Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5440
Revised: June 5, 2010
Accepted: June 12, 2010
Published online: November 21, 2010
AIM: To study a novel technique to record spinal and cortical evoked potentials (EPs) simultaneously in response to electrical stimulation in the human rectum.
METHODS: Eight male and nine female healthy volunteers participated. Stimulating electrodes were attached to the rectal mucosa at 15 cm and 12 cm above the dentate line. Recording skin electrodes were positioned over vertebrae L4 through S2. The electrical stimulus was a square wave of 0.2 ms duration and the intensity of the stimulus varied between 0 and 100 mA. EP responses were recorded using a Nicolet Viking IV programmable signal conditioner.
RESULTS: Simultaneous recording of cortical and spinal EPs was obtained in > 80% of the trials. The EP responses increased with the intensity of the electrical stimulation, were reproducible overtime, and were blocked by application of Lidocaine jelly at the site of stimulation. The morphology (N1/P1), mean ± SD for latency (spinal N1, 4.6 ± 0.4 ms; P1, 6.8 ± 0.5 ms; cortical N1, 136.1 ± 4.2 ms; P1, 233.6 ± 12.8 ms) and amplitude (N1/P1, spinal, 38 ± 7 μV; cortical 19 ± 3 μV) data for the EP responses were consistent with those in the published literature. Reliable and reproducible EP recordings were obtained with the attachment of the electrodes to the rectal mucosa at predetermined locations between 16 and 8 cm above the anal verge, and the distance between the attachment sites of the electrodes (the optimal distance being approximately 3.0 cm between the two electrodes).
CONCLUSION: This technique can be used to assess potential abnormalities in primary afferent neural pathways innervating the rectum in several neurodegenerative and functional pain disorders.
- Citation: Garvin B, Lovely L, Tsodikov A, Minecan D, Hong S, Wiley JW. Cortical and spinal evoked potential response to electrical stimulation in human rectum. World J Gastroenterol 2010; 16(43): 5440-5446
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5440
Evoked potential (EP) recordings in response to peripheral nerve stimulation are useful measures of the conduction characteristics of afferent sensory pathways. EP responses at the cortical level have been recorded in response to mechanical (balloon) or electrical stimulation in the gastrointestinal tract at several locations including the esophagus, colon and rectum[1-4]. These responses are reproducible, at least with electrical stimulation in the rectum[5,6]. The cortical EP responses reflect the cumulative involvement of peripheral and central afferent pathways. Recording spinal EPs that measure the conduction characteristics of the first order primary afferent neurons has been challenging because of technical difficulties. For example, recording spinal EPs in response to balloon distention in the rectum is problematic because the stimulation artifact associated with balloon distension overlaps with the ability to record spinal waveforms with latencies typically < 20 ms[7]. The availability of a reliable method to record visceral EPs at the spinal level would help address an important unresolved issue that involves several gastrointestinal disorders in which dysfunction of primary afferent neurons has been implicated. For instance, visceral hypersensitivity has been reported in patients with irritable bowel syndrome but it remains unresolved whether this disorder is associated with distinct abnormalities involving the first order primary afferent neurons that transmit pain signals and/or higher order processing in pain signaling pathways[8]. We could identify only one publication that has reported simultaneous recording of cortical and spinal EPs in response to visceral stimulation[7]. In that study, recording of cortical EPs in response to rectal electrical stimulation was observed in approximately 90% of subjects, whereas spinal EPs were recorded simultaneously with cerebral EPs in approximately 40% of the subjects (personal communication). This supports the need for improved methods to record spinal EPs in response to stimulation of visceral primary afferent neurons.
Here, we report a new technique to record spinal and cortical EPs simultaneously in response to electrical stimulation in the rectum, which increased the percentage of successful spinal EP recordings to approximately 80%. We propose that the reliability of this method was related to the direct attachment of stimulating electrodes to the rectal mucosa via rigid sigmoidoscopy at specific locations, and the distance between the sites of electrode attachment.
Seventeen healthy male (n = 8) and female (n = 9) subjects, with a mean age of 32 years (range: 20-44 years), completed the recording session. None of the volunteers had a history of medical, gastrointestinal, or neurological problems, or were taking any medication on a regular basis. The protocol was approved by the Institutional Review Board at the University Michigan Medical Center. Written informed consent was obtained from all participants.
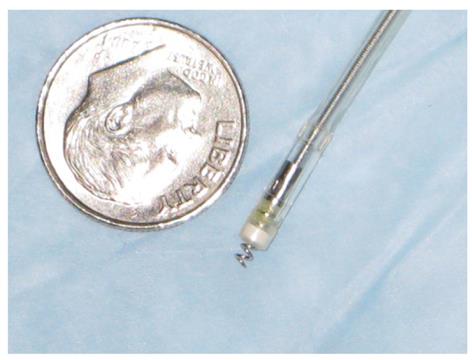
Subjects fasted overnight before the study to eliminate the confounding influence of recent food consumption. A 200-mL tap water enema was administered prior to the recording session to ensure adequate visualization of the rectum. The participant was positioned in the left lateral decubitus position. All studies were performed in a quiet room with dimmed lighting. Two electrodes (Medtronic CapSureFix Model 5076, unipolar configuration) were used in this study (Medtronic Inc., Minneapolis, MN, USA) (Figure 1). This model has an electrically active helix that is designed to extend easily (up to 2 mm) for active fixation and retraction. The electrodes were attached to the rectal mucosa via a disposable rigid sigmoidoscope (Welch Allyn, Model 53130; All Medical Supply, Westland, MI). Typically, placement of the stimulating electrodes was accomplished in 2-3 min with minimal discomfort to the participant.
In preliminary studies, we examined placement of the electrodes at varying distances above the dentate line from 6 to 20 cm, and varied the proximal-distal distance between the attachment sites of the two electrodes from 0.5 to 4 cm. Preliminary studies revealed that the optimal placement to record cortical and spinal EPs simultaneously was between 8 and 16 cm above the dentate line, and 2.5-3.5 cm separation between the two electrodes. Recording of spinal EPs was progressively unreliable at distances < 2.0 cm between the electrode attachment sites. Separation distances > 3.0 cm between the stimulating electrodes required progressively higher intensities of electrical stimulus to produce a threshold response. Based on the preliminary studies, we routinely attached the stimulating electrodes at 15 and 12 cm above the dentate line on the anterior wall of the rectum. A ground electrode was positioned at the back of the neck. Cortical and spinal EPs were recorded simultaneously using Ag/AgCl-skin-electrodes positioned at the vertex [midway between the auricular prominences (Cz)] referenced to linked ears (AlA2) and L4 and L5 and S1 and S2. In some studies, skin electrodes were also attached at L1-3 to provide additional spinal EP data. EP responses were recorded using a Nicolet Viking IV programmable signal conditioner (Nicolet Biomedical, Madison, WI, USA). The electrode impedances were < 5 kΩ. Each trial represented the final average of 50 randomly applied stimulations (every 1-3 s) that was repeated three times to ensure intra-run reproducibility. The random stimulation paradigm was employed to minimize any potential contribution of stimulus anticipation by the participant.
The electrical stimulus was a square wave of 0.2 ms duration and the intensity of the stimulus varied between 0 and 100 mA. Recording parameters: sample frequency 2 kHz, amplifier gain 100 K, bandpass filters at 0.5-500 Hz for cortical EPs and 30-10 kHz for spinal EPs. EP waveforms exhibited characteristic prominent negative (N) and positive (P) peaks numbered in order of occurrence, i.e. N1, P1, etc. All amplitude and latency measures were calculated from the responses obtained at the Cz and L5 locations because in most subjects, the waveforms were best defined and highest in amplitude at these locations. Relative positivity resulted in a downward deflection in the waveform. The latency (ms) of the spinal and cortical responses was measured at the peak of the major negative deflection (N1) and the positive peak (P1), respectively. The spinal and cortical response amplitude (μV) was measured peak-to-peak (N1/P1). The latency reflects the conduction velocity characteristics of the afferent pathway being investigated and, in the absence of temporal dispersion, the amplitude of a signal reflects the number of afferents contributing to the response[5,6].
Volunteers were instructed before stimulation to report when they first perceived any sensation in the pelvic area. Electrical pulses were initiated at a current shown to be sub-threshold (< 5 mA) and gradually increased by 1-mA increments until perception was reported. All subjects reported a threshold non-painful pulse deep in the pelvis. This was identified as the threshold intensity. The paradigm was repeated three times to ensure reproducibility of the threshold sensation and associated EP profile, and again at current intensities of 1.5 and 2 times above the threshold intensity. The threshold for sensation always corresponded to the initial appearance of the EP waveform. It is noteworthy that in two recording sessions, the EP responses became erratic. In both cases, one of the stimulating electrodes had detached from the mucosa. Reattachment of the electrode to the mucosa resulted in restoration of a reproducible cortical and spinal EP response.
After documenting that the EP waveform was reproducible by repeating each trial three times, the stimulating electrodes were removed in six participants and the rectal mucosa at the site of attachment was swabbed with Lidocaine (Xylocaine) 2% topical jelly (AstraZeneca, Wilmington, DE, USA), using a cotton applicator under direct visualization using the rigid sigmoidoscope. The stimulating electrodes were then reattached at their original sites and the response to electrical stimulation repeated using the same parameters (1.5 times threshold intensity) that were employed to produce the initial EP signal. In addition, six participants underwent repeat testing between 1 and 6 mo after the first recording session, to evaluate reproducibility of the initial EP responses.
For acquisition and analysis of spinal and cortical EP recordings, the stimulus intensity used was 50% (1.5 ×) greater than threshold stimulus. Results were compared by two-tailed Student’s t test for paired observations. Repeated measures analysis of variance was used to compare the latency and amplitude data from the two recording sessions. The level of significance for all calculations was set at the 95% confidence level (P < 0.05).
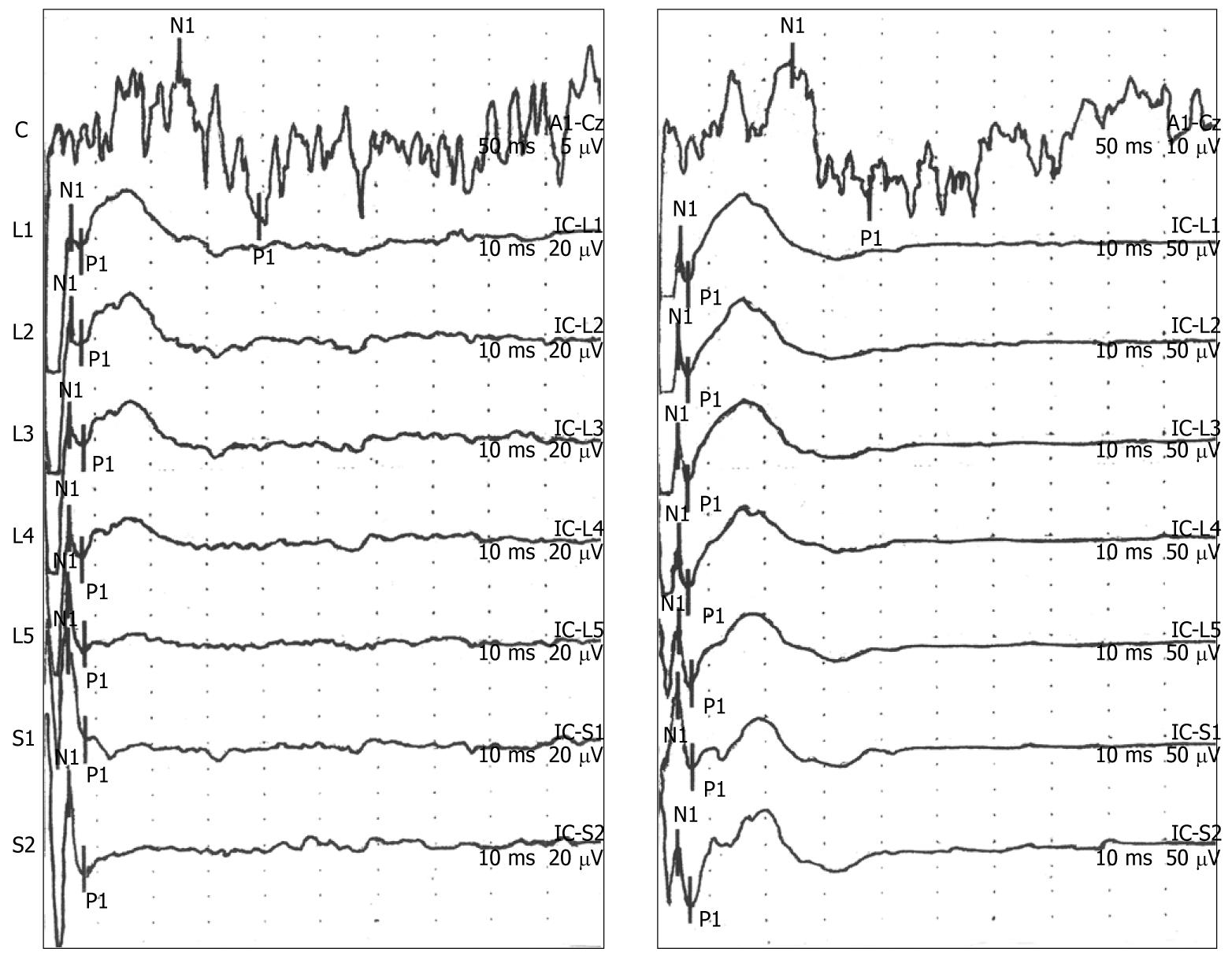
Reproducible polyphasic cerebral EPs were recorded in 16 of the 17 subjects (94%) who participated in the study. Simultaneous spinal and cortical EPs were recorded in 14 of the 17 participants (82%). All participants demonstrated the typical morphology for cortical and spinal EP recordings of an N1/P1 wave form depicted in Figure 2, which increased with stimulus intensity (threshold intensity, left panel; 2 × threshold intensity, right panel). Note the different scales for measuring the amplitude of EP response in the left panel (cortical, 5 μV/division; spinal, 20 μV/division) and right panel (cortical, 10 μV/division; spinal, 50 μV/division). Summary amplitude data for graded electrical stimulation (threshold and 2 × threshold) are presented in Table 1. The mean threshold stimulus intensity was 28 mA (range: 12-47 mA), which was experienced as a non-painful pulse deep within the pelvis; typically in the midline or left of midline. Participants used the descriptors of a “tapping” or “poking” sensation that increased in intensity and corresponded to the intensity of electrical stimulation. The mean ± SD values for latency and amplitude of the EP responses obtained at a stimulus intensity of 1.5 × threshold for the 14 participants who demonstrated both cortical and spinal EP responses are presented in Table 2.
| N1/P1 (μV) | Spinal | Cortical |
| Threshold | 21 ± 4 | 12 ± 3 |
| 2.0 × threshold | 49 ± 6 | 26 ± 5 |
| Spinal | Cortical | |
| N1 latencies (ms) | 4.6 ± 0.4 | 136.1 ± 4.2 |
| P1 latencies (ms) | 6.8 ± 0.5 | 233.6 ± 12.8 |
| N1/P1 amplitude (μV) (1.5 × threshold) | 38 ± 7 | 19 ± 3 |
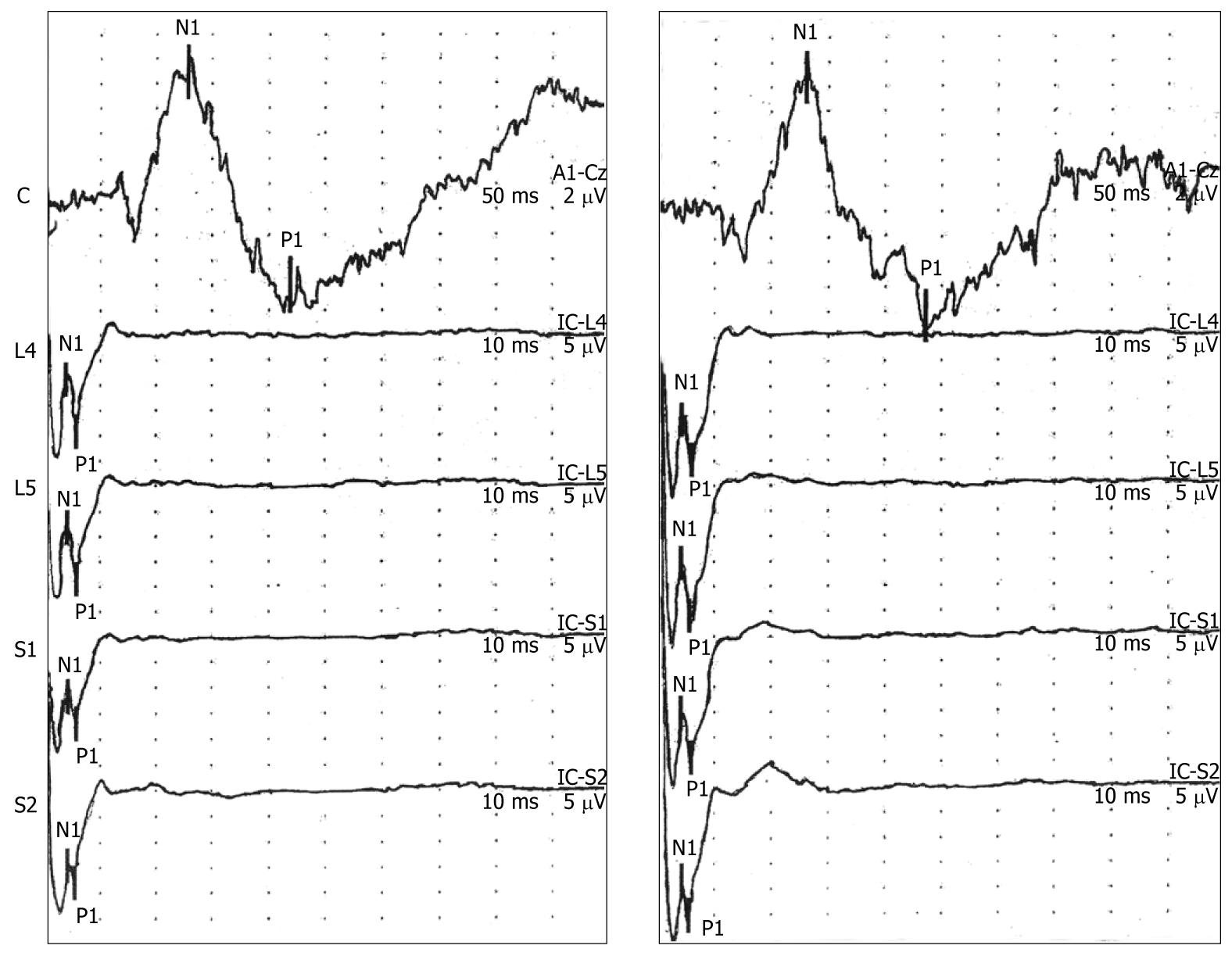
The six participants who underwent a second recording session demonstrated reproducible EP wave forms and no significant changes in the latency and amplitude values compared to the first recording session (Table 3). Representative EP responses from two recording sessions for one volunteer are presented in Figure 3.
| Spinal | Cortical | |
| N1 latencies (ms) | 4.4 ± 0.5 | 132.1 ± 6.1 |
| P1 latencies (ms) | 6.6 ± 0.6 | 229.8 ± 15.8 |
| N1/P1 amplitude (μV) | 35 ± 8.5 | 18.6 ± 3.4 |
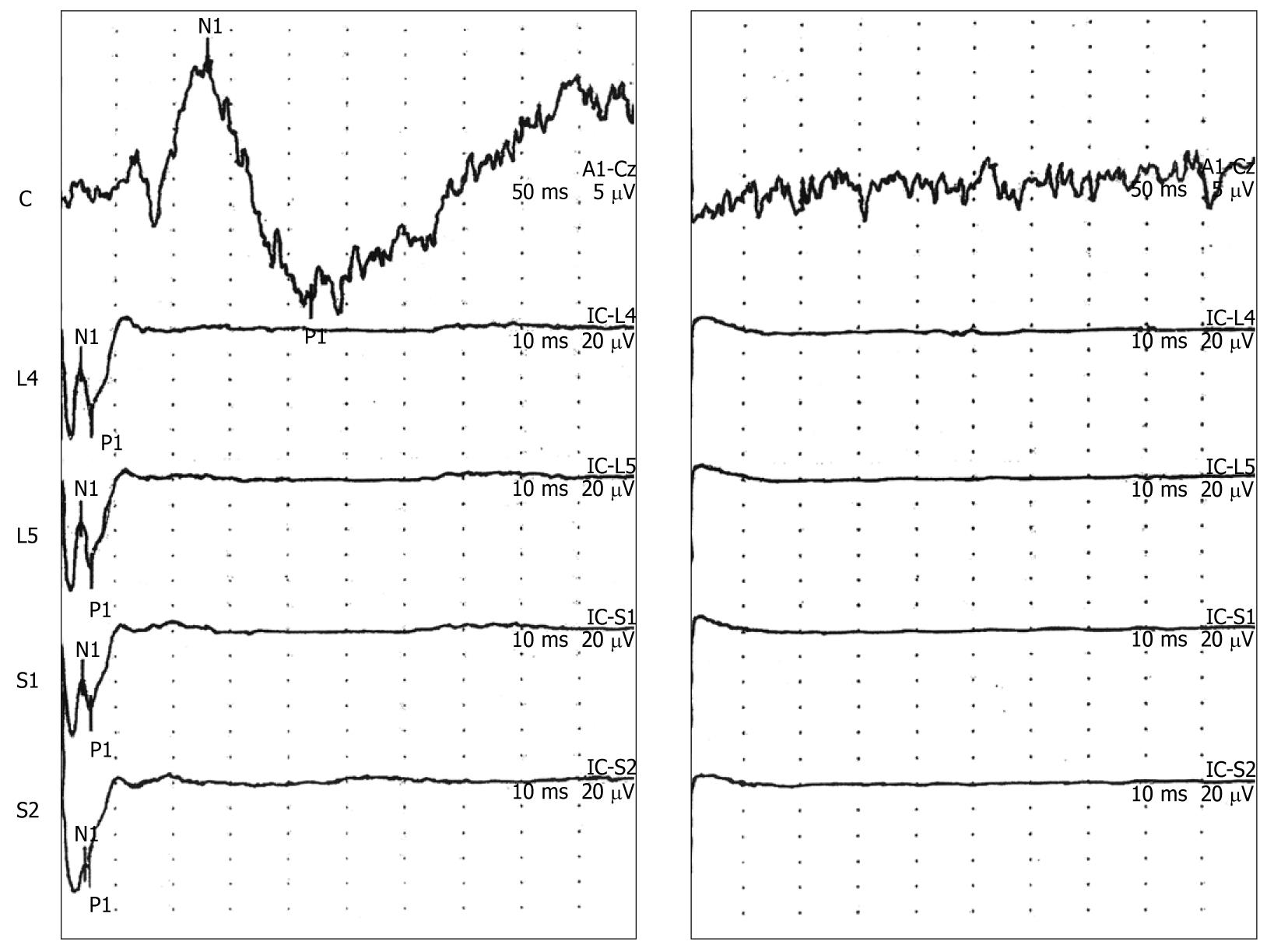
Application of Lidocaine jelly to the rectal mucosa at the sites of initial stimulation followed by reattachment of the electrodes at the original locations, and restimulation using the same parameters resulted in loss of the EP wave forms at the spine and vertex. In addition, all participants experienced a loss in the perception of the tapping or poking sensation in the pelvis after application of Lidocaine (Figure 4, right panel, n = 6).
The goal of this study was to evaluate a novel approach to stimulate the rectal mucosa electrically and simultaneously record EPs at the level of the lower spinal column and vertex. We were able to record successfully simultaneous cortical and spinal EPs in approximately 80% of the volunteers, which represents a twofold improvement over a previous study[7]. We were unable to record reliable spinal EPs in three of 17 participants. In these three individuals, we were able to record a cortical EP waveform. We speculate that the inability to record a reproducible spinal wave form in these three individuals may be related to body habitus. All three individuals had a body mass index > 25.
The polyphasic cortical EP wave forms that we recorded demonstrated similar morphology, latencies and amplitudes as described in previous studies[1-4]. We believe that any differences compared to the published literature can be attributed to the methodology that we employed, which involved direct attachment of the electrodes to the rectal mucosa, and the distance between attachment of the electrodes. The sensory innervation of the rectum involves afferent fibers that travel with sympathetic and parasympathetic spinal afferent nerves. The pelvic nerve afferents originate from the inferior hypogastric plexus[9] include a mixture of unmyelinated C fibers and poorly myelinated Aδ fibers that transmit a variety of sensory modalities that range from innocuous to painful[10-12]. Stimulation in this area is complicated by the proximity of somatic nerves that could be activated by high-intensity electrical stimulation.
Very few published data exist with regard to the characteristic wave form, latencies and amplitudes for spinal EPs using rectal stimulation. The characteristics of the spinal EP wave forms that we recorded were quite similar to those in a previous study that used a different electrical stimulation methodology[7]. Neurophysiological testing has been employed in the diagnoses of neurogenic disorders that involve the lower urinary tract and spinal cord, including somatosensory EP measurements[13,14]. However, the performance of cortical and spinal EP testing in response to bladder or urethra stimulation has not gained broad acceptance in clinical practice because of technical challenges.
We employed electrical stimulation in this study because currently available technologies using balloon distention obscure the recording of spinal EP wave forms. A potential drawback of using electrical stimulation is that this approach can result in nonspecific activation of all afferent pathways, depending on the stimulation parameters. On the other hand, it is possible that specific electrical stimulation parameters can be used to stimulate preferentially subpopulations of primary afferent neurons. For example, using our stimulation parameters, participants reported the perception of a non-painful “tapping” or “poking” sensation that increased with the intensity of electrical stimulus. This is similar to previous studies[1,3,4]. Others have used electrical stimulation protocols (typically involving high stimulation intensities) that evoke the perception of pain[8]. The sensations reported by our participants may be mediated by Aδ-fiber somatic afferents that are thought to encode non-painful stimuli, but saturate at stimulation levels well below pain threshold. It is possible that customized electrical stimulus parameters might prove useful to assess Aδ-fiber afferents in isolation. This may be important in sensitization states because this population of afferents can undergo phenotypic changes after the development of central sensitization[12]. Pain perception is thought to involve slower conducting small myelinated Aδ and unmyelinated C fibers and require more intense stimulation for activation[15]. It remains to be determined whether individuals with neurodegenerative disorders or chronic pain syndromes will demonstrate detectable changes in latency or amplitude of EP wave forms recorded at the lower spinal.
In conclusion, this study demonstrates that is possible to record cortical and spinal EPs simultaneously using rectal electrical stimulation with a relatively high level of reliability, e.g. approximately 80%. We believe that this methodology can be employed to improve our understanding of the potential contribution of rectal primary afferent neurons in various neurodegenerative and functional pain disorders. We anticipate that combining this technology with other assessments of anorectal physiology and functional neuroimaging techniques will result in novel insights regarding the neurophysiological characteristics of visceral afferent pathways in both health and disease.
Recording of evoked potentials (EPs) is a broadly applied technique to study central and peripheral neural function in health and disease in humans. Recording of EPs at the level of the spine in response to visceral stimulation has been technically challenging. The absence of a reliable method to record spinal EPs in response to visceral stimulation has placed limitations on the study of primary afferent neurons and their role in a variety of neurodegenerative and functional pain disorders. This article reports a new technique that represents a significant improvement in our ability to record cortical and spinal EPs simultaneously in response to electrical stimulation in the human rectum.
At the present time, we have a limited understanding of the role of the primary sensory neurons that innervate the lower gastrointestinal tract in neurodegenerative diseases such as diabetes mellitus or functional pain disorders such as irritable bowel syndrome. The availability of a technique to record spinal EPs reliably in response to visceral stimulation will help address this deficit in our knowledge base.
The authors believe that the improved reliability of this method to record spinal EPs in response to visceral stimulation is related to the attachment of the stimulating electrodes to the rectal mucosa at specific locations, and the distance between the stimulating electrodes.
The authors believe that this methodology can be employed to improve their understanding of the potential contribution of rectal primary afferent neurons in various neurodegenerative and functional pain disorders. The authors anticipate that combining this technology with other assessments of anorectal physiology and functional neuroimaging techniques will result in novel insights regarding the neurophysiological characteristics of visceral afferent pathways in both health and disease.
An EP is an electrical potential recorded from the nervous system of a human or other animal following presentation of a stimulus such as sound (auditory EP), light (visual EP), or physiological [chemical, mechanical, heat/cold or electrical (somatosensory EP)]. They can be contrasted to spontaneous potentials detected by electroencephalography or electromyography.
The authors of the present study report a new technique to measure spinal and cortical EPs simultaneously in response to visceral stimulation. This technique could enable us to determine whether alterations in visceral hypersensitivity involve abnormalities in the first-order primary afferent neurons.
Peer reviewers: Inge I Depoortere, PhD, Centre for Gastroenterological Research, Gasthuisberg OandN, bus 701, Leuven 3000, Belgium; Ted Dinan, Professor, Department of Psychiatry, Cork University Hospital, Wilton, Cork, C1, Ireland
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Loening-Baucke V, Read NW, Yamada T. Cerebral evoked potentials after rectal stimulation. Electroencephalogr Clin Neurophysiol. 1991;80:490-495. |
| 2. | Loening-Baucke V, Anderson RH, Yamada T, Zhu YX. Study of the afferent pathways from the rectum with a new distention control device. Neurology. 1995;45:1510-1516. |
| 3. | Hobday DI, Hobson A, Furlong PL, Thompson DG, Aziz Q. Comparison of cortical potentials evoked by mechanical and electrical stimulation of the rectum. Neurogastroenterol Motil. 2000;12:547-554. |
| 4. | Hobday DI, Hobson AR, Sarkar S, Furlong PL, Thompson DG, Aziz Q. Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol. 2002;283:G335-G339. |
| 5. | Rogers J, Laurberg S, Misiewicz JJ, Henry MM, Swash M. Anorectal physiology validated: a repeatability study of the motor and sensory tests of anorectal function. Br J Surg. 1989;76:607-609. |
| 6. | Harris ML, Hobson AR, Hamdy S, Thompson DG, Akkermans LM, Aziz Q. Neurophysiological evaluation of healthy human anorectal sensation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G950-G958. |
| 7. | Chey WD, Beydoun A, Roberts DJ, Hasler WL, Owyang C. Octreotide reduces perception of rectal electrical stimulation by spinal afferent pathway inhibition. Am J Physiol. 1995;269:G821-G826. |
| 8. | Rössel P, Pedersen P, Niddam D, Arendt-Nielsen L, Chen AC, Drewes AM. Cerebral response to electric stimulation of the colon and abdominal skin in healthy subjects and patients with irritable bowel syndrome. Scand J Gastroenterol. 2001;36:1259-1266. |
| 9. | Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559-578. |
| 10. | Loening-Baucke V, Read NW, Yamada T. Further evaluation of the afferent nervous pathways from the rectum. Am J Physiol. 1992;262:G927-G933. |
| 11. | Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40-52. |
| 12. | Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765-1769. |
| 13. | Podnar S. Neurophysiology of the neurogenic lower urinary tract disorders. Clin Neurophysiol. 2007;118:1423-1437. |
| 14. | Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine (Phila Pa 1976). 2010;35:1122-1127. |
| 15. | Hollerbach S, Tougas G, Frieling T, Enck P, Fitzpatrick D, Upton AR, Kamath MV. Cerebral evoked responses to gastrointestinal stimulation in humans. Crit Rev Biomed Eng. 1997;25:203-242. |