Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2011; 17(11): 1383-1399
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
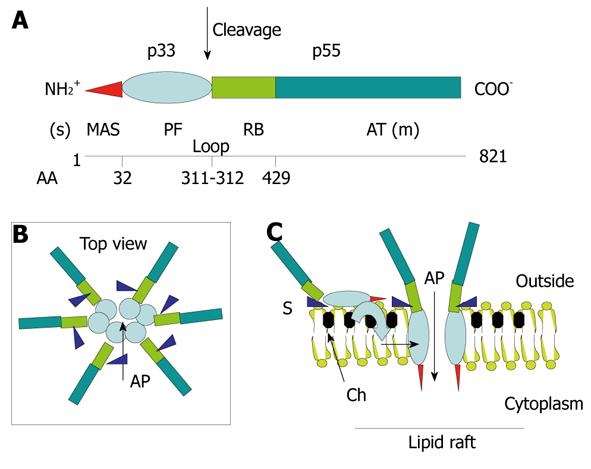
Figure 4 Structure-function relationships in the VacA toxin.
A: VacA is an 88-kDa protein that can be cleaved into two subunits designated p33 (red and light blue) and p55 (light green and dark green). The cleavage between the subunit occurs in a flexible loop between residues 311 and 312. The two subunits are probably attached by non-covalent bonds between the p33 carboxy terminus and the N-p55 terminus. The amino-terminal part of p33 consists of 32 hydrophobic amino-acid stretch that is involved in the recognition of mitochondria (red) (MAS) (where the “s” toxin subtype site is located) followed by the p33 “core” subunit that forms, upon entry into the lipid membrane and oligomerization, an anionic channel (PF) (light blue). The toxin nicking site is located in the flexible loop domain. The amino-terminal part of p55 contains the cell receptor domain (about 110 amino acids) (RB; light green) followed by the type Va autotransporter domain (AT; dark green) that is involved in the secretion of the toxin by the bacterium (where the “m” toxin subtype site is located); B and C: VacA binds as a monomer to its cell surface receptor sphingomyelin (S) with low affinity, then the p33 core progressively is embedded (light blue curve arrow) in the lipid membrane bilayers, at the level of lipid rafts [containing saturated lipid such as sphingomyelin and cholesterol (Ch)] and forms an anionic channel (AP) by oligomerization of p33. This multiplies by 6 the number of toxin cell receptors associated with the oligomerized VacA, thus increasing greatly the toxin affinity for the target cells.
- Citation: Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol 2011; 17(11): 1383-1399
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1383