Published online Jan 7, 2011. doi: 10.3748/wjg.v17.i1.111
Revised: October 16, 2010
Accepted: October 23, 2010
Published online: January 7, 2011
AIM: To investigate the effect of transgenic expression of kallistatin (Kal) on carbon tetrachloride (CCl4)-induced liver injury by intramuscular (im) electrotransfer of a Kal-encoding plasmid formulated with poly-L-glutamate (PLG).
METHODS: The pKal plasmid encoding Kal gene was formulated with PLG and electrotransferred into mice skeletal muscle before the administration of CCl4. The expression level of Kal was measured. The serum biomarker levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), malonyldialdehyde (MDA), and tumor necrosis factor (TNF)-α were monitored. The extent of CCl4-induced liver injury was analyzed histopathologically.
RESULTS: The transgene of Kal was sufficiently expressed after an im injection of plasmid formulated with PLG followed by electroporation. In the Kal gene-transferred mice, protection against CCl4-induced liver injury was reflected by significantly decreased serum ALT, AST, MDA and TNF-α levels compared to those in control mice (P < 0.01 to 0.05 in a dose-dependent manner). Histological observations also revealed that hepatocyte necrosis, hemorrhage, vacuolar change and hydropic degeneration were apparent in mice after CCl4 administration. In contrast, the damage was markedly attenuated in the Kal gene-transferred mice. The expression of hepatic fibrogenesis marker transforming growth factor-β1 was also reduced in the pKal transferred mice.
CONCLUSION: Intramuscular electrotransfer of plasmid pKal which was formulated with PLG significantly alleviated the CCl4-induced oxidative stress and inflammatory response, and reduced the liver damage in a mouse model.
- Citation: Diao Y, Zhao XF, Lin JS, Wang QZ, Xu RA. Protection of the liver against CCl4-induced injury by intramuscular electrotransfer of a kallistatin-encoding plasmid. World J Gastroenterol 2011; 17(1): 111-117
- URL: https://www.wjgnet.com/1007-9327/full/v17/i1/111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i1.111
Gene therapy refers to a therapeutic approach by introduction and expression of genetic material in particular cells or tissues. Over the past two decades, great efforts and abundant resources have been invested into the development of an effective, safe and relatively long-lasting gene therapy strategy. Great success has been made recently so that gene therapy was elected as one of the breakthroughs of 2009 by Science[1]. In order to achieve the goal of therapy, a carrier, or “vector”, is the key required to deliver the genetic material into the target cells. Whilst exciting progress in the development of gene therapy have been made, a safe and efficient method of gene delivery has been elusive thus far. Initially, a number of viral vectors were chosen, owing to their high efficiency in gene transfer. Unfortunately, they also bring added risks to the patients, even though their virulence was genetically disabled. Non-viral vectors avoid the risks. However, lower transferring efficiency has limited their clinical use thus far. To boost efficacy of non-viral gene therapies several delivery methods have been under investigation. Electro-gene therapy is one of the most efficient non-viral approaches for gene therapy[2-4]. It has already been considered a safe method because naked DNA is not immunogenic and plasmid DNA does not have the risk of insertional mutagenesis[5]. Moreover, DNA electrotransfer can be accomplished using devices that have already been used on human cancer patients to deliver cytotoxic molecules such as bleomycin and cisplatin to solid tumors by electrochemotherapy[6,7]. Thus all elements are available for safe and widespread use of this efficient technology and for implementing the use of genes for medical purposes[8]. To further improve the transfer efficiency, plasmids were formulated in poly-L-glutamate (PLG) prior to intramuscular (im) injection and electroporation. Such a combined approach significantly elevated transgene expression in myofibers[9].
Kallistatin (Kal) that belongs to the serine protease inhibitor family is widely expressed in organs such as the liver, kidneys, and blood vessels. Previous studies have demonstrated kallistatin to be a potent anti-inflammatory agent. Kallistatin administration by gene delivery attenuates oxidative stress, apoptosis, inflammation, and organ damage in animal models[10-13]. These findings indicate that kallistatin may play an important role as an antioxidant in maintaining oxidative balance and preventing oxidative endothelial and tissue injury.
Oxidative stress is a state of redox imbalance caused by increased reactive oxygen species (ROS) generation and decreased antioxidant capacity. Administration of carbon tetrachloride (CCl4) is an established experimental model of severe toxic liver injury involving generation of oxidative stress and is frequently used for the screening of anti-hepatotoxic and/or hepatoprotective activities of drugs[14]. Antioxidants and anti-inflammatory agents can play a role in liver protection by scavenging active oxygen and free radicals and neutralizing lipid peroxides.
In the present study, we first investigated the feasibility of improving the expression of the transgene after introducing the PLG formulated plasmid into mouse skeletal muscle by electroporation, then investigated the therapeutic efficacy of Kal expression in the circulation on CCl4-induced liver injury. After CCl4 had been injected into mice to induce liver injury, evaluations of liver marker enzymes, the extent of oxidative stress and liver histology were performed, revealing that elevated levels of human Kal were effective in alleviating oxidative stress and protecting liver against CCl4-induced liver damage.
Sodium salt of PLG (15-50 kDa) was purchased from Stsicn Co (Nanjing, China); EndoFree plasmid Giga kit was purchased from Qiagen GmbH (Hilden, Germany); DNA Delivery Device was purchased from TERESA Healthcare Sci-Tech Company (Shanghai, China); reporter lysis buffer and β-galactosidase enzyme assay system was purchased from Promega (Madison, USA); BCA Protein Assay Kit was purchased from Pierce (Rockford, USA); Quantikine kit was purchased from R&D Systems Inc (Minneapolis, USA).
Balb/c mice (6- to 8-wk-old females, Slaccas Company, Shanghai) were used throughout this study. The animals were maintained on a 12/12 h day/night cycle at room temperature 18-24°C. Food and water were provided ad libitum. The Animal Studies Ethics Committee of Huaqiao University approved all the experiments reported here.
The expression plasmid pLac encoding β-galactosidase under the control of the cytomegalovirus immediate-early promoter, and the pKal under the control of the same promoter and driving a coding sequence for the human Kal, were constructed as reported previously[15] and kept in our laboratory. The integrity of the sequence was determined by DNA sequencing. Plasmids were transformed and expanded into E. coli strain JM-109 and purified with the EndoFree plasmid Giga kit in accordance with the supplier’s protocol. DNA was dissolved in Endofree TE buffer and kept frozen in aliquots at a concentration of 2 mg/mL.
Formulation was made by mixing the plasmid DNA with the sodium salt of PLG before adjusting the NaCl concentration to 0.15 mol/L with a 5 mol/L stock solution. The plasmid and polymers were allowed to incubate at room temperature for 15 min prior to adding NaCl for tonicity adjustment. The pH of the formulation was adjusted using dilute hydrochloric acid or sodium hydroxide to 7.0. Sterility of the formulation was achieved by sterile filtration through sterile 0.22 μm pore size filters, and was injected immediately after preparation.
Mice were anesthetized by intraperitoneal (ip) injection of pentobarbital sodium at a dose of 30 mg/kg body weight. The plasmid was injected directly into the tibialis anterior (TA) muscles of the mice using a 500 μL syringe. The injected volumes were 50 μL for each side of the TA muscles.
Two minutes after the im injection of plasmid DNA, an electrical field was applied to the area around the injection. Two silver needle electrodes were inserted 3 mm apart into the TA muscles and 6 electric pulses were applied using the TERESA DNA delivery device. The electric pulses were 50 ms in duration at a voltage of 60 V.
The TA muscles treated with plasmid pLac were excised at different time points post delivery, and were analyzed for β-galactosidase expression. The samples were collected immediately after euthanizing the animals. The samples were frozen by keeping in liquid nitrogen and then stored at -70°C until further use. These samples were weighed, minced into small pieces and homogenized with reporter lysis buffer using a homogenizer. The tissue homogenate was then centrifuged at 15 000 g and 4°C for 15 min and the clear supernatant was separated for further analysis. The β-galactosidase activity was measured using the β-galactosidase enzyme assay system. The total protein content of the samples was measured with the BCA protein assay kit. Finally, the enzyme activity in the samples was expressed as milliunits/mg of protein.
The mice were divided into the following groups: (1) control; (2) CCl4; (3) pLac (50 μg) + CCl4; (4) pKal (100 μg) + CCl4; (5) pKal (50 μg) + CCl4; and (6) pKal (25 μg) + CCl4. Each group was composed of 8 mice. CCl4 was dissolved in corn oil vehicle (1:1, v/v). The mice received a single ip injection with the CCl4 preparation at a dose of 0.8 mL/kg body weight to induce liver injury 6 d after plasmid administration. Control mice received an ip injection of an equal volume of corn oil alone. The mice were sacrificed and the blood samples were collected via the inferior vena cava 24 h after CCl4 treatment. The livers were sampled immediately afterwards.
The serum concentration of kallistatin and tumor necrosis factor (TNF)-α were measured with the Quantikine Immunoassay kit following the manufacturer’s instructions. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using a Hitachi 7020 biochemical analyzer. Lipid peroxidation was detected by measuring the serum level of malonyldialdehyde (MDA) spectrophotometrically, the end product derived from the breakdown of polyunsaturated fatty acids and related esters, with the classical thiobarbituric acid method[16].
The excised liver was fixed in buffered formalin, embedded in paraffin, cut into 5 μm-thick sections, and examined with hematoxylin eosin (HE) staining. Pathology was scored in a blinded manner by a trained pathologist by counting the number of necrotic or inflammatory foci per microscopic field[17]. Five fields were checked at 200 × magnification as follows: 0 (absent), 1 (< 2 foci), 2 (2-4 foci), and 3 (> 4 foci). The inflammatory response and muscle damage arising from plasmid injection and electroporation was evaluated by analysis of HE-stained slides of TA muscles.
After deparaffinization in xylene and rehydration in graded ethanols, endogenous peroxidase activity of the paraffin-embedded slides was blocked by incubation with 3% hydrogen peroxide (H2O2) at 37°C for 10 min. The slides were then treated twice in a microwave oven for 5 min in citrate buffer (pH 6.0) at high power to retrieve antigens. After blocking with goat serum at 37°C for 30 min, the specimens were incubated with the rabbit polyclonal antibodies (primary antibody) against transforming growth factor (TGF)-β1 overnight at 4°C, followed by incubation with biotinylated anti-rabbit antibody at 37°C for 30 min and then horseradish peroxidase conjugated streptavidin at 37°C for 10 min. The slides were stained using 3,3’-diaminobenzidine-H2O2, counterstained with hematoxylin, and examined under a light microscope.
Data are given as means ± SD. Analysis of variance was performed to test the significance; P-values were considered significant when less than 0.05.
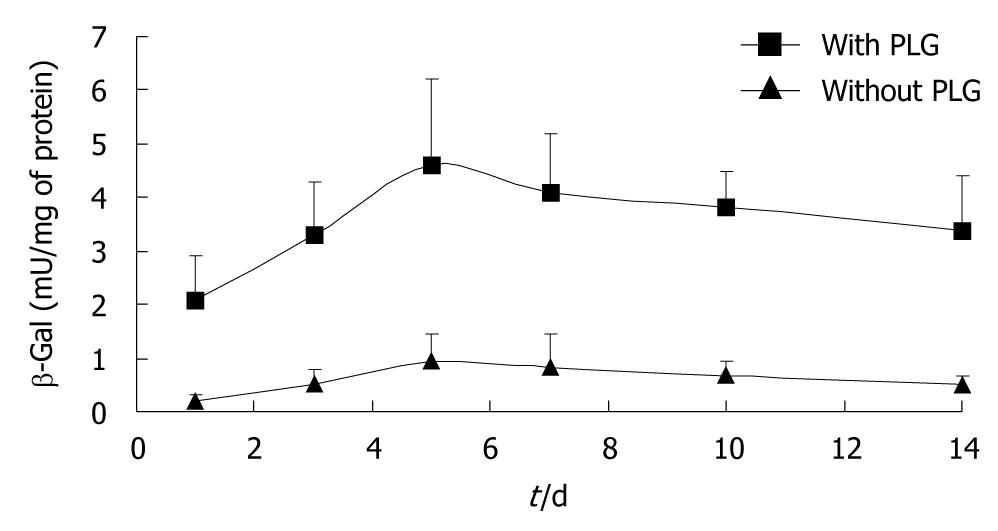
The transgene expression in the TA muscle was enhanced by the PLG formulation (Figure 1). The expression of β-galactosidase in the animals that received plasmid formulated in 6 mg/mL PLG reached its peak level at 5 d post-delivery and was 5-fold higher than that in the mice who received naked plasmid. Thus PLG could enhance the expression of an electroplated plasmid injected im in muscles.
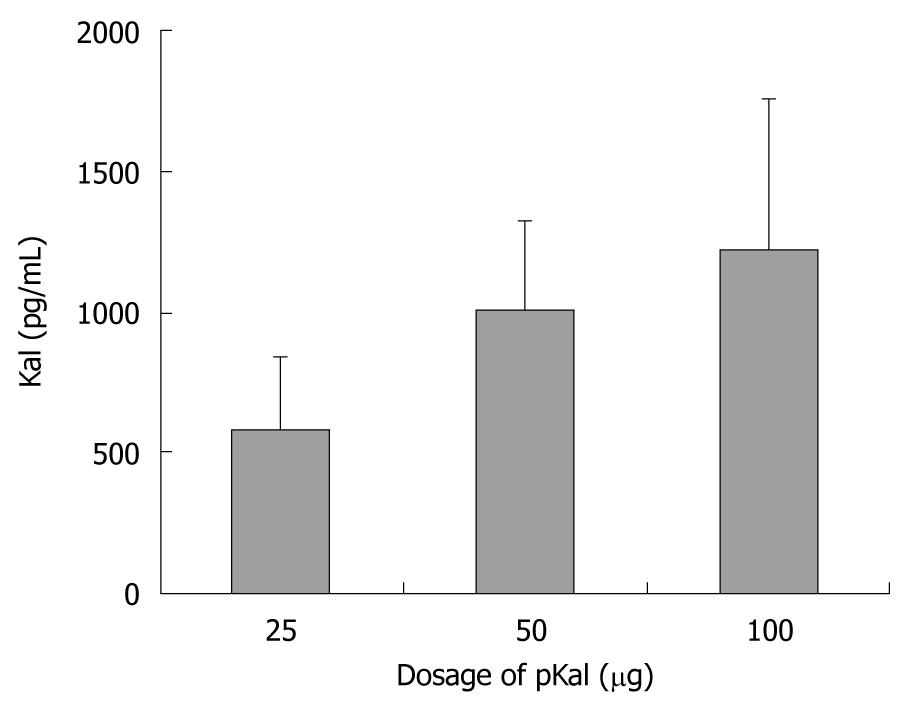
The electroporation of an increasing dose of plasmid pKal (25-100 μg) into TA muscle led to a dose-dependent increase in human Kal plasma concentration, detected on the 7th day after plasmid injection (Figure 2). The level of serum Kal in the 50 μg group was almost 2-fold higher than that in the 25 μg group, but was only slightly lower than in the 100 μg group, suggesting there was a saturation of expression such that the relationship of plasmid dose and transgene expression level was not linear.
The mice induced with a single dose of CCl4 developed hepatic damage as compared with the normal control group (P < 0.01), as shown by marked changes in ALT and AST activities in the serum (Table 1). Injection of pKal resulted in a significant reduction in ALT and AST activities with each of the 3 doses compared with the CCl4 alone group (P < 0.01-0.05).
| Group | ALT (U/L) | AST (U/L) | MDA (nmol/mg protein) | TNF-α (pg/mL) |
| Control | 37 ± 7 | 48 ± 7 | 0.46 ± 0.10 | 2.9 ± 2.1 |
| CCl4 | 134 ± 26b | 183 ± 20b | 1.15 ± 0.29b | 28.1 ± 11.8b |
| pLac + CCl4 | 123 ± 18b | 181 ± 28b | 1.07 ± 0.33b | 28.8 ± 9.4b |
| pKal (100 μg) + CCl4 | 95 ± 24d | 121 ± 35d | 0.48 ± 0.27d | 12.4 ± 4.0d |
| pKal (50 μg) + CCl4 | 98 ± 28c | 143 ± 37c | 0.65 ± 0.25d | 14.5 ± 3.5d |
| pKal (25 μg) + CCl4 | 101 ± 32c | 151 ± 37c | 0.82 ± 0.21c | 17.3 ± 4.8c |
TNF-α is one of the pro-inflammatory cytokines which are early mediators of tissue damage and repair. Mouse serum TNF-α concentration was measured to evaluate the influence of Kal on CCl4-induced inflammatory responses (Table 1). CCl4 exposure markedly stimulated TNF-α releasing in comparison with the control group (P < 0.01). The serum TNF-α levels in the 3 pKal + CCl4 groups were significantly lower than that in the CCl4 group (P < 0.01-0.05) in a dose-dependent manner. These results clearly suggested that the CCl4-induced inflammatory response could be suppressed by pretreatment with pKal.
The generation of ROS and increase of hepatic lipid peroxidation are important features of chronic liver diseases. To examine the effects of Kal on hepatic oxidative stress, liver tissue was homogenized to determine the hepatic MDA level 24 h after CCl4 administration (Table 1). MDA is the end product of lipid peroxidation; its level indirectly reflects the degree of oxidative stress. The hepatic MDA concentration in the group treated with CCl4 was only significantly higher than in the control group (P < 0.01). The 3 pKal pretreatment groups significantly attenuated the elevated MDA compared with CCl4 and pLac + CCl4 groups (P < 0.01-0.05).
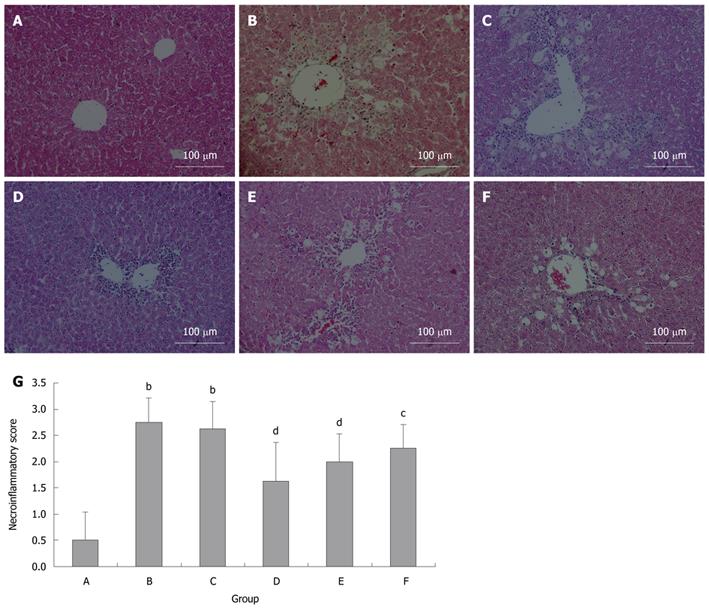
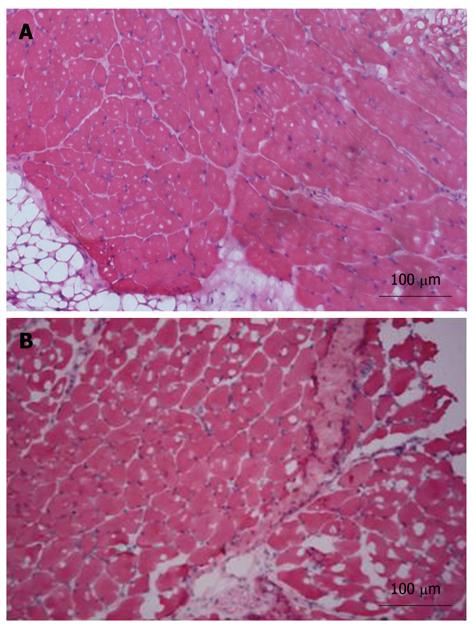
To analyze the extent of liver injury, liver sections were stained with hematoxylin and eosin (Figure 3). No apparent damage was found in the liver sections from the control mice (Figure 3A). In contrast, extensive damage was detected in the sections from CCl4 and pLac + CCl4 groups (Figure 3B and C). Hepatocyte necrosis was the predominant histopathologic lesion, and the affected livers displayed hemorrhage, vacuolar change, hydropic degeneration of hepatocytes and infiltration of inflammatory cells. All these lesions in the pKal + CCl4 groups were significantly attenuated (Figure 3D-G).
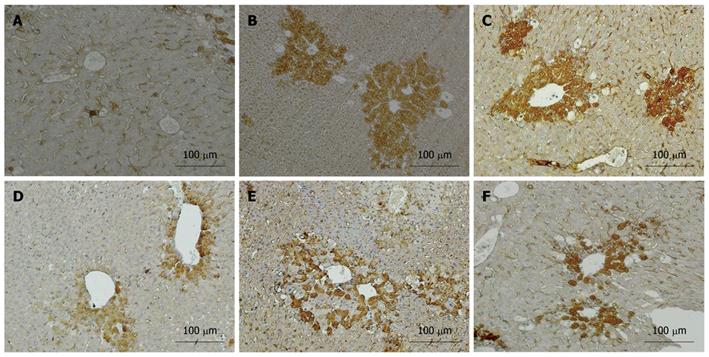
In liver tissue from the control group, the expression of TGF-β1 was negative in liver cells and weakly positive in stromal cells of the portal area (Figure 4A). In the CCl4 and pLac + CCl4 groups, the expression of TGF-β1 was strongly positive in the cytoplasm of both hepatic parenchymal cells and stromal cells (Figure 4B and C). Compared with the CCl4 group, the expression of TGF-β1 was significantly reduced in the pKal treatment groups (Figure 4D-F).
The safety of plasmid injection and electroporation was evaluated by im injection of 50 μg pLac followed by electroporation. Fifty μL saline injections were used as a control. The injected TA muscles were harvested on day 7, fixed, dehydrated, and analyzed by HE staining. The representative images of the muscle samples in various groups are shown in Figure 5. The treatment induced low inflammatory responses at the injection site. No necrosis was observed for any of the groups.
The transfer ability of the recombinant plasmid can be improved by the combination of proper formulation, im injection, and electroporation. To improve the efficacy and reproducibility of plasmid delivery, protective, interactive, non-condensing polymers and non-ionic block co-polymers, consisting of ethylene oxide and propylene oxide monomers, have been developed[18,19]. Both systems have increased distribution of DNA within the muscle tissue and augmented transgene expression compared with DNA in saline. Negatively charged polymers, such as PLG, could also improve the expression of genes delivered by im injection and electroporation, and elevated levels of secreted gene products in multiple in vivo models have been reported[18,20]. Although the mechanism by which PLG enhances transgene expression is unknown, based on our data we concur with other authors’ findings that the formulation of PLG can (1) disperse plasmids throughout the electrical field at the time of electroporation; (2) protect plasmids from nuclease degradation; and (3) facilitate intracellular uptake and trafficking of transcriptional active plasmid in muscle cells[9].
The technique of electroporation has been used for nearly 30 years as a means of introducing DNA into cells in vitro, and is now widely used for transfection of plasmids into different tissues in vivo. More recently, electroporation has been used for the treatment of cutaneous and subcutaneous tumors in humans and its safety was proved clinically[21]. Our results also showed that electroporation treatment-associated muscle damage was minimal. These experiences have paved the way for the clinical use of gene electroporation in humans. The DNA electroporation is reaching the clinical stage as several clinical trials to transfer genes in tumors and in muscle are ongoing[22,23].
Using an intramuscular electrotransfer method, we demonstrated that the PLG formulation of pKal provided a satisfactory expression level of Kal. The expressed Kal possesses antioxidant properties and the secreted Kal could contribute to protection of liver against CCl4-induced damage in a mouse model, as indicated by both histological observation and biochemical evaluation. The Kal gene-transferred mice demonstrated a significant reduction in the level of serum peroxidation marker MDA, with a concomitant improvement in the activities of the hepatic antioxidative defense system. This suggests that Kal is able to protect against hepatic cellular membrane oxidative damage via a free radical scavenging property.
Our results also exhibited the anti-inflammatory activity of Kal. TNF-α is a proinflammatory cytokine produced predominantly by macrophages and plays a key role in the host defense response to injury and infection. The expression of Kal in the circulation inhibited the increase in TNF-α production from Kupffer cells following CCl4 injection, due to its anti-inflammatory activity.
The efficiency of most protein drugs, whose half-life in vivo is generally shorter than chemical drugs, markedly depends on their plasma kinetics. Taking the advantages of gene transfer, a steady-state therapeutic level of the recombinant protein in the circulation could be maintained for a long time. This is especially important in the treatment of chronic disease or the prevention of disease. Our results showed that the constant expression level of Kal in the pKal group with the lowest dosage was already sufficient to prevent CCl4-induced liver damage. These data provided compelling and mechanistic evidences for the importance of Kal in regulation of liver injury.
TGF-β1 in the liver is secreted by hepatocytes, Kupffer cells, stellate cells (HSC), endothelial cells and infiltrating mononuclear cells, and plays a pivotal role in hepatic fibrogenesis. Among many inflammatory cytokines involved in liver fibrosis, TGF-β1 appears to be the most important, because (1) there is higher TGF-β1 expression in activated HSC; (2) TGF-β1 has potency in upregulating extracellular matrix expression; (3) there is higher expression of TGF-β receptors on HSC; and (4) TGF-β1 increases the expression of tissue inhibitor of metalloproteinases-1. Therefore, many antifibrosis strategies focus on reducing the secretion of TGF-β1 and blocking the TGF-β signal transduction pathway to reduce TGF-β1-induced HSC proliferation. Our results clearly demonstrated the inhibitory effect of Kal expression on the hepatic TGF-β1 level in an experimental animal model of liver damage induced by CCl4.
In conclusion, the data of this study provided the evidence that the combination of PLG formulation, im injection and electroporation of plasmid encoding the Kal gene is an effective gene therapeutic method in a CCl4-induced liver damage model. The beneficial effects of this technique to reduce the expression of TGF-β1 also make it a promising strategy for the treatment of hepatic fibrosis in the future.
Acute and chronic hepatic injuries cause high morbidity and mortality worldwide. Although the pathogenesis is not fully understood, it is clear that reactive oxygen species play a key function in the pathological changes in the liver. Kallistatin is a member of the serine proteinase inhibitor superfamily and was shown to have pleiotropic effects, including anti-oxidative stress, anti-inflammation and angiogenesis. Studies show it can attenuate oxidative stress, apoptosis, inflammation, and organ damage. However, the short half-life of kallistatin protein in vivo limits its efficiency. In contrast, long-term transgene expression can be achieved by gene therapy.
Gene therapy has already proven to be a novel and promise modality in reducing liver injury, but is still in its infancy, and ideal gene delivery systems for gene transfer with high and prolonged gene expression, as well as less cytotoxicity or immunogenicity remain to be developed. Viral vectors are so far the most efficient tools for delivery of genes into mammalian cells. Drawbacks such as cost, immunogenicity, and difficulties in manufacture have shifted the interest towards nonviral vectors. However, the low efficiency of gene transfer hampers the development of nonviral vectors severely.
In order to boost the efficacy of non-viral gene therapy delivery, DNA electrotransfer technology was applied and the plasmid formulation was also optimized. The kallistatin expression in vivo was increased greatly; the animal model showed that the gene therapy strategy was effective in alleviating oxidative stress and protecting the liver against carbon tetrachloride (CCl4)-induced liver damage.
Although additional studies are necessary to translate this technology into clinic trials in humans, the results provide a rationale to develop new pharmacological strategies in the clinical management of patients with acute and chronic liver injury.
Electro-gene therapy: a method involving injection of a naked plasmid encoding a marker gene or a therapeutic gene, followed by in vivo electroporation, where short electrical pulses are applied to the injected tissue. The gene expression of the injected plasmid was reported to increase more than 100-fold.
The paper described the improvement of the intramuscular electrotransfer of a kallistatin-encoding plasmid by using poly-L-glutamate in plasmid formulation. The ensuing increased presence of kallistatin in plasma protected those transfected mice from the hepatotoxic effects of a single dosage of CCl4. In general, results are sound and their interpretation is correct.
Peer reviewers: Dr. Juan Carlos Laguna Egea, Professor of Pharmacology, Unitat de Farmacologia, Facultat de Farmàcia, Universitat de Barcelona, Avenida Diagonal 643, Barcelona 08028, Spain; Valentina Medici, MD, Assistant Professor, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of California Davis, 4150 V Street, Suite 3500, Sacramento, CA 95817, United States
S- Editor Sun H L- Editor Cant MR E- Editor Lin YP
| 2. | Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262-4267. [Cited in This Article: ] |
| 3. | Bloquel C, Fabre E, Bureau MF, Scherman D. Plasmid DNA electrotransfer for intracellular and secreted proteins expression: new methodological developments and applications. J Gene Med. 2004;6 Suppl 1:S11-S23. [Cited in This Article: ] |
| 4. | André FM, Gehl J, Sersa G, Préat V, Hojman P, Eriksen J, Golzio M, Cemazar M, Pavselj N, Rols MP. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum Gene Ther. 2008;19:1261-1271. [Cited in This Article: ] |
| 5. | Cukjati D, Batiuskaite D, André F, Miklavcic D, Mir LM. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry. 2007;70:501-507. [Cited in This Article: ] |
| 6. | Miklavcic D, Snoj M, Zupanic A, Kos B, Cemazar M, Kropivnik M, Bracko M, Pecnik T, Gadzijev E, Sersa G. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010;9:10. [Cited in This Article: ] |
| 7. | Hui SW. Overview of drug delivery and alternative methods to electroporation. Methods Mol Biol. 2008;423:91-107. [Cited in This Article: ] |
| 8. | Ferber D. Gene therapy. Safer and virus-free? Science. 2001;294:1638-1642. [Cited in This Article: ] |
| 9. | Nicol F, Wong M, MacLaughlin FC, Perrard J, Wilson E, Nordstrom JL, Smith LC. Poly-L-glutamate, an anionic polymer, enhances transgene expression for plasmids delivered by intramuscular injection with in vivo electroporation. Gene Ther. 2002;9:1351-1358. [Cited in This Article: ] |
| 10. | Chao J, Yin H, Yao YY, Shen B, Smith RS Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201-1213. [Cited in This Article: ] |
| 11. | Gao L, Yin H, S Smith R Jr, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008;88:1157-1166. [Cited in This Article: ] |
| 12. | Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358-1365. [Cited in This Article: ] |
| 13. | Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005;52:1319-1324. [Cited in This Article: ] |
| 14. | Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086-3098. [Cited in This Article: ] |
| 15. | Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu KW, Fung PW, Habib N, Farzaneh F, Xu RA. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol. 2007;13:4615-4619. [Cited in This Article: ] |
| 16. | Xu R, Harrison PM, Chen M, Li L, Tsui TY, Fung PC, Cheung PT, Wang G, Li H, Diao Y. Cytoglobin overexpression protects against damage-induced fibrosis. Mol Ther. 2006;13:1093-1100. [Cited in This Article: ] |
| 17. | de Meijer VE, Sverdlov DY, Popov Y, Le HD, Meisel JA, Nosé V, Schuppan D, Puder M. Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. PLoS One. 2010;5:e11256. [Cited in This Article: ] |
| 18. | Fewell JG, MacLaughlin F, Mehta V, Gondo M, Nicol F, Wilson E, Smith LC. Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation. Mol Ther. 2001;3:574-583. [Cited in This Article: ] |
| 19. | Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30:1232-1245. [Cited in This Article: ] |
| 20. | Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34:232-240. [Cited in This Article: ] |
| 21. | Testori A, Tosti G, Martinoli C, Spadola G, Cataldo F, Verrecchia F, Baldini F, Mosconi M, Soteldo J, Tedeschi I. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach. Dermatol Ther. 2010;23:651-661. [Cited in This Article: ] |
| 22. | Villemejane J, Mir LM. Physical methods of nucleic acid transfer: general concepts and applications. Br J Pharmacol. 2009;157:207-219. [Cited in This Article: ] |
| 23. | Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896-5903. [Cited in This Article: ] |