease the conversion activity in our study, it was not completely abrogated. Accordingly, we could not rule out that a fraction of converting Treg cells may be generated in the presence of other unknown tumor-derived soluble factors besides TGF-β1. It is likely that multiple cytokines are involved in the induction of Foxp3 expression[30]. TGF-β1, together with other factors, seemed to account for the induction of Treg cells in the GC microenvironment. Further research is needed to elucidate the potential mechanism of GC derived other factors for induction of Tregs. Additionally, what should be noted is that TGF-β1 derived from GC can also account for immunosuppression of other cell types, and besides GC cells, macrophages and stromal cell also can secrete TGF-β1 in tumor environments[31]. It is documented that TGF-β1 is important for the inhibition of CD8+ CTL and NK cells, which play a critical role in the prevention and clearance of tumors[32]. A better understanding of the mechanisms of the Treg increase in GC may allow for future immunotherapeutic and diagnostic opportunities in this population. Recent reports have shown that functional polarization of Th subsets of lymphocytes has been implicated in tumor promotion. Tumor derived TGF-β1 in the tumor microenvironment could promote tumor eradication by influencing the polarization of Th1/Th2 and controlling Treg/Th17 cell polarization[33]. This study only focuses on the specific role of TGF-β1 in the induction of Tregs. However, the polarization of other Th cells in the tumor environment by TGF-β1 needs to be further elucidated. A complete understanding the role of TGF-β1 in controlling T cell polarization in tumors is crucial for dissecting the beneficial use of TGF-β1 in future immunotherapies against gastric cancer.
In conclusion, we provide evidence that a higher TGF-β1 level is related to the increased population of CD4+Foxp3+ Tregs. GC cell supernatants can stimulate induction of human CD4+Foxp3+ Treg cells. This study suggests that gastric cancer cell supernatants can induce the conversation of Tregs from CD4+CD25- naive T cells partly via mechanisms involving TGF-β1. Our data support the existence of intercellular cross-talk between the tumor cell and Tregs that might regulate anti-tumor immune responses.
Regulatory T cells (Tregs) accumulate in the tumor environment and suppress tumor-specific T-cell responses. Previous studies have suggested that Tregs were elevated in gastric cancer and increased populations of Tregs impaired anti-tumor immunity. However, the molecular and cellular features responsible for the increase and maintenance of Treg cell levels in gastric cancer remain elusive.
Recently, emerging evidence suggests that Tregs play an important role in tumor escape from immunological control. Although the precise mechanism causing the increased numbers of Treg cells is unknown, transforming growth factor-β1 (TGF-β1), as well as other mediators, has been reported in inducing Treg cells. However, there are contradictory reports regarding the role of TGF-β1 in the induction of Tregs in cancer. The research aimed to explore whether or not gastric cancer cells producing this cytokine would account for the increased Treg and promote tumor progression.
In this study, the data confirmed the higher level of serum TGF-β1 in patients with gastric cancer. Moreover, the higher TGF-β1 level correlated with the increased population of CD4+Foxp3+ Tregs in advanced gastric cancer. Gastric cancer cell induced the increase of functional CD4+Foxp3+ Tregs, mainly from the conversion of CD4+CD25- naive T cells. Furthermore, gastric cancer cells can induce Tregs development via production of TGF-β1.
The results indicated that the gastric cancer cell played a pivotal role in impairing the antitumor T cell response by induction of Tregs. This study supports the existence of intercellular cross-talk between the tumor cell and Tregs that might regulate anti-tumor immune responses. A complete understanding of the role of TGF-β1 in tumors is crucial for dissecting the beneficial use of TGF-β1 in future immunotherapies against gastric cancer.
Regulatory T cell (Treg cell) is functionally defined as a T cell that inhibits an immune response by influencing the activity of another cell type. Tregs are characterized by specific expression of the forkhead transcription factor Foxp3, and make up 5%-10% of the normal peripheral CD4+ T cell population. Treg cells within the tumor microenvironment are a crucial component of the tumor immunosuppressive network.
The authors examined the level of serum TGF-β1, and found that it was higher in patients with gastric cancer than in healthy controls. In addition, they also found that gastric cancer cells induced the increased CD4+Foxp3+ Tregs via production of TGF-β1. Gastric cancer cells upregulated the production of TGF-β1 and blockage of TGF-β1 partly abrogated Tregs phenotype. These experiments are well-designed and results are clear.
Peer reviewers: Fabrizio Montecucco, MD, Assistant, Division of Cardiology, Department of Internal Medicine, University of Geneva, Avenue de la Roseraie 64, 1211 Geneva, Switzerland; Ki-Baik Hahm, MD, PhD, Professor, Gachon Graduate School of Medicine, Department of Gastroenterology, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon, 406-840, South Korea
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269-1276. |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. |
| 3. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 4. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 5. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. |
| 6. | von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338-344. |
| 7. | Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131:109-118. |
| 8. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. |
| 9. | Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064-1071. |
| 10. | Tokuno K, Hazama S, Yoshino S, Yoshida S, Oka M. Increased prevalence of regulatory T-cells in the peripheral blood of patients with gastrointestinal cancer. Anticancer Res. 2009;29:1527-1532. |
| 12. | Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526-6531. |
| 13. | Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889-899. |
| 14. | Mamura M, Lee W, Sullivan TJ, Felici A, Sowers AL, Allison JP, Letterio JJ. CD28 disruption exacerbates inflammation in Tgf-beta1-/- mice: in vivo suppression by CD4+CD25+ regulatory T cells independent of autocrine TGF-beta1. Blood. 2004;103:4594-4601. |
| 15. | Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM. A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol Biol Cell. 2004;15:4682-4694. |
| 16. | Lin Y, Kikuchi S, Obata Y, Yagyu K. Serum levels of transforming growth factor beta1 are significantly correlated with venous invasion in patients with gastric cancer. J Gastroenterol Hepatol. 2006;21:432-437. |
| 17. | Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, Verheijen JH, Hommes DW, Lamers CB, Sier CF. Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97:398-404. |
| 18. | Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983-2990. |
| 19. | Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostics factors and patient survival. J Surg Res. 2007;139:182-188. |
| 20. | Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061-1067. |
| 21. | Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133-143. |
| 22. | Hong S, Lee HJ, Kim SJ, Hahm KB. Connection between inflammation and carcinogenesis in gastrointestinal tract: focus on TGF-beta signaling. World J Gastroenterol. 2010;16:2080-2093. |
| 23. | Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350-1358. |
| 24. | Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262-5270. |
| 25. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. |
| 26. | Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286-2293. |
| 27. | Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, Nelson DR. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest. 2007;87:582-590. |
| 28. | Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455-471. |
| 29. | Mao C, Wang S, Jiang Q, Tong J, Ma J, Yang M, Xu X, Qiu G, Shao Q, Li L. Increased CD4CD25+FOXP3+ regulatory T Cells in cancer patients from conversion of CD4+CD25- T cells through tumor-derived factors. Onkologie. 2008;31:243-248. |
| 30. | Zhao X, Ye F, Chen L, Lu W, Xie X. Human epithelial ovarian carcinoma cell-derived cytokines cooperatively induce activated CD4+CD25-CD45RA+ naïve T cells to express forkhead box protein 3 and exhibit suppressive ability in vitro. Cancer Sci. 2009;100:2143-2151. |
| 31. | Reimann M, Lee S, Loddenkemper C, Dörr JR, Tabor V, Aichele P, Stein H, Dörken B, Jenuwein T, Schmitt CA. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell. 2010;17:262-272. |
| 32. | Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129-141. |
| 33. | Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554-567. |