INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies and causes of cancer deaths in Europe[1] and the United States[2]. However, there has been a notable rise in the incidence of CRC in Asia over the last few decades[3]. Almost all CRCs develop from normal colonic epithelial cells with an adenomatous polyp as an intermediate step. Current efforts are directed towards detection and removal of adenomatous polyps. Therefore, endoscopy has become a part of routine clinical practice. Unfortunately, the polyp often remains indolent for years and is generally discovered only during routine screening. Even after removal, polyps can still lead to recurrence. Endoscopy has its financial limitations; therefore, chemoprevention might be crucial in reducing the incidence of CRC. In this context, chemoprevention refers to the use of chemical compounds to prevent, inhibit, or reverse carcinogenesis. The focus of chemoprevention in CRC is bioactive food components.
Bioactive food components are constituents in commonly consumed foods or dietary supplements, which result in the promotion of better health. It is clear that bioactive food components can play an important role and influence cancer outcomes[4], as previously demonstrated by cytochrome p450 inhibitors, inducers of cell cycle arrest and apoptosis, and inhibitors of angiogenesis[5]. Bioactive food components involved in CRC chemoprevention include folate, fiber, and short-chain fatty acids (SCFA, e.g. butyrate, which is decomposed product of fiber in large intestine), methionine, bilineurine, vitamin D, and calcium. Epigenetic changes play a very important role in the evolution from normal intestinal epithelium to colon cancer[6]. The main chemoprevention drugs for epigenetic changes are folate and butyrate[6]. This review discusses the effect of folate and fiber on CRC chemoprevention.
FOLATE
Folate is a water-soluble B vitamin found abundantly in fresh fruits and leafy green vegetables, and provides one-carbon groups in methylation of DNA[7], and contributes to DNA synthesis and replication as well as epigenetic regulation of gene expression[8]. Therefore, folate deficiency might impair these processes and cause chromosomal breaks, as well as deleterious alterations in gene expression. For example, folate depletion has been shown to induce the upregulation of p16INK4A, p21WAF1 and p53 tumor suppressor genes, which are involved in DNA damage signaling, inhibition of cell-cycle progression through checkpoints, and apoptosis[9]. Folate deficiency also induces genomic instability and aberrant DNA methylation, which might contribute to colorectal carcinogenesis[10]. However, whether folate can prevent the occurrence of CRC should take the following into consideration:
Firstly, several epidemiologic and clinical studies have found a relationship between folate deficiency and CRC risk[7,8,11-13]. Low levels of folate in the diet or blood have been shown to be associated with a higher risk of CRC[14,15]. In contrast, high intake of dietary folate has been inversely associated with the risk of CRC[8,14]. Multiple case-control and observational cohort studies suggest a reduction of 30%-40% in CRC risk for participants with high levels of folate intake compared to those with low levels[11-13]. According to some data[16], the risk of CRC decreases 11% for every 400 μg of total folate ingested. It has also been shown to elicit various responses that might be beneficial in reducing the risk of CRC, where these effects might be mediated through increased concentrations of colonic mucosal folate[17].
Secondly, although a protective role against CRC has been suggested for high dietary folate intake, epidemiological evidence has not consistently shown a protective effect of high folate intake against CRC[8,18,19]. As such, high folate intake might enhance colorectal tumor recurrence and progression[20-22]. Animal studies have suggested that high-dose folic acid might promote colorectal tumorigenesis[23], as there is a very close relationship between increases in the incidence of CRC and the remarkable increase in dietary folate intake and blood folate levels[22]. A large, placebo-controlled multicenter trial has shown that high-dose folate might potentially increase the risk of neoplastic transformation[24] and one study has reported a significantly increased risk of CRC in subjects with high plasma folate concentrations[6].
Thirdly, in two large prospective cohorts[25], the Nurses’ Health Study and Health Professionals Follow-Up Study, higher prediagnostic levels of plasma folate were not associated with an increased risk of CRC-specific or overall mortality. Moreover, a low folate diet induced genomic uracil misincorporation and hypomethylation in Big Blue Mice and uracil DNA glycosylase deficiency (Ung-/-) mice, which is insufficient to promote tumor development[26]. A multicenter, randomized, double-blind trial has shown that folate supplementation was found to have no effect on adenoma recurrence [relative risk (RR) = 1.07, 95% CI: 0.85-1.34][27].
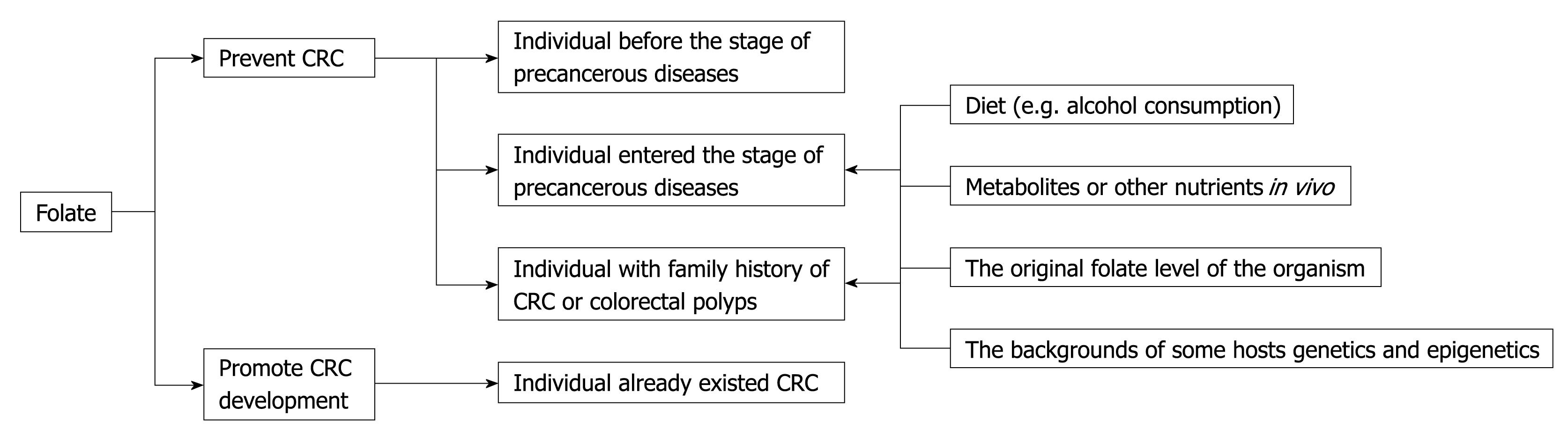
Why did above-mentioned differences emerge? Some scholars have suggested that dietary folate supplementation protection against colonic carcinogenesis might depend on the stage of colorectal carcinogenesis, and would protect against carcinogenesis in normal colorectal tissue, but that folate might enhance pre-existing lesions[23,24,28]. Findings from several studies indicate that any relationship between folate intake and CRC risk is a complex association that might depend on many factors, including gender, age, alcohol consumption, and smoking, all of which interfere with folate metabolism (Figure 1).
Figure 1 Folate supplementation protection against colorectal cancer (CRC) might depend on the stage of colorectal carcinogenesis and might be influenced by many factors.
Gender
One of the earliest epidemiological papers put forth a hypothesis that men might benefit from folate more than women in colorectal neoplasia. There was a significantly reduced risk for adenomatous polyps in men, but no such association was observed for women[29]. Another study, based on the data from the National Health and Nutrition Examination Survey I (NHANES I), revealed that dietary intake of greater than 249 μg/d of folate in men was inversely associated with colon cancer, but not in women[30]. However, most investigations with regard to the relationship between colorectal neoplasia and gender revealed that men and women have the same susceptibility[31,32].
Age
Lower folate intake might have a more pronounced effect on aging colonic mucosa; a folate-depleted diet reduced colonic folate concentrations in older, but not younger rats[33]. Age related changes in the metabolism of folate might explain the link between age and CRC risk.
Alcohol
Alcohol consumption was found to be a risk factor for developing CRC. There might also be an interaction between low folate levels and high alcohol consumption and CRC[34]. Folate absorption in the intestine and its availability in the body can be modified by alcohol consumption[35].
Folate stores
Folate stores might influence tumor responsiveness to chemotherapy. Even under conditions of high systemic folate status, transient localized folate depletion might occur[36], especially in colonic epithelium that demonstrates rapid rates of proliferation[37]. Localized inflammation is also known to deplete folate[38]. These factors could act together to substantially reduce localized intracellular folate bioavailability.
Metabolites
Scientists have shown that folate deficiency is significantly more frequently associated with oncogenesis when combined with hyperhomocysteinemia. Patients with folate deficiency associated with hyperhomocysteinemia had 17 times as many carcinogenic lesions as patients with normal homocysteinemia, regardless of the folate status of the disease[39]. Inflammatory bowel disease patients with folate deficiency and hyperhomocysteinemia might be associated with increased colorectal carcinogenesis[39]. A study in the general population has shown that low folate status with hyperhomocysteinemia increased the risk of recurrence of adenoma[40].
Apart from the above-mentioned factors, distinctions between dietary folate levels and chemical drug effects, a patient’s folate levels before treatment, the occurrence of folate supplementation in patients and some genetic and epigenetic discrepancies of organisms might also be causes of variances in these findings.
In summary, we think that folate deficiency makes the body inclined to generate DNA repair defects and heterotypic cells due to abnormal methylation, which develops in the early stage of abnormal cells. Folate supplementation might have preventive effects on the individual before the stage of precancerous diseases, but for those who have entered this stage (such as adenomatous polyposis), the effect is still difficult to determine. Therefore, folate should be included in the discussion of those factors that might promote CRC tumor development. On the other hand, even in high-risk groups with a family history of CRC or colorectal polyps (for example, adenomatous polyposis and Peutz-Jaegher hamartoma syndrome), folate is supplied to prevent CRC. Precancerous diseases are still influenced by a number of factors, such as diet, metabolites or other nutrients in vivo (such as choline and vitamin B12), folate levels of the organism, and the predisposed backgrounds of some host’s genetics and epigenetics.
FIBER
The protective effects of bioactive food components against the development of CRC are due to not only their folate content, but also their fiber content. Fiber has been investigated as a CRC chemopreventive agent in many clinical trials. As such, it is speculated that increased dietary fiber intake can reduce the risk of CRC through a variety of mechanisms[6,41-43]. These include dilution or adsorption of fecal potential carcinogens, which potentially inhibit chemically-induced carcinogenesis; decreasing the exposure period of colonic epithelial cells to carcinogens, co-carcinogens, or promoters through reduction of contact time between intraluminal contents and the colonic mucosa; alterations in bile acid metabolism, by binding potential carcinogens like secondary bile acids; increasing the production of SCFA; and promoting a favorable colonic microflora by modifying its metabolic activities and composition.
Studies on the protective role of dietary fiber and its main end product of intestinal microbial fermentation, butyrate, against CRC remain controversial. Butyrate, an SCFA, is an important energy source for intestinal epithelial cells and plays a role in the maintenance of colonic homeostasis[44]. It induces potent effects, such as inhibition of inflammation and carcinogenesis of colonic mucosa, reinforcing various components of the colonic defense barrier, decreasing oxidative stress, as well as promotion of cancer cell growth arrest, differentiation, and apoptosis[45].
Several studies have linked higher fiber intake to a reduced risk for CRC[46,47]. A multiethnic cohort study[48] showed that dietary fiber was inversely associated with a CRC risk after adjustment for age and ethnicity in men (RR = 0.49, 95% CI: 0.41-0.60, highest vs lowest quintile) and women (RR = 0.75, 95% CI: 0.61-0.92). After further adjustment for lifestyle and dietary factors, the inverse association remained significant in men (RR = 0.62, 95% CI: 0.48-0.79), but not in women (RR = 0.88, 95% CI: 0.67-1.14). These authors presumed that this phenomenon is related to women’s hormone use. The data from the Japan Collaborative Cohort Study supported the potential protective effects of dietary fiber against CRC, mainly against colon cancer[49]. Other studies showed that high fiber and calcium intakes were more markedly associated with a lower risk of CRC in patients carrying the D1822V APC allele [odds ratio (OR): 0.50, 95% CI: 0.27-0.94 for fiber; OR: 0.51, 95% CI: 0.28-0.93 for calcium] than in those without this allele[50].
The role of fiber in the prevention of colorectal diseases remains controversial. There is some evidence that fiber is not effective for primary prevention of CRC[51,52]. In a large prospective cohort study, total dietary fiber intake was not associated with CRC risk either[53]. Another continued follow-up study failed to show any effect of a low-fat (20% of total energy), high-fiber (18 g/1000 kcal), high-fruit and -vegetable (3.5 servings/1000 kcal) eating pattern on adenoma recurrence even after 8 years of follow-up[54].
When analyzing the differences in the above-mentioned studies, the following aspects should be addressed: (1) Patients with different types of CRCs might be associated with the histone acetylation state of the promoters of tumor-related genes (especially apoptosis-related genes); (2) The findings are different between in vitro and in vivo experiments, possibly due to the direct administration of butyrate to in vitro cells compared to preventing CRC or adenoma with a high-fiber diet in vivo. If we use sodium butyrate interference directly in patients, we might discover different effects; (3) The timing of the different interventions will produce different results: in the early stage of DNA damage, butyrate supplementation can induce chromosomes to remain in the open stage, via inhibition of histone deacetyltransferase, where it is easy to repair DNA damage. Therefore, the best opportunity for butyrate prevention of CRC might be in the early stages of polyp formation, rather than during the transformation stage of adenomatoid polypus to adenocarcinoma; (4) The status of DNA mismatch repair might affect the results of butyrate prevention of CRC[55]; (5) The dose of sodium butyrate suitable for research in vitro and in vivo might be different[56]; (6) The concentration of SCFA formed in the intestinal tract is variable; (7) The complexity of dietary interactions might have an effect; and (8) The duration of the study might also influence the protective role of fiber against CRC[44]. Although the effects of increased dietary fiber intake on reducing the risk of CRC has not been unified in studies conducted to date, more studies are needed to increase knowledge in this area.
CONCLUSION
Although treatment continues to improve, CRC remains a major cause of death throughout the world. A number of studies have demonstrated the potential chemopreventive effect of bioactive food components, but many challenges still remain. For example, there is very little evidence from clinical studies, such that most of the available information is based on epidemiological and experimental data. The value of folate and fiber has not yet been confirmed in epidemiological and clinical studies; therefore, it cannot be accepted as standard medical practice and cannot replace endoscopic screening. Therefore, we should lucubrate the timing of medication, drug dose, interaction mechanisms, and molecular regulatory network of folate and fiber to further advance the field of CRC chemoprevention research. We should then be able to identify compounds and/or molecules using high-throughput techniques, and validate them using the large number of clinical cases resources. Thus we will obtain new individual clinical application programs of CRC chemoprevention to combat this major health problem and minimize the risk of CRC. Future studies and clinical trials must be conducted in a variety of study settings and among different population groups, which will help to elucidate the role of bioactive food components in CRC.