Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.571
Revised: December 11, 2009
Accepted: December 18, 2009
Published online: February 7, 2010
AIM: To explore the relationship between Cripto-1 (CR-1) and tyrosine phosphorylation STAT3 (p-STAT3) expressions in gastric cancer (GC) and gastric carcinogensis and metastasis.
METHODS: The PV9000 immunohistochemical method was used to detect the expression of CR-1 and p-STAT3 in 178 cases of GC, 95 matched normal gastric mucosa, 40 chronic atrophic gastritis (CAG), 48 intestinal metaplasia (IM) and 25 dysplasia (DYS).
RESULTS: The positive rates of CR-1 and p-STAT3 expression were significantly higher in CAG (65.0% and 60.0%), in IM (83.3% and 77.1%), DYS (80.0% and 68%) and GC (71.3% and 60.1%) than in normal gastric mucosa (43.2% and 41.1%, P < 0.05), respectively. The expressions of CR-1 and p-STAT3 (78.3% and 66.7%) were significantly higher in GC with lymph node metastasis than in those without metastasis (53.1% and 42.9%, P < 0.05). CR-1 expression was also related to histological and Lauren’s types of GC (P < 0.001). Furthermore, there was positive relationship between CR-1 and p-STAT3 expressions in GC (rk = 0.189, P = 0.002).
CONCLUSION: The up-regulation of CR-1 and p-STAT3 may play important roles in gastric carcinogenesis and lymph node metastasis. CR-1 and p-STAT3 expression in GC was positively correlated, and the relevant molecular mechanism requires further investigations.
- Citation: Zhang JG, Zhao J, Xin Y. Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol 2010; 16(5): 571-577
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.571
Human Cripto-1 (CR-1), the founder member of the epidermal growth factor-Cripto-FRL1-Cryptic family, plays an important role during early embryonic development and cellular transformation[1]. CR-1 is also an oncogenic growth factor involved in tumorigenesis through influencing cell proliferation, survival, migration and invasion[2-4]. CR-1 is absent or in low levels in normal tissues but over-expressed in most malignant tumors, such as breast, colon, stomach, pancreas, lung, cervix and ovary cancers, which is being assessed as a tumor-specific target for immunotherapy[1,5]. STAT3, a well recognized oncogene, is an important member of the STAT family that are normally inactive within the cytoplasm and become activated by tyrosine phosphorylation in response to cytokines and growth factors[6]. Its signal pathway is closely associated with the proliferation, differentiation and apoptosis, and constant activation of STAT3 can promote cell proliferation and carcinogenesis[7,8]. Given the importance of CR-1 over-expression and STAT3 activation in carcinogenesis, we investigated the expression and clinicopathological significance of CR-1 and phosphorylation STAT3 (p-STAT3) in gastric cancer (GC), and also explored the relationship between abnormal expressions of the two oncoproteins in GC.
Surgically resected GC specimens were collected from the First Affiliated Hospital of China Medical University, including 178 cases of GC, 95 matched normal gastric mucosa (obtained at > 5 cm apart from the edge of primary tumor focus), 40 chronic atrophic gastritis (CAG), 48 intestinal metaplasia (IM), and 25 dysplasia (DYS). There were 121 males and 57 females. The mean age of patients was 61 years. According to Borrmann’s classification, gross types of primary tumors were classified as follows: 4 cases of Borrmann I, 22 cases of Borrmann II, 140 cases of Borrmann III, and 12 cases of Borrmann IV. In the light of the WHO’s histological classification of GC, 178 cases were classified as follows: 2 papillary adenocarcinoma, 9 well and 65 moderately and 74 poorly differentiated adenocarcinoma, 3 undifferentiated adenocarcinoma, 19 mucinous adenocarcinoma and 6 signet ring cell carcinoma. Samples were fixed in 10% formalin, embedded in paraffin, cut into 4 μm thick sections and constructed in blocks for tissue microarray. All the samples were evaluated by two experienced pathologists for diagnosis. None of the patients had received chemotherapy or radiation therapy preoperatively.
Expression of CR-1 and p-STAT3 in GC, precancerous lesions and normal gastric mucosa were detected using an IHC method. The PV-9000 kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Company. Mouse monoclonal antibody against human CR-1 was from the R&D systems (working dilution 1:80). Rabbit monoclonal antibody against human p-STAT3 was from the Signalway Antibody (working dilution 1:25). All procedures were implemented according to the manufacturer’s instructions. For negative controls, sections were treated with 0.01 mol/L phosphate-buffered saline instead of primary antibodies.
Specific immunoreactivity of CR-1 protein was located in the cytoplasm, while p-STAT3 protein was located in the nucleus and cytoplasm. Two hundred cells from two selected representative fields of each section were counted by two independent observers for the determination of their immunostaining intensity. Staining intensity (A) was classified as 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The percentage of positive cells (B) examined in 200 cells were divided into 0 (< 5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%) and 4 (> 75%). According to the product of A and B, the IHC result was classified as 0, negative (-); 1-4, weakly positive (+); 5-8, moderately positive (++) and 9-12, strongly positive (+++).
Statistical analysis was performed using SPSS 11.5 Package, and χ2 test, Fisher’s exact test and Kendall’s tau-b test were used to differentiate the rates of different groups and test the correlation between the two factors. P < 0.05 was considered statistically significant.
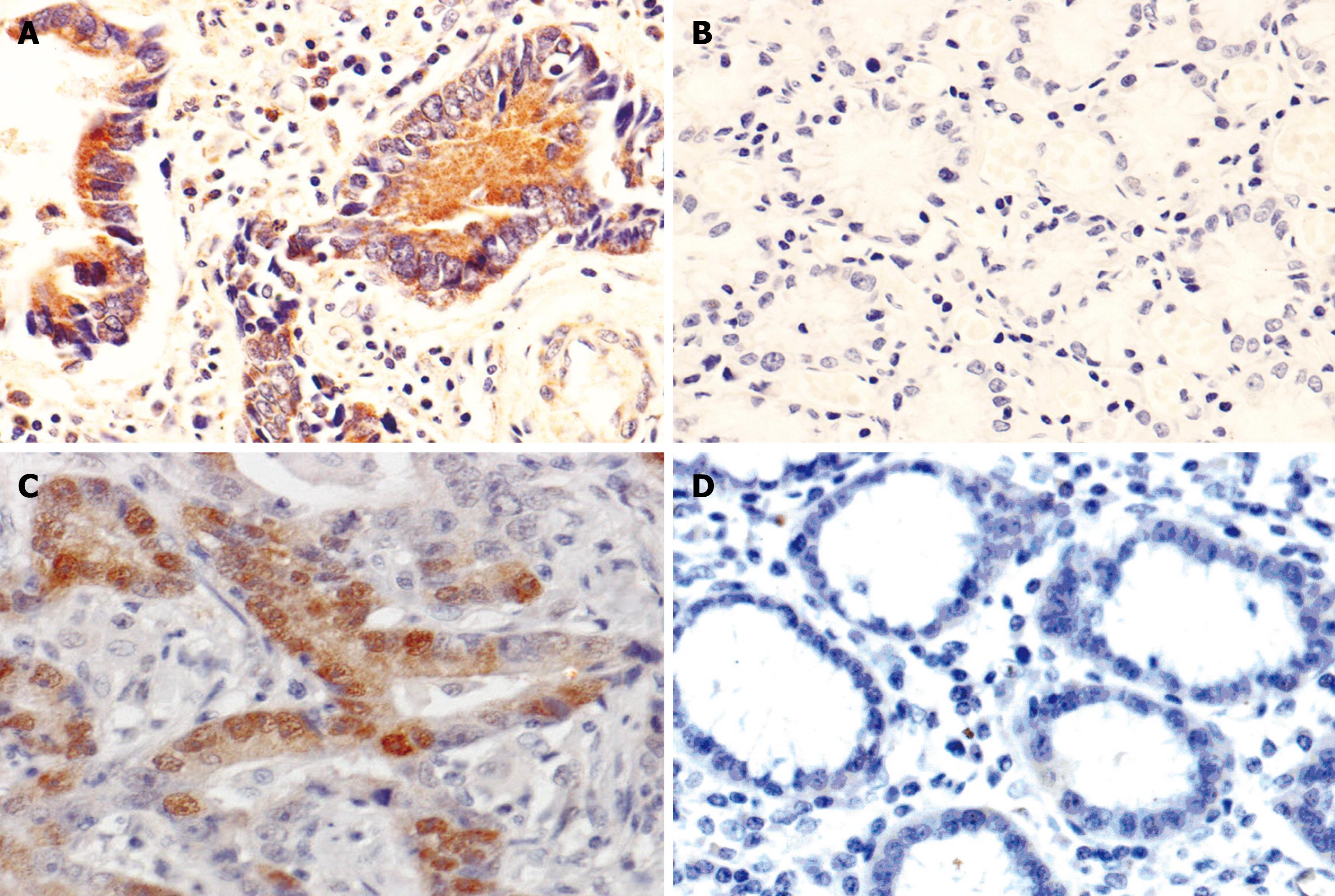
The immunoreactivity of CR-1 protein was located diffusely in the cytoplasm. The positive rates of CR-1 presence in CAG (65.0%, 26/40), IM (83.3%, 40/48), DYS (80.0%, 5/25) and GC (71.3%, 127/178, Figure 1A) were significantly higher than that in normal gastric mucosa (43.2%, 41/95, Figure 1B), respectively, P < 0.05; there was no statistically significant difference in CR-1 expression between DYS and GC (P = 0.246), but the positive rate of CR-1 in IM was significantly higher than that in GC (Table 1).
The immunoreactivity of p-STAT3 protein was located in the nucleus and cytoplasm. The positive rates of p-STAT3 expression in CAG (60%, 24/40), IM (77.1%, 37/48), DYS (68.0%, 17/25) and GC (60.1%, 107/178, Figure 1C) were significantly higher than that in normal gastric mucosa (41.1%, 35/95, Figure 1D), respectively, P < 0.05. There was no significant difference in p-STAT3 expression among IM, DYS and GC (P = 0.087, P = 0.103, Table 2).
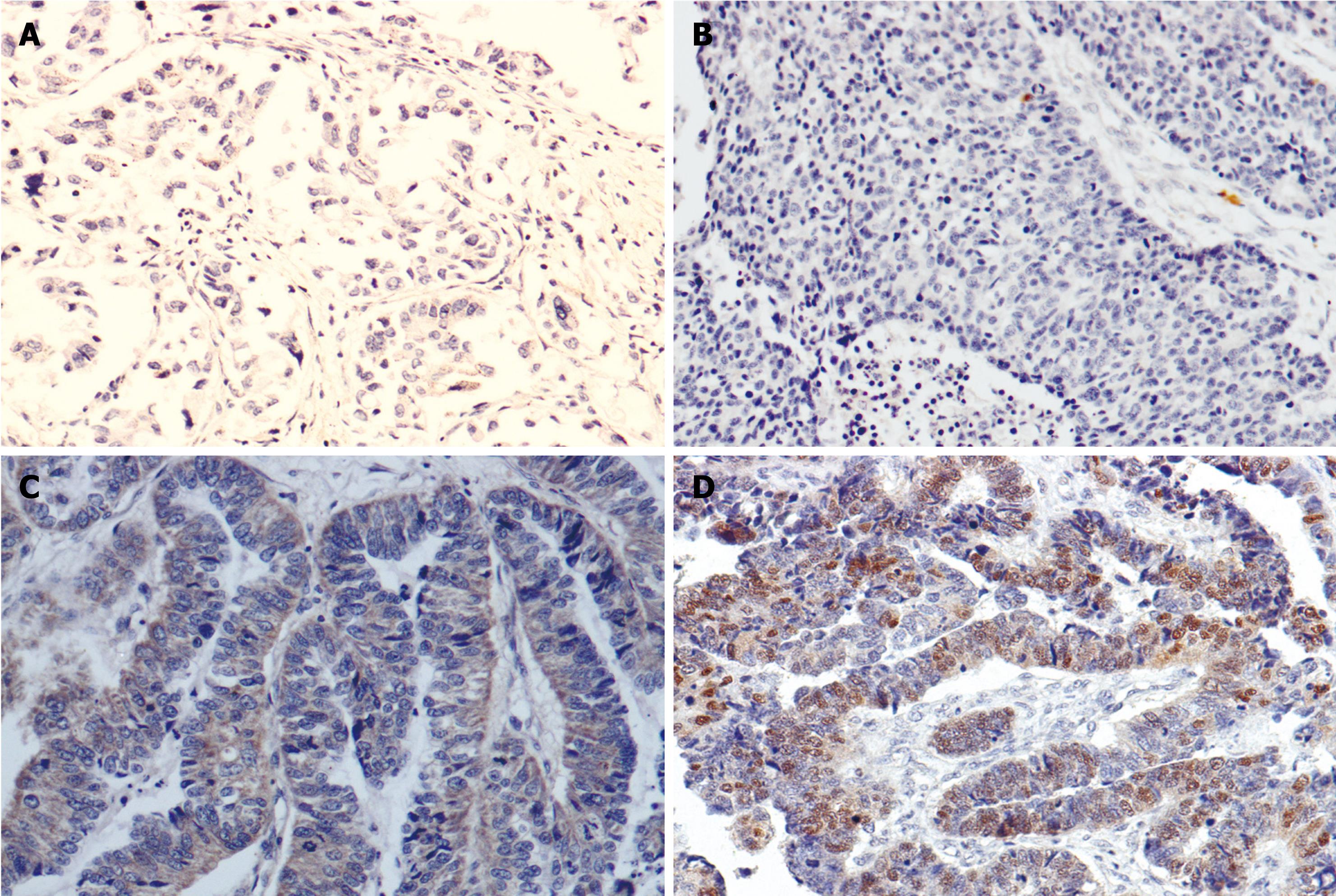
Tables 3 and 4 showed the correlation of immunohistochemical (IHC) expression of CR-1 and p-STAT3 with clinicopathological parameters. Statistical analysis showed the expression of CR-1 was related to histological differentiation (P < 0.001), Lauren’s types (P < 0.001) and lymph node metastasis (P = 0.006) (Figure 2A and C), but not related to the age and gender of patients, or Borrmann’s classification of GC. There was no relation between p-STAT3 expression and gender, age, Borrmann’s classification, histological types or Lauren’s types, but p-STAT3 expression was significantly higher in tumors with lymph node metastasis (66.7%) than in those without metastasis (42.9%), P < 0.05 (Figure 2B and D).
| Groups | n | Cripto-1 expression | +→+++ (%) | χ² | P | |||
| - | + | ++ | +++ | |||||
| Gender | 4.052 | 0.269 | ||||||
| Female | 57 | 11 | 24 | 14 | 8 | 80.7 | ||
| Male | 121 | 40 | 39 | 24 | 18 | 66.9 | ||
| Age (yr) | 2.920 | 0.417 | ||||||
| ≤ 61 | 90 | 22 | 33 | 23 | 12 | 75.6 | ||
| > 61 | 88 | 29 | 30 | 15 | 14 | 67.0 | ||
| Borrmann type | 0.1951 | |||||||
| Bor I type | 4 | 0 | 3 | 0 | 1 | 100 | ||
| Bor II type | 22 | 6 | 9 | 7 | 0 | 72.7 | ||
| Bor III type | 140 | 41 | 48 | 27 | 24 | 70.7 | ||
| Bor IV type | 12 | 4 | 3 | 4 | 1 | 66.7 | ||
| WHO’s histological types | < 0.0011 | |||||||
| Papillary. ade. | 2 | 0 | 1 | 1 | 0 | 100 | ||
| Well-diff. ade. | 9 | 3 | 2 | 2 | 2 | 66.7 | 17.872 | < 0.0012 |
| Moderately-diff. ade. | 65 | 8 | 28 | 11 | 18 | 87.7 | ||
| Poorly-diff. ade. | 74 | 27 | 27 | 16 | 4 | 63.5 | ||
| Undiff. ade. | 3 | 0 | 1 | 2 | 0 | 100 | ||
| Mucinous ade. | 19 | 11 | 3 | 3 | 2 | 42.1 | ||
| SRC | 6 | 2 | 1 | 3 | 0 | 66.7 | ||
| Lauren’s types | 28.523 | < 0.001 | ||||||
| Intestinal type | 74 | 11 | 29 | 14 | 20 | 68.4 | 19.566 | < 0.0013 |
| Diffused type | 85 | 29 | 31 | 21 | 4 | 65.9 | ||
| Mixed type | 19 | 11 | 3 | 3 | 2 | 42.1 | ||
| Lymph node metastasis | 12.394 | 0.006 | ||||||
| No | 49 | 23 | 14 | 9 | 3 | 53.1 | ||
| Yes | 129 | 28 | 49 | 29 | 23 | 78.3 | ||
| Groups | n | P-STAT3 expression | +→+++ (%) | χ² | P | |||
| - | + | ++ | +++ | |||||
| Gender | 3.091 | 0.391 | ||||||
| Female | 57 | 18 | 27 | 7 | 5 | 68.4 | ||
| Male | 121 | 53 | 42 | 16 | 10 | 56.2 | ||
| Age (yr) | 2.177 | 0.544 | ||||||
| ≤ 61 | 90 | 37 | 35 | 13 | 5 | 58.9 | ||
| > 61 | 88 | 34 | 34 | 10 | 10 | 61.4 | ||
| Borrmann type | 0.9091 | |||||||
| Bor I type | 4 | 1 | 2 | 0 | 1 | 75.0 | ||
| Bor II type | 22 | 11 | 8 | 2 | 1 | 61.2 | ||
| Bor III type | 140 | 55 | 53 | 20 | 12 | 60.7 | ||
| Bor IV type | 12 | 4 | 6 | 1 | 1 | 66.7 | ||
| WHO’s histological types | 0. 1001 | |||||||
| Papillary. ade. | 2 | 0 | 0 | 0 | 2 | 100 | ||
| Well-diff. ade. | 9 | 4 | 4 | 0 | 1 | 55.6 | ||
| Moderately-diff. ade. | 65 | 23 | 23 | 10 | 9 | 64.5 | ||
| Poorly-diff. ade. | 74 | 35 | 29 | 7 | 3 | 52.7 | ||
| Undiff. ade. | 3 | 1 | 2 | 0 | 0 | 66.7 | ||
| Mucinous ade. | 19 | 7 | 8 | 4 | 0 | 63.2 | ||
| SRC | 6 | 1 | 3 | 2 | 0 | 83.3 | ||
| Lauren’s types | 11.417 | 0.076 | ||||||
| Intestinal type | 74 | 27 | 26 | 9 | 12 | 63.5 | ||
| Diffused type | 85 | 37 | 35 | 10 | 3 | 56.5 | ||
| Mixed type | 19 | 7 | 8 | 4 | 0 | 63.2 | ||
| Lymph node metastasis | 9.632 | 0.021 | ||||||
| No | 49 | 28 | 16 | 3 | 2 | 42.9 | ||
| Yes | 129 | 43 | 53 | 20 | 13 | 66.7 | ||
The positive relationship was observed between CR-1 and p-STAT3 expression in GC (rk = 0.189, P = 0.002) (Table 5).
| Cripto-1 expression | p-STAT3 expression | Total | |||
| - | + | ++ | +++ | ||
| - | 24 | 21 | 6 | 0 | 51 |
| + | 28 | 22 | 9 | 4 | 63 |
| ++ | 13 | 17 | 3 | 5 | 38 |
| +++ | 6 | 9 | 5 | 6 | 26 |
| Total | 71 | 69 | 23 | 15 | 178 |
Human CR-1, also known as teratocarcinoma-derived growth factor-1 (TDGF-1), was originally isolated and cloned from a human NTERA-2 teratocarcinoma cDNA library and was classified among the epidermal growth factor (EGF) family of peptides due to amino acid sequence similarities within the EGF-like domain[9]. CR-1 maps to human chromosome 3p21.3 and encodes a 188-amino acid glycosylphosphatidylinositol-linked glycoprotein. CR-1 protein contains a signal sequence, a characteristic EGF-like domain, a second cysteine-rich region motif (CFC domain), and a hydrophobic COOH-terminus[1]. Several findings suggested that CR-1 can specifically bind to Glypican-1, a membrane-associated heparan sulfate proteoglycan, and activate the tyrosine kinase c-Src, triggering the mitogen-activated protein kinase and Akt signaling pathways and then promote cell proliferation, survival, migration, and invasion[4,10]. In addition, by forming complex with activin and growth factor β (TGF-β) ligand and the receptor, CR-1 inhibited activin and TGF-β-signaling, while activin and TGF-β are potent inhibitors of cell growth in various target tissues[11,12]. Disruption of their signaling was associated with carcinogenesis. CR-1 may be a potential target for therapy in human malignancies[5]. In fact, anti-CR-1 antisense oligonucleotides or neutralizing blocking anti-CR-1 monoclonal antibodies have been shown to strongly inhibit the in vitro and in vivo growth of human breast, colon, ovarian, testicular, and leukemia carcinoma cells[5,13,14].
Bianco et al[15] determined CR-1 plasma levels using a sandwich-type ELISA in 21 healthy volunteers, 54 patients with breast cancer, 33 patients with colon carcinoma, and 21 patients with benign breast lesions. Very low levels of CR-1 were detected in the plasma of healthy volunteers (0.32 ± 0.19 ng/mL). A significant increase in the levels of plasma CR-1 was found in patients with colon carcinoma (4.68 ± 3.5 ng/mL) and in patients with breast carcinoma (2.97 ± 1.48 ng/mL, P < 0.001), indicating that measurement of plasma CR-1 level can help in early detection of malignancies. Similar observations were made in IHC analysis and real-time reverse transcription-PCR detection. Zhong et al[16] evaluated CR-1 expression in 118 cases of GC, and found that the positive rate of CR-1 was associated with lymph node metastasis, liver metastasis and late TNM stage (P < 0.05). Our IHC investigation showed that the expression of CR-1 in CAG, IM, DYS and GC was significantly higher than that in normal gastric mucosa, suggesting the over-expression of CR-1 may contribute to malignant transformation of the gastric mucosa. We speculated that CR-1 may play an important role as a tumorigenic factor and early gastric tumorigenic molecule during gastric carcinogenesis. In IM, the positive rates of CR-1 was significantly higher than in normal gastric mucosa (P < 0.05), and in GC of the intestinal type was significantly higher than that in the diffused type (P < 0.001), indicating that CR-1 may be involved in the occurrence of intestinal type of GC. The expression of CR-1 in the well-to-moderately differentiated cancer group was higher than in the poorly differentiated cancer group (P < 0.001), which showed that CR-1 expression was correlated with differentiation of GC. Moreover, our results showed a positive correlation between CR-1 expression and lymph node metastasis, in GC with lymph node metastasis, the positive rates of CR-1 was significantly higher than that without metastasis (P < 0.05), which was consistent with the reported findings[16], indicating that CR-1 may participate in the development and lymph node metastasis of GC. CR-1 can act as a prognostic indicator for GC patients.
STAT3 maps to human chromosome 2q13-14.1 and encodes 750-795 amino acids protein. STAT3 protein contains six basic domains: an N-terminal conserved sequence, a helix domain, a DNA binding domain, a joining region, a Src homology 2 domain (SH2) and a transcription activation domain. The SH2 domain, being most conserved region in STAT3, acts as the cardinal part in STAT3 function by participating in tyrosin phosphorylation. STAT3 is mainly located in the cytoplasm when the cells are in resting state. Upon induction (activation) of tyrosine phosphorylation by a wide variety of cytokines or growth factors, STAT3 is activated and forms p-STAT3. p-STAT3 dimerizes and translocates to the nucleus, further regulating transcription of target genes[17,18]. Many reports have shown that p-STAT3 is abnormally activated in most malignant tumors, including squamous cell carcinoma of the head and neck, breast and GCs[7,19,20]. Lee et al[20] detected p-STAT3 expression in 307 cases of GC, correlative analyses between p-STAT3 and clinical parameters demonstrated a positive correlation with prognosis of patients with GC. In this study, we found that the expression of p-STAT3 in CAG, IM, DYS and GC was significantly higher than that in normal gastric mucosa (P < 0.05), but there was no significant difference in p-STAT3 expression between DYS and GC, suggesting that DYS of gastric mucosa with p-STAT3 abnormal activation has a canceration tendency, and p-STAT3 may be involved in the early events of gastric carcinogenesis. There was no relation between p-STAT3 expression and gender, age, Borrmann’s types, histological types or Lauren’s types, but p-STAT3 expression was significantly higher in GC with lymph node metastasis than without metastasis (P < 0.05), which suggested that the level of p-STAT3 expression may indicate the potential for lymph node metastasis and facilitate the prognostic judgment of GC.
CR-1 can induce activation of the cytoplasmic tyrosine kinase c-Src through specifically binding to Glypican-1[21], and also can indirectly interact with epidermal growth factor receptor (EGFR) to promote cell survival and proliferation[22]; STAT3 can be activated by both EGFR and c-Src[23-25]. Based on the previous findings mentioned above and the results in our study that there is a significant association between CR-1 expression and p-STAT3 activation in GC (rk = 0.189, P = 0.002), we presume that STAT3 may be the downstream molecule of CR-1, and over-expressed CR-1 protein participated in occurrence and development of GC through activating STAT3 signaling pathway. The relevant molecular mechanism requires further investigations.
In conclusion, CR-1 and p-STAT3 may take part in the occurrence, development and metastasis of GC, which can provide theoretical foundation for early diagnosis and judging the prognosis of GC. We discovered that the CR-1 expression in GC was positively related to p-STAT3 expression. These findings provide new insights into understanding the molecular mechanism involved in gastric carcinogenesis and progression, and may lead to the development of new approaches for early detection and effective therapy. However, whether CR-1 and p-STAT3 collaborate to contribute to gastric carcinogenesis and development need further studies.
Gastric cancer (GC) is one of the malignant diseases with highest incidence and mortality rates and it is of great significance to investigate the mechanisms behind the occurrence and development of GC. The over-expression of Cripto-1 (CR-1) and phosphorylation STAT3 (p-STAT3) has been shown to play an important role in the tumorigenesis and cancer progression. Therefore, the exploration into the correlation and significance of the expression of CR-1 and p-STAT3 in GC and precancerous lesions may sheds a new light on early detection and therapy of GC.
CR-1 and p-STAT3 have been shown to exert oncogenic effects in various human neoplasms. In this study, the authors investigated the expression of CR-1 and p-STAT3 proteins in atrophic gastritis, normal mucosa, intestinal metaplasia (IM) and GC to explore its correlation with gastric carcinogenesis and metastasis. They found that over-expression of CR-1 and p-STAT3 may take part in early carcinogenesis and metastasis of GC.
CR-1 and STAT3 are over-expressed in a wide range of human cancers such as gastric, colorectal and breast carcinomas, and may play a key role in cancer pathogenesis. Over-expression of CR-1 and STAT3 increases cancer cell proliferation, migration, invasion and angiogenesis. This is the first study to report that the expression of CR-1 and p-STAT3 in GC was positively correlated, and speculate that there might be a functional relationship between CR-1 and STAT3, where STAT3 might be a the downstream molecule of CR-1.
The positive rates of CR-1 and p-STAT3 expression were significantly higher in chronic atrophic gastritis, IM, dysplasia and GC than that in normal gastric mucosa. And the expression rates of CR-1 and p-STAT3 protein was also significantly higher in GC with lymph node metastasis when compared with those without metastasis. Detection of CR-1 and p-STAT3 protein expressions might be helpful in early detection and prognosis judgment of GC patients. In addition, CR-1 and p-STAT3 may serve as a potential therapeutic target for GC.
Human CR-1, also known as teratocarcinoma-derived growth factor-1 (TDGF-1), is a member of the epidermal growth factor-Cripto-FRL1-Cryptic family including mouse CR-1, cryptic Xenopus FRL-1, zebrafish one-eyed pinhead and chick Cripto. CR-1 functions as an oncogene involved in in vitro cellular transformation and enhancement of cancer cell proliferation, migration, invasion and angiogenesis. STAT3 belongs to the STAT family of signal transducers and activators of transcription, which play a critical role in mediating cellular responses to various stimuli, mainly from those of growth factors and cytokines.
In this study, Zhang et al investigated the expression of CR-1 and p-STAT3 proteins in normal mucosa, IM and GC to explore its correlation with gastric carcinogenesis and metastasis. In general, this is an interesting study with new precision on known markers for GC progression.
Peer reviewers: Masanori Hatakeyama, MD, PhD, Professor, Department of Microbiology, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; Vittorio Ricci, MD, PhD, Department of Physiology, Human Physiology Section, University of Pavia Medical School, Via Forlanini 6, Pavia 27100, Italy; Nathalie Perreault, PhD, Associate Professor, Department of Anatomy and Cell Biology, University of Sherbrooke, 3001 12th Avenue North, Sherbrooke, J1H5N4, Canada
S- Editor Wang JL L- Editor Ma JY E- Editor Ma WH
| 1. | Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731-5741. [Cited in This Article: ] |
| 2. | Watanabe K, Bianco C, Strizzi L, Hamada S, Mancino M, Bailly V, Mo W, Wen D, Miatkowski K, Gonzales M. Growth factor induction of Cripto-1 shedding by glycosylphosphatidylinositol-phospholipase D and enhancement of endothelial cell migration. J Biol Chem. 2007;282:31643-31655. [Cited in This Article: ] |
| 3. | Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094-4105. [Cited in This Article: ] |
| 4. | Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, Guedez L, Salloum R, Ginsburg E, Sun Y. Role of human cripto-1 in tumor angiogenesis. J Natl Cancer Inst. 2005;97:132-141. [Cited in This Article: ] |
| 5. | Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer Res. 2004;64:4018-4023. [Cited in This Article: ] |
| 6. | Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651-662. [Cited in This Article: ] |
| 7. | Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11-19. [Cited in This Article: ] |
| 8. | Smirnova OV, Ostroukhova TY, Bogorad RL. JAK-STAT pathway in carcinogenesis: is it relevant to cholangiocarcinoma progression? World J Gastroenterol. 2007;13:6478-6491. [Cited in This Article: ] |
| 9. | Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. EMBO J. 1989;8:1987-1991. [Cited in This Article: ] |
| 10. | Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, Gray PC. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324-2336. [Cited in This Article: ] |
| 11. | Gray PC, Shani G, Aung K, Kelber J, Vale W. Cripto binds transforming growth factor beta (TGF-beta) and inhibits TGF-beta signaling. Mol Cell Biol. 2006;26:9268-9278. [Cited in This Article: ] |
| 12. | Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666-677. [Cited in This Article: ] |
| 13. | De Luca A, Arra C, D’Antonio A, Casamassimi A, Losito S, Ferraro P, Ciardiello F, Salomon DS, Normanno N. Simultaneous blockage of different EGF-like growth factors results in efficient growth inhibition of human colon carcinoma xenografts. Oncogene. 2000;19:5863-5871. [Cited in This Article: ] |
| 14. | Hu XF, Li J, Yang E, Vandervalk S, Xing PX. Anti-Cripto Mab inhibit tumour growth and overcome MDR in a human leukaemia MDR cell line by inhibition of Akt and activation of JNK/SAPK and bad death pathways. Br J Cancer. 2007;96:918-927. [Cited in This Article: ] |
| 15. | Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, De Luca A, Jones B, Balogh G, Russo J. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res. 2006;12:5158-5164. [Cited in This Article: ] |
| 16. | Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ, Du H, Zhang GG, Hu Y, Lu AP, Li JY. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology. 2008;52:560-568. [Cited in This Article: ] |
| 17. | Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-2488. [Cited in This Article: ] |
| 18. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. [Cited in This Article: ] |
| 19. | Scheper MA, Nikitakis NG, Sauk JJ. Survivin is a downstream target and effector of sulindac-sensitive oncogenic Stat3 signalling in head and neck cancer. Int J Oral Maxillofac Surg. 2007;36:632-639. [Cited in This Article: ] |
| 20. | Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, Kim J, Kong J, Choi MG, Sohn TS. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS. 2009;117:598-606. [Cited in This Article: ] |
| 21. | Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192-1197. [Cited in This Article: ] |
| 22. | Bianco C, Kannan S, De Santis M, Seno M, Tang CK, Martinez-Lacaci I, Kim N, Wallace-Jones B, Lippman ME, Ebert AD. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J Biol Chem. 1999;274:8624-8629. [Cited in This Article: ] |
| 23. | Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017-8023. [Cited in This Article: ] |
| 24. | Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311-319. [Cited in This Article: ] |