Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3584
Revised: April 6, 2010
Accepted: April 13, 2010
Published online: July 28, 2010
AIM: To investigate whether tumor debris created by high-intensity focused ultrasound (HIFU) could trigger antitumor immunity in a mouse hepatocellular carcinoma model.
METHODS: Twenty C57BL/6J mice bearing H22 hepatocellular carcinoma were used to generate antitumor vaccines. Ten mice underwent HIFU ablation, and the remaining 10 mice received a sham-HIFU procedure with no ultrasound irradiation. Sixty normal mice were randomly divided into HIFU vaccine, tumor vaccine and control groups. These mice were immunized with HIFU-generated vaccine, tumor-generated vaccine, and saline, respectively. In addition, 20 mice bearing H22 tumors were successfully treated with HIFU ablation. The protective immunity of the vaccinated mice was investigated before and after a subsequent H22 tumor challenge. Using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, the cytotoxicity of splenic lymphocytes co-cultured with H22 cells was determined in vitro before the tumor challenge, and tumor volume and survival were measured in vivo after the challenge in each group. The mechanism was also explored by loading the vaccines with bone marrow-derived dendritic cells (DCs).
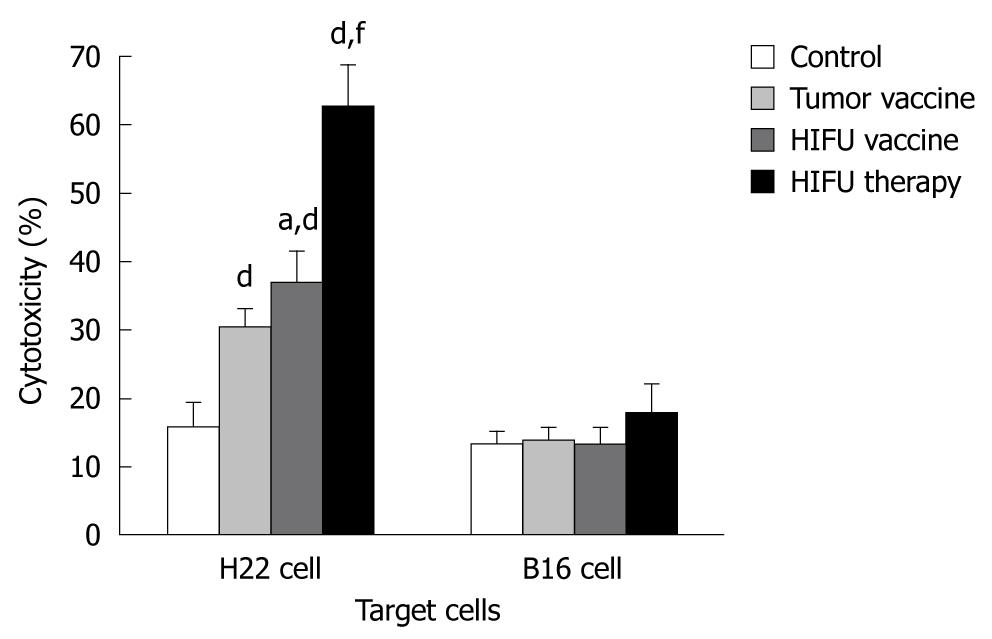
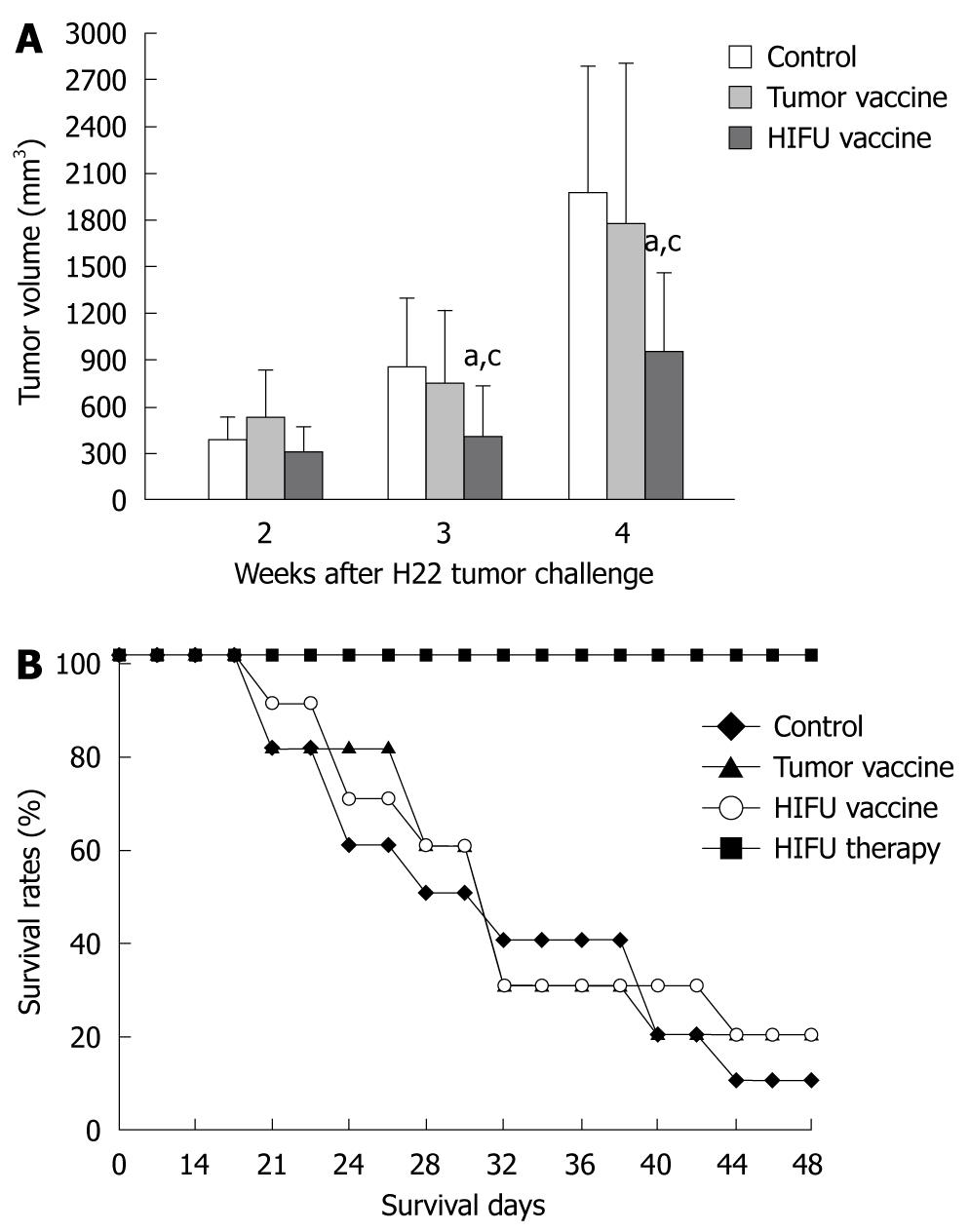
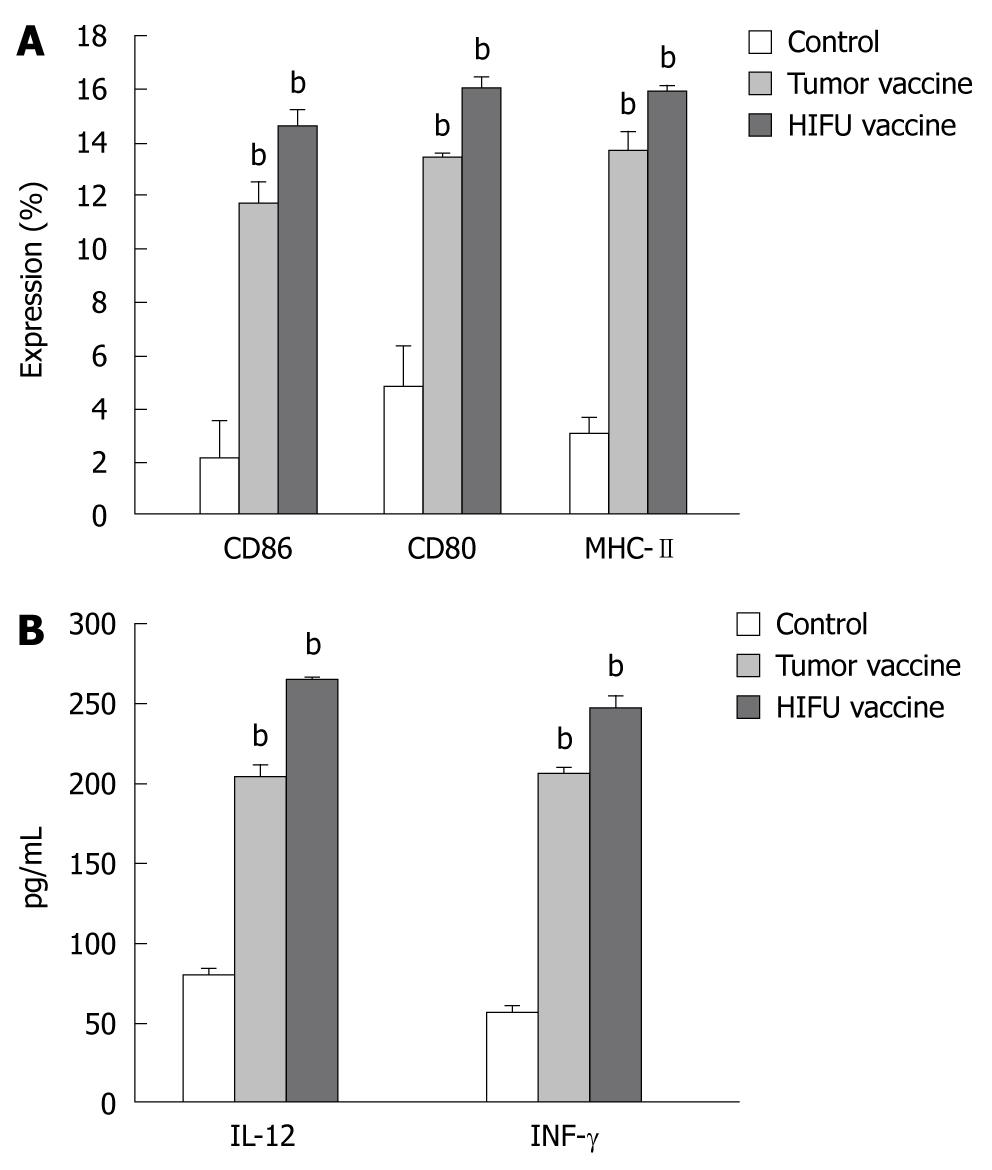
RESULTS: Compared to the control, HIFU therapy, tumor-generated and HIFU-generated vaccines significantly increased cytolytic activity against H22 cells in the splenocytes of the vaccinated mice (P < 0.001). The tumor volume was significantly smaller in the HIFU vaccine group than in the tumor vaccine group (P < 0.05) and control group (P < 0.01). However, there was no tumor growth after H22 rechallenge in the HIFU therapy group. Forty-eight-day survival rate was 100% in mice in the HIFU therapy group, 30% in both the HIFU vaccine and tumor vaccine groups, and 20% in the control group, indicating that the HIFU-treated mice displayed significantly longer survival than the vaccinated mice in the remaining three groups (P < 0.001). After bone marrow-derived DCs were incubated with HIFU-generated and tumor-generated vaccines, the number of mature DCs expressing MHC-II+, CD80+ and CD86+ molecules was significantly increased, and interleukin-12 and interferon-γ levels were significantly higher in the supernatants when compared with immature DCs incubated with mouse serum (P < 0.001). However, no differences of the number of mature DCs and cytokine levels were observed between the HIFU-generated and tumor-generated vaccines (P > 0.05).
CONCLUSION: Tumor debris remaining after HIFU can improve tumor immunogenicity. This debris releases tumor antigens as an effective vaccine to develop host antitumor immune response after HIFU ablation.
- Citation: Zhang Y, Deng J, Feng J, Wu F. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol 2010; 16(28): 3584-3591
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3584
As a non-invasive thermal ablation, high-intensity focused ultrasound (HIFU) therapy has received increasing attention for the clinical management of patients with solid tumors, including those of the prostate, liver, pancreas, breast, kidney, uterus, bone and soft tissue[1-3]. Under real-time imaging guidance, this technique uses ultrasound energy locally to ablate a targeted tumor at depth, with no damage to overlying tissues. In addition, it has been shown that HIFU ablation can enhance host antitumor immune response[4-13], which may be of benefit in local recurrence and metastasis control in cancer patients who have previous dysfunction of antitumor immunity.
Selective recognition and destruction of tumor cells by the host immune system is a major role of antitumor immunity, and tumor antigens expressed by tumor cells are essential to achieve this antitumor immune response specific to tumor cells. After HIFU ablation, large amounts of tumor debris remain in situ, and the host gradually reabsorbs them as a normal process of the healing response. Our previous findings revealed a variety of tumor antigen expressions on HIFU-ablated breast cancer cells[15]. Some tumor antigens disappeared completely, others remained in their entirety such as heat shock protein (HSP) 70, while most remained partially in the tumor debris after HIFU ablation. However, it is still unknown whether the remaining tumor debris may be a potential antigen source available for the induction of host antitumor immunity. To test HIFU effects on the inherent immunogenicity of the tumor debris, we performed HIFU to ablate in vivo hepatocellular carcinoma (HCC), and then used the tumor debris to inoculate naïve animals against subsequent tumor challenge. The purpose of this study was to investigate whether the remaining tumor debris created by in situ HIFU ablation could be strongly immunogenic as an effective tumor vaccine to stimulate host antitumor immunity, and to provide potential benefit in long-term survival in a murine tumor model.
The animal study was approved by the Chongqing Experimental Animal Committee (Chongqing, China). Male and female C57BL/6J mice (6-8 wk old) were obtained from the Experimental Animal Center of Chongqing Medical University (Chongqing, China), and housed in microisolator cages in a laminar flow unit under ambient light in the same animal center. All animal experiments adhered to the Animal Welfare Committee guidelines.
The H22 HCC cell line was provided by the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). 2 × 106 H22 cells (0.02 mL) were injected subcutaneously into the right flank of syngeneic C57BL/6J mice to establish a tumor model. Palpable tumors started to develop after 3 d, and reached the size of 5-7 mm after 7 d.
Twenty C57BL/6J mice bearing H22 tumors were used to generate antitumor vaccines at day 7 after tumor implantation. Under general anesthesia 10 mice underwent HIFU ablation, and the remaining 10 mice received a sham-HIFU procedure with no ultrasound irradiation. HIFU energy was produced by a 2-cm diameter transducer with a focal length of 8 mm, operating at a frequency of 9.5 MHz. Acoustic power was 5 W, and median exposure time was 220 s (range: 180-240 s). All mice were sacrificed 1 d after treatment, and tumor samples were harvested. The tissues were brought up to the same weight in both treated and untreated tumors, and they were respectively minced and homogenized. Repetitive freeze-thaw cycles were performed for the preparation of cellular lysate. Using the Bradford assay (Bio-Rad, Hercules CA, USA), the same protein concentration (0.5 mg/mL) was also achieved in both lysates, where the treated and untreated tumor tissues had the milligram per milliliter protein concentration in RPMI 1640.
Sixty C57BL/6J mice were randomly divided into three groups: control group, tumor vaccine group and HIFU vaccine group. Each group had 20 mice, including 10 for cytotoxic T lymphocyte (CTL) assay 15 d after vaccination and 10 for long-term follow-up after tumor challenge. By using subcutaneous injection, the mice in the tumor group and HIFU group received either 10 μg untreated H22 vaccine or 10 μg HIFU-treated H22 vaccine in the left flank of each mouse. Those in the control group received only injection with same amount of saline solution. The vaccination times were 2 sessions, once a week for 2 consecutive weeks.
Ten mice were sacrificed 7 d after the final vaccination in each group, and spleens were harvested to assess the activity of splenic CTL. Single cell suspensions were generated by passage through a metallic mesh. Erythrocytes were lysed with 0.87% ammonium chloride for 1 min, and macrophages were removed by exposure to plastic plates for 2 h. The nonadherent lymphocytic population was collected, washed, and resuspended at 2 × 106 cells/mL as CTL effectors. H22 and B16 (a mouse melanoma cell line) cells were used as targeted cells, and the effector/target cell ratio was 10:1, which was the best cellular ratio in our pre-experimental study. The splenic lymphocytes were then co-cultured with either H22 or B16 cells in 96-well plates for 24 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed to determine the cytotoxicity of the CTLs in each group. This is a standard colorimetric assay for determining in vitro cytotoxicity of CTLs against tumor cells in experiments[16,17]. As negative control groups, an equal number of target cells were cultured alone and an equal number of effector cells were cultured without target cells in a total of 200 μL. Using a multiwell spectrophotometer reader (Molecular Devices, Menlo Park, California, USA), the optical intensity at 570 nm was measured. Each assay was performed in triplicate, and RPMI 1640 medium was used as a blank control. According to the optical intensity in each group, the cytotoxicity calculation was determined by the following equation: Cytotoxicity (%) = (Effector spontaneous + Target spontaneous - Experimental)/(Target spontaneous - Target maximal) × 100%.
Seven days after the final vaccination, 10 mice were challenged in each group by subcutaneous injection of 2 × 106 H22 cells (0.02 mL) in the right flank of each mouse. The tumor growth was detected every 3 d by measuring its diameter with a Vernier caliper. Tumor volume was calculated using the following formula: tumor volume (mm3) = d2× D/2, where d and D are the shortest and longest diameters of the measured tumor, respectively. All mice were followed up for 48 d to observe the survival data.
Twenty C57BL/6J mice bearing H22 tumors were treated with HIFU at day 7 after tumor implantation to determine whether specific antitumor reactivity could be detected directly after HIFU ablation. These mice were classified as the HIFU therapy group in this study, and HIFU therapeutic parameters were similar to those used in generating the HIFU-related tumor vaccine. Fifteen days after HIFU ablation, 10 mice were sacrificed for the assessment of in vitro CTL cytotoxicity as described above, and the remaining 10 were rechallenged with 2 × 106 H22 tumor cells to follow long-term survival.
Bone marrow-derived dendritic cells (DCs) were isolated from C57BL/6J mice, as described by Inaba and colleagues[18]. Briefly, DCs were obtained from bone marrow precursors by flushing mouse femur and tibia bones with cold PBS. After erythrocytes were lysed with ammonium chloride, erythrocyte-depleted bone marrow cells (4 × 106/2 mL per well) were cultured in 6-well plates (Nunc, Roskilde, Denmark) in complete medium (RPMI 1640 supplemented with 10% FBS, L-glutamine, 5 mmol/L 2-mercaptoethanol, and antibiotics) at 37°C in a humidified atmosphere with 50 mL/L CO2. The culture medium also contained 20 ng/mL mouse recombinant granulocyte macrophage colony-stimulating factor (mrGM-CSF; PeproTech, London, UK) and 20 ng/mL mouse recombinant interleukin-4 (mrIL-4; PeproTech, London, UK). The cultures were fed every 2 d with fresh medium containing 10 ng/mL mrGM-CSF and 10 ng/mL mrIL-4. On day 7, nonadherent and loosely adherent cells were collected, washed, and resuspended in PBS at 1 × 106/mL. These cells presented the typical morphological characteristics of immature DCs, and flow cytometry analysis showed that a majority (75%-80%) of them had positive expression of CD11c and CD205 molecules.
2 × 106/mL immature DCs were primed with either 5 μg H22 tumor vaccine (tumor vaccine group) or 5 μg HIFU-generated vaccine (HIFU vaccine group) in complete medium at 37°C. DCs co-cultured with the same amount of mouse serum alone were classified as the control group. These cells were incubated for 5 d in a humidified atmosphere with 50 mL/L CO2. Using flow cytometry (FACSCalibur™ Flow Cytometer, BD Biosciences, San Jose, CA, USA), the cells in each group were then analyzed for the expression of MHC class II, CD80, and CD86 molecules. Culture supernatants were harvested in each group, and cytokine production was determined in the supernatants by enzyme-linked immunosorbent assay using murine kits from R&D Systems (Minneapolis, MN, USA) for interleukin (IL)-12 and interferon (IFN)-γ, according to the manufacturer’s recommendations. Each assay was performed in triplicate with separate DC preparations.
All observed data are displayed as mean ± SD. Statistical analysis was performed using the Student’s t test. A cumulative survival rate was calculated by using the Kaplan-Meier method, and the statistical significance of any survival difference was evaluated by the log-rank test. Differences were considered statistically significant when the P value was less than 0.05.
As shown in Figure 1 and Table 1, HIFU therapy, tumor- and HIFU-generated vaccines significantly increased cytolytic activity against H22 cells in the splenocytes of the vaccinated mice when compared with the activity in mice vaccinated with saline alone (P < 0.001). None of the vaccines elicited CTL cytotoxicity to control target B16 cells (P > 0.05), suggesting that HIFU therapy and the HIFU-generated vaccine could induce specific antitumor immunity. The splenocytes isolated from the HIFU-treated mice produced significantly stronger anti-H22 CTL activity than that of either the HIFU vaccine or tumor vaccine group (P < 0.001). The HIFU-generated vaccine was significantly better than the tumor-generated vaccine at increasing the cytotoxicity of CTLs (P = 0.013).
The tumors were monitored with the caliper for 4 wk until mean tumor size was too variable due to death of the mice. As shown in Figure 2 and Table 2, vaccination with HIFU-treated tumor had a marked inhibitory effect on tumor growth in the 3rd and 4th wk of the tumor challenge when compared with the control (saline) (P < 0.01) and tumor-generated groups (P < 0.05). The strongest inhibition was observed in the HIFU therapy group because after H22 rechallenge no tumor growth was detected during the follow-up period. An inhibition of tumor growth was observed in the tumor-generated vaccine group. However, there was no statistical difference between the control and tumor-generated vaccine groups (P > 0.05).
Survival of the vaccinated mice and HIFU-treated mice was also recorded for up to 48 d after tumor challenge with H22 cells. Survival curves (Figure 2) showed that 100% of HIFU-treated mice, 30% of HIFU vaccinated mice, and 30% of tumor vaccinated mice survived for 48 d, whereas 20% of saline vaccinated mice (control) survived 48 d. The HIFU-treated mice displayed significantly longer survival than the vaccinated mice in the remaining three groups (P < 0.001). The mice inoculated with either the HIFU-generated vaccine or tumor-generated vaccine survived a little longer than the mice vaccinated with saline (control). However, no statistical differences were observed among them.
The expression of MHC class II, CD80 and CD86 molecules on DCs was determined by flow cytometry after incubation for 5 d. As shown in Figure 3 and Table 3, incubation with either the HIFU-generated vaccine or tumor-generated vaccine significantly increased the number of mature DCs (MHC-II+, CD80+ and CD86+) when compared with incubation with mouse serum (P < 0.001), suggesting both vaccines could induce phenotypic maturation of DCs. However, no differences in the expression of MHC-II, CD80 and CD86 were observed between the HIFU- and tumor-generated vaccines (P > 0.05).
HIFU-generated vaccine induces IL-12 and IFN-γsecretion by DCs
As shown in Figure 3 and Table 4, after immature DCs were incubated with HIFU- and tumor-generated vaccines, IL-12 and IFN-γ levels were significantly higher in the supernatants compared to DCs incubated with mouse serum (P < 0.001). However, there was no statistical difference in IL-12 and IFN-γ secretion between the HIFU and tumor vaccine groups (P > 0.05). These results demonstrated that both HIFU- and tumor-generated vaccines could induce the functional maturation of DCs.
The concept of HIFU as a noninvasive therapy for the local destruction of diseased tissue dates back more than 60 years. Much of the clinical application is recent, where clinical results are very promising in the treatment of solid malignancies[1-3]. It is obvious that large amounts of in situ tumor debris remain after HIFU ablation, however, little is known about whether this debris may be a potential antigen source for triggering host antitumor immune response. Using a newly developed tumor destruction model, we demonstrated that the remaining tumor debris can be immunogenic as an effective vaccine to elicit tumor-specific immune responses, including induction of CTL cytotoxic activity in the spleen, protection against a lethal tumor challenge in naïve mice, activation of immature DCs, and secretion of Th1-associated cytokines by mature DCs for the development of cell-mediated immune response. To our knowledge, this is the first report of the use of HIFU ablation to generate an in situ tumor vaccine, and the first report of the use of this crude tumor vaccine which is functional in stimulating tumor-specific immunity in naïve animals in the absence of immune adjuvant. Our findings may contribute greatly to the understanding of how in situ HIFU ablation triggers the host antitumor immune response. However, with the use of flow cytometry, peptide MHC tetramers analysis is needed in the future to measure the number of antigen-specific CD8+ T lymphocytes.
Thermal and nonthermal effects are two major mechanisms related to HIFU-induced coagulation necrosis. During HIFU exposure, the absorption of ultrasound energy in a targeted tumor leads to a rapid temperature rise above 56°C within the focal volume[19], and thus induces complete coagulation necrosis of the targeted cancer, with no direct evidence of apoptotic cells detected by the TUNEL method in the treated tumors[20].
It is postulated that antitumor immunity enhanced by the ultrasound thermal effect would be similar to those observed in other thermal therapies such as radiofrequency[21-23]. Cavitation is the most important nonthermal mechanism for HIFU-induced tissue destruction. It can cause membranous organelles, including mitochondria, endoplasmic reticulum, cell and nuclear membranes to collapse instantaneously, and thus lead to tumor cells breaking up into small pieces, on which the tumor antigens may remain intact[24]. Recent studies have revealed that acoustic cavitation can upregulate expression of tumor antigens such as heat shock proteins[4,5,8,14,15]. If heat shock proteins (HSPs) remain and upregulate as intracellular molecular chaperones in the tumor debris after HIFU ablation, they may bind tumor peptide antigens, and act as tumor vaccines to produce a potent cellular immune response[25,26]. However, as overexpression of HSPs may have deleterious effects on antitumor immunity after heat treatments such as hyperthermia, further studies are needed to evaluate whether HSPs could play an important role in the induction of host antitumor immune response after HIFU therapy.
Although the mechanism behind this enhanced immune response is still unknown, our findings reveal that it should be specific antitumor immunity. We have demonstrated that the HIFU-generated tumor vaccine can significantly elicit cytotoxicity of CTLs to H22 cells, whereas cytolytic activity against control target B16 cells was not observed in the splenocytes of vaccinated mice. Compared to tumor lysate, in vitro anti-H22 CTL activity was stronger in mice receiving the HIFU-generated tumor vaccine. Similar results were also observed in a mouse H22 tumor model after the vaccinated mice were challenged with H22 cells. Vaccination with HIFU-treated tumor caused a stronger inhibition of tumor growth than the control and tumor-generated vaccine groups, indicating the involvement of a tumor-specific immune response. However, this immune protection was still weak, because no significant survival benefit was observed after a lethal H22 challenge in the HIFU-generated vaccine group when compared with the control and tumor-generated vaccine groups.
We have found that the mice bearing H22 tumors, which were treated by HIFU previously, had the strongest protection against a second H22 cell challenge. The most potent cytotoxicity of CTLs against H22 cells in vitro was detected in mice who received previous HIFU ablation. Compared to the HIFU- and tumor-generated vaccine groups, in situ HIFU ablation of H22 tumors resulted in complete protection against a subsequent H22 tumor rechallenge. All mice survived during the follow-up period, with no evidence of tumor growth. These data suggest that once mice bearing H22 tumors are cured by HIFU treatment, a bona fide systemic memory response may be generated. In addition, tumor debris remaining after in situ ablation may continuously stimulate the host immune system during the reabsorption of dead tissue, leading to a stronger antitumor immune response. However, to support our speculation, further studies are needed to investigate the mechanisms behind this. Furthermore, as follow-up time was limited in this study, a longer period is necessary in future studies to observe the survival benefit and tumor development after tumor rechallenge in the HIFU-treated mice. Both the HIFU- and tumor-generated vaccine groups developed a relatively weak immune response, because the vaccination times were very limited, only performed once a week for 2 consecutive weeks. Therefore, further studies are necessary in this mouse H22 tumor model to optimize the vaccination method including the number of sessions, dosage and interval time. In order to induce a stronger immunological response, a longer vaccination time with the HIFU-treated tumor will be investigated, and immunoadjuvants will be used in combination with HIFU therapy.
Similar to other thermal therapies, a marked inflammatory reaction, with abundant leukocytic infiltration, has been histologically observed at the margins of coagulation necrosis in HIFU ablation[27-30]. We have recently found that HIFU ablation can significantly induce local infiltration of activated DCs within the marked inflammatory reaction in patients with breast cancer[31]. DCs are the most potent antigen-presenting cells for induction of adaptive immunity against cancer[32]. They infiltrate local tumors and present tumor antigens to naïve T lymphocytes in a MHC restricted fashion. Activating signals, delivered directly or indirectly by tumor cells including apoptotic and necrotic tumor cells, can induce the progression of infiltrating DCs from an immature to a mature stage[33]. During maturation DCs increase the expression of costimulatory molecules such as CD80 and CD86, and mature DCs secrete Th1-associated cytokines to induce cell-mediated immunity[34]. This study produced direct evidence that with no immune adjuvant, the remaining tumor debris can activate immature DCs, and thus induce secretion of IL-12 and IFN-γ by mature DCs for the development of cellular antitumor immune response. However, it is still unknown whether the activated DCs could induce an in situ antitumor immune response by presenting tumor antigens directly to lymphocytes. Further studies are necessary to investigate the potential role of activated DCs in the induction of specific antitumor immunity in vivo.
Our findings indicate that a weak but tumor-specific immune response was produced by the HIFU-generated tumor vaccines after in situ tumor destruction. Therefore, active immunological stimulation such as immunoadjuvants, in combination with HIFU, could augment the efficacy of HIFU-induced antitumor immunity specifically against the targeted tumors, if the destruction of tumors releases tumor antigens or improves tumor immunogenicity.
HCC is one of the most common malignancies worldwide. Local tumor recurrence and metastasis are usually the cause of failure of multidisciplinary treatments of HCC in clinical practice. Using a mouse HCC model, we found that HIFU ablation can trigger host tumor-specific immune response. This may decrease or perhaps even eliminate residual and metastatic tumor cells in HCC patients who have had original antitumor immunity dysfunction.
In summary, this study demonstrated that tumor debris remaining after in situ HIFU ablation may improve tumor immunogenicity. This debris may release tumor antigens as an effective vaccine to elicit tumor-specific immune responses. However, further studies are needed to explore the nature of the “activation” factors in HIFU-generated tumor debris.
As a non-invasive thermal ablation, high-intensity focused ultrasound (HIFU) therapy has received increasing attention for the clinical management of patients with hepatocellular carcinoma (HCC). After HIFU ablation, large amounts of tumor debris remain in situ, and the host gradually reabsorbs them as a normal process of the healing response. However, it is still unknown whether the remaining tumor debris may be a potential antigen source available for the induction of host antitumor immunity.
In the present study, the authors investigated whether tumor debris created by in situ HIFU could be an effective tumor vaccine to stimulate antitumor immunity in a mouse HCC model.
This study demonstrated that the remaining tumor debris after HIFU can be immunogenic as an effective vaccine to elicit tumor-specific immune responses, including induction of cytotoxic T lymphocyte cytotoxic activity in the spleen, protection against a lethal tumor challenge in naïve mice, activation of immature dendritic cells (DCs), and secretion of Th1-associated cytokines by mature DCs for the development of cell-mediated immune response. These findings may contribute greatly to the understanding of how in situ HIFU ablation triggers the host antitumor immune response.
These findings contribute greatly to the understanding of how in situ HIFU ablation triggers the host antitumor immune response. In addition, they suggest that HIFU ablation combined with subsequent immunotherapy such as immunoadjuvants may augment the efficacy of HIFU-induced antitumor immunity specifically against the targeted tumor, leading to a decrease of local recurrence and metastasis in HCC.
It is a very interesting paper with adequately described and written all necessary parts of a manuscript.
Peer reviewer: Tamara M Alempijevic, MD, PhD, Assistant Professor, Clinic for Gastroenterology and Hepatology, Clinical Centre of Serbia, 2 Dr Koste Todorovica St., 11000 Belgrade, Serbia
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
| 1. | Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321-327. [Cited in This Article: ] |
| 2. | Chaussy C, Thüroff S, Rebillard X, Gelet A. Technology insight: High-intensity focused ultrasound for urologic cancers. Nat Clin Pract Urol. 2005;2:191-198. [Cited in This Article: ] |
| 3. | Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol. 2006;15:26-35. [Cited in This Article: ] |
| 4. | Hu Z, Yang XY, Liu Y, Morse MA, Lyerly HK, Clay TM, Zhong P. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun. 2005;335:124-131. [Cited in This Article: ] |
| 5. | Madersbacher S, Gröbl M, Kramer G, Dirnhofer S, Steiner GE, Marberger M. Regulation of heat shock protein 27 expression of prostatic cells in response to heat treatment. Prostate. 1998;37:174-181. [Cited in This Article: ] |
| 6. | Kramer G, Steiner GE, Gröbl M, Hrachowitz K, Reithmayr F, Paucz L, Newman M, Madersbacher S, Gruber D, Susani M. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate. 2004;58:109-120. [Cited in This Article: ] |
| 7. | Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, Lyerly HK, Clay TM, Zhong P. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. [Cited in This Article: ] |
| 8. | Hundt W, O'Connell-Rodwell CE, Bednarski MD, Steinbach S, Guccione S. In vitro effect of focused ultrasound or thermal stress on HSP70 expression and cell viability in three tumor cell lines. Acad Radiol. 2007;14:859-870. [Cited in This Article: ] |
| 9. | Keshavarzi A, Vaezy S, Noble ML, Chi EY, Walker C, Martin RW, Fujimoto VY. Treatment of uterine leiomyosarcoma in a xenograft nude mouse model using high-intensity focused ultrasound: a potential treatment modality for recurrent pelvic disease. Gynecol Oncol. 2002;86:344-350. [Cited in This Article: ] |
| 10. | Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, Jin CB. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217-1222. [Cited in This Article: ] |
| 11. | Rosberger DF, Coleman DJ, Silverman R, Woods S, Rondeau M, Cunningham-Rundles S. Immunomodulation in choroidal melanoma: reversal of inverted CD4/CD8 ratios following treatment with ultrasonic hyperthermia. Biotechnol Ther. 1994;5:59-68. [Cited in This Article: ] |
| 12. | Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J (Engl). 2002;115:1332-1335. [Cited in This Article: ] |
| 13. | Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P, Wu F. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34:81-87. [Cited in This Article: ] |
| 14. | Kruse DE, Mackanos MA, O'Connell-Rodwell CE, Contag CH, Ferrara KW. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys Med Biol. 2008;53:3641-3660. [Cited in This Article: ] |
| 15. | Wu F, Wang ZB, Cao YD, Zhou Q, Zhang Y, Xu ZL, Zhu XQ. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann Surg Oncol. 2007;14:1237-1242. [Cited in This Article: ] |
| 16. | Heo DS, Park JG, Hata K, Day R, Herberman RB, Whiteside TL. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 1990;50:3681-3690. [Cited in This Article: ] |
| 17. | Hussain RF, Nouri AM, Oliver RT. A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods. 1993;160:89-96. [Cited in This Article: ] |
| 18. | Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693-1702. [Cited in This Article: ] |
| 19. | ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111-129. [Cited in This Article: ] |
| 20. | Wu F, Wang ZB, Cao YD, Zhu XQ, Zhu H, Chen WZ, Zou JZ. "Wide local ablation" of localized breast cancer using high intensity focused ultrasound. J Surg Oncol. 2007;96:130-136. [Cited in This Article: ] |
| 21. | Wissniowski TT, Hänsler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496-6500. [Cited in This Article: ] |
| 22. | den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024-4029. [Cited in This Article: ] |
| 23. | Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139-1146. [Cited in This Article: ] |
| 24. | Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia. 2007;23:165-171. [Cited in This Article: ] |
| 25. | Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469-476. [Cited in This Article: ] |
| 26. | Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192-201. [Cited in This Article: ] |
| 27. | Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol. 2001;27:1099-1106. [Cited in This Article: ] |
| 28. | Madersbacher S, Kratzik C, Marberger M. Prostatic tissue ablation by transrectal high intensity focused ultrasound: histological impact and clinical application. Ultrason Sonochem. 1997;4:175-179. [Cited in This Article: ] |
| 29. | Fried NM, Roberts WW, Sinelnikov YD, Wright EJ, Solomon SB. Focused ultrasound ablation of the epididymis with use of thermal measurements in a canine model. Fertil Steril. 2002;78:609-613. [Cited in This Article: ] |
| 30. | Wu F, Wang ZB, Cao YD, Chen WZ, Bai J, Zou JZ, Zhu H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89:2227-2233. [Cited in This Article: ] |
| 31. | Xu ZL, Zhu XQ, Lu P, Zhou Q, Zhang J, Wu F. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol. 2009;35:50-57. [Cited in This Article: ] |
| 32. | Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372-383. [Cited in This Article: ] |
| 33. | Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445-449. [Cited in This Article: ] |
| 34. | Wan H, Dupasquier M. Dendritic cells in vivo and in vitro. Cell Mol Immunol. 2005;2:28-35. [Cited in This Article: ] |