Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3267
Revised: April 26, 2010
Accepted: May 3, 2010
Published online: July 14, 2010
AIM: To identify the key cytokines involved in hepatic differentiation of mouse bone marrow mesenchymal stem cells (mBM-MSCs) under liver-injury conditions.
METHODS: Abdominal injection of CCl4 was adopted to duplicate a mouse acute liver injury model. Global gene expression analysis was performed to evaluate the potential genes involved in hepatic commitment under liver-injury conditions. The cytokines involved in hepatic differentiation of mBM-MSCs was functionally examined by depletion experiment using specific antibodies, followed by rescue experiment and direct inducing assay. The hepatic differentiation was characterized by the expression of hepatic lineage genes and proteins, as well as functional features.
RESULTS: Cytokines potentially participating in hepatic fate commitment under liver-injury conditions were initially measured by microarray. Among the up-regulated genes determined, 18 cytokines known to closely relate to liver growth, repair and development, were selected for further identification. The fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF) and oncostatin M (OSM) were finally found to be involved in hepatic differentiation of mBM-MSCs under liver-injury conditions. Hepatic differentiation could be dramatically decreased after removing FGF-4, HGF and OSM from the liver-injury conditioned medium, and could be rescued by supplementing these cytokines. The FGF-4, HGF and OSM play different roles in the hepatic differentiation of mBM-MSCs, in which FGF-4 and HGF are essential for the initiation of hepatic differentiation, while OSM is critical for the maturation of hepatocytes.
CONCLUSION: FGF-4, HGF and OSM are the key cytokines involved in the liver-injury conditioned medium for the hepatic differentiation of mBM-MSCs.
- Citation: Dong XJ, Zhang H, Pan RL, Xiang LX, Shao JZ. Identification of cytokines involved in hepatic differentiation of mBM-MSCs under liver-injury conditions. World J Gastroenterol 2010; 16(26): 3267-3278
- URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3267
It is generally believed that the bone marrow mesenchymal stem cells (BM-MSCs) provide an appropriate hematopoietic microenvironment that exerts regulatory effects on the self-renewal and differentiation of hematopoietic stem/progenitor cells. They are capable of differentiating into mesoderm cell lineages, including osteoblasts, chondrocytes and adipocytes[1-3]. However, emerging new findings suggest that BM-MSCs are able to give rise to a more broad range of cells, including hepatocytes, neurons, epithelial cells and keratinocytes[4-8]. This plasticity of BM-MSCs has attracted much attention to their in vivo new functions under either metabolic or pathologic conditions, and their clinical therapy for tissue repair. In fact, several studies in animal models have suggested that endogenous MSCs may “naturally” be involved in wound healing and tissue regeneration, and the engrafted exogenous MSCs have beneficial effects in tissue repair, including that of bone, myocardial tissue, skin, kidney and liver[9-19]. These may encourage further studies on the new insight into MSCs biology and the mechanisms underlying MSCs differentiation, which are still poorly understood at present.
Recently, by an in vivo tracing technology, we have demonstrated that BM-MSCs could be recruited from the bone marrow into peripheral blood, and toward into the wounded sites in response to the injured-liver signals, which indicated a close relationship between BM-MSCs and liver repair[20]. Moreover, we have also found that the engrafted exogenous BM-MSCs could be recruited to the injured liver, and were able to differentiate into multiple hepatic-lineage cells, which greatly improved the wound healing, providing further insight into the relationship between BM-MSCs and injured liver[20]. Our previous reports also support the idea that the liver-injury conditioned culture medium can induce the differentiation of BM-MSCs into functional hepatic cells in an in vitro experiment[4]. These observations indicated that the hepatic differentiation of BM-MSCs may be induced by the cytokines secreted from the injured liver cells, since no cellular interactions existed in such cell-free cultural medium. However, which cytokines direct hepatic fate specification of BM-MSCs still remains unclear. In the present study, we identified the key cytokines that play a crucial role in the differentiation of mBM-MSCs in the liver-injury conditioned medium. We hope our finding will benefit the better understanding of the novel mechanisms underlying BM-MSCs involved liver repair and regeneration, and help improve the cytokine-based hepatic inducing strategy and provide a rich cellular resource from BM-MSCs for cytotherapy of acute liver diseases.
Eight to ten-week-old male ICR mice obtained from the Laboratory Animal Unit of Zhejiang Academy of Medical Sciences (Hangzhou, China) were used in the experiments. Animals were housed under specified pathogen-free conditions. All animal experiments were done in accordance with a legal regulation, which includes approval by a local ethical committee.
The mouse bone marrow MSCs (mBM-MSCs) were prepared as described previously[4]. Briefly, the bone marrow was extruded by clipping of the epiphysial ends of the bones and flushing with IMDM (Sigma, St. Louis, MO), supplemented with 10% fetal bovine serum (Hyclone, Rockville, MD), 1% penicillin/streptomycin (Medium A). After 3 d, non-adherent cells and debris were removed, and the adherent cells were cultured continuously. At near confluence, the cells were replated at 5 × 104 cells/cm2. Osteogenic, chondrogenic and adipogenic differentiations were examined for functional identification[5].
The acute liver-injury mouse model was prepared according to the method described previously[21]. Briefly, the mice were treated with CCl4 (1.0 mL/kg body weight of a 10% solution in mineral oil injected intraperitoneally) twice a day and then sacrificed by cervical vertebrae luxation on the 24th h after the last injection.
The hepatocytes were isolated by the two-step collagenase perfusion from healthy mice (as control) or liver-injury mouse model prepared by the method described above. Briefly, donor animals received 25 U heparin (Sigma) prior to cell isolation. After cannulation of the portal vein, the liver was perfused with a calcium-free buffer solution, 3 mL/min at 37°C for 10 min. Then, the liver was perfused with 0.025% collagenase IV (Invitrogen, Carlsbad, CA), 2 mL/min at 37°C for 15 min. The perfused liver was resected, and the cells were released by gentle shaking and collected in 20 mL IMDM. The supernatant cell suspension was filtered using a 200 mm nylon mesh and the filtrate was washed twice with PBS by centrifugation at 50 ×g for 45 s to remove cell debris, damaged cells, and non-parenchymal cells. After washing, the hepatocytes were cultured in medium A at 5 × 104 cells/cm2. Forty-eight hours later, the supernatant was collected and passed through a 0.25 mm filter. The filtrate was finally defined as hepatocyte-injury conditioned medium and stored in aliquots at -20°C for future use.
The mBM-MSCs of passage 3 were inoculated in differentiation medium at 5 × 104 cells/cm2 on culture flasks. The differentiation medium consisted of 50% fresh IMDM medium (Medium A) and 50% conditioned medium. As a negative control, mBM-MSCs were cultured in medium A only. Cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Cultures were maintained by medium exchange every 3 d. The cell morphology was observed under a confocal laser-scanning microscope (LSM 510; Carl Zeiss Inc., Jena, Germany).
The expressions of hepatic linage genes [α-fetoprotein (AFP), albumin (ALB), hepatocyte nuclear factor 3β (HNF3β), tyrosine aminotransferase (TAT)] and cytokine genes [fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF) and oncostatin M (OSM)] involved in hepatic differentiation and commitment were analyzed by real-time polymerase chain reaction (PCR). For this, total RNA was extracted from undifferentiated control or differentiated cells, normal or injured liver cells, respectively, using NucleoSpin® RNAIIKits, and then 5 μg of which was reversely transcribed into cDNA with SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The transcripts normalized to β-actin were measured by real-time PCR using Mastercyclerep realplex system and Real-Time Detection software (Eppendorf, Germany), in which the standard double-stranded DNA dye (SYBR Grean I) was used. Gene-specific primers were designed using the Primer Premier software (Table 1).
| Gene | Sequence (5’-3’) | Annealing (°C) | Product (bp) |
| AFP-F | CACTGCTGCAACTCTTCGTA | 52 | 300 |
| AFP-R | CTTTGGACCCTCTTCTGTGA | ||
| HNF3b-F | GACCTCTTCCCTTTCTACCG | 51 | 551 |
| HNF3b-R | TTGAAGGCGTAATGGTGC | ||
| ALB-F | TCTTCGTCTCCGGCTCTG | 55 | 475 |
| ALB-R | CTGGCAACTTCATGCAAAT | ||
| TAT-F | CTTCAGTCCTGGATGTTCGC | 55 | 619 |
| TAT-R | CAGGGATTGGACGGGTTGTT | ||
| FGF4-F | CTGGTGGCTCACAGGACAATAAGAT | 62 | 483 |
| FGF4-R | GCTGGCTGAAGAAACAGGTAATAGGT | ||
| HGF-F | GTGCCAACAGGTGTATCAG | 62 | 399 |
| HGF-R | TGTCACAGACTTCGTAGCG | ||
| OSM-F | CTCACGGTCCACTACAACAC | 62 | 123 |
| OSM-R | GAGCCATCGTCCCATTCC | ||
| β-actin-F | TTCCTTCTTGGGTATGGAAT | 55 | 200 |
| β-actin-R | GAGCAATGATCTTGATCTTC |
The expressions of hepatic linage proteins (AFP and ALB) in differentiated hepatocytes and cytokine proteins (FGF-4, HGF and OSM) in injured liver cells were further analyzed by ELISA. The total proteins from differentiated cells or undifferentiated control cells, normal or injured liver cells were extracted using T-PER (a protein extraction reagent) (Qiagen, Germany) according to the manufacturer’s manual, and were coated on a 96-well polystyrene plate at 4°C overnight. The wells were washed with PBST (500 μL Tween 20/L PBS) and then blocked with 0.5% BSA for 1 h at 37°C. The hepatic linage proteins (AFP and ALB) or cytokines (FGF-4, HGF and OSM) were detected using the polyclonal antibodies, including rabbit anti-mouse HGF and FGF4, goat anti-mouse OSM (abcam, UK) rabbit anti-AFP and goat anti-ALB (Biodesign, Saco, Maine, USA) after washing with PBST for 3 times. The secondary antibodies, including goat anti-rabbit and rabbit anti-goat (Santa Cruz Biotechnology, Santa Cruz, CA, USA), labeled with horseradish peroxidase (HRP) were added at a proper dilution. Finally, the TMB was added, and the observation density was detected by a microplate reader at 450 nm after 2 mol/L sulphuric acid was added to stop the reaction.
The hepatic differentiation was functionally determined by glycogen storage. For this, the culture dishes containing differentiated cells were fixed in 95% alcohol for 10 min. Samples were then oxidized in 1% periodic acid for 5 min, rinsed three times in deionized (d) H2O, treated with Schiff’s reagent for 15 min, and rinsed in dH2O for 5 min. Finally, the preparations were assessed under light microscope, and the positive rate of differentiated cells was counted as previously described[4].
The hepatic differentiation was functionally determined by urea synthesis. The mBM-MSCs were plated at 5 × 104 cells/cm2 on collagen coated six-well plates in differentiation medium or control medium. After washing extensively with PBS, cells differentiated at days 0, 4, 8, 16, 20 and 21 were incubated in 2 mL of serum-free Hanks’ buffered salt solution containing 5 mmol/L NH4Cl for 2 h at 37°C. After incubation, the urea concentrations in the supernatant were measured according to the method described previously[22].
A global gene expression analysis was performed by microarray to identify the potential cytokines responsible for hepatic commitment. Total RNA and complementary DNA were prepared from CCl4-treated and untreated mouse livers. An Illumina Mouse WG-6 v2.0 BeadChip (Illumina, San Diego, CA, USA) was used to generate expression profiles of more than 48 000 transcripts with 500 ng of labelled cDNA for each sample, following manufacturer’s recommended protocols. A randomized design was used to minimize chip effects. Four individuals were replicated in the two batches. Expression intensity was measured with an average of 30 beads for each transcript. The BeadChips were imaged with an Illumina BeadArray Reader. The raw intensities were extracted with the Gene Expression Module in Illumina’s BeadStudio software. Expression intensities were log2 transformed and median-centered by subtracting the mean value of each array from each intensity value.
The cytokines involved in hepatic specification in liver-injury conditioned medium were initially identified in a depletion experiment. The liver-injury conditioned medium was incubated with a number of different cytokine antibodies (anti-HGF, -FGF-4, -OSM, -FGF-3, -FGF-10, -FGF-12, -FGF-13, -FGF-14, -FGF-15, -FGF-17, -FGF-18, -FGF-20, -FGF-21, -bNGF, -IGF-1, -TGF-β1, -TGF-β2, and -TGF-β3, abcam and Biodesign) at different concentrations (1:100, 1:200 and 1:500) overnight at 4°C under agitation. Then, the cytokines were removed by affinity co-immunoprecipitation. The protein A-coupled sepharose beads were prepared according to the manufacturer’s instructions (Abcam), and were added into the antibody pretreated liver-injury conditioned medium. After being incubated at 4°C for 4 h, the samples were centrifuged at 10 000 ×g for 5 min. Supernatants were collected for hepatic differentiation induction assay. The hepatic differentiation was examined based on the hepatic lineage gene and protein expressions and functional characterizations as described above. In parallel, a non-specific rabbit antibody (IgG isotype) was used in control groups.
To confirm the observation from depletion experiment that HGF, FGF-4 and OSM may be involved in hepatic differentiation in liver-injury conditioned medium, a rescue experiment was performed by adding back these three cytokines into the co-immunoprecipitated liver-injury conditioned medium. The liver-injury conditioned medium was pretreated with anti-HGF, anti-FGF-4 and anti-OSM, followed by protein A-coupled Sepharose beads as described above, and mouse FGF-4 (0, 1.25, 2.5, and 5 μg/mL), HGF (0, 2.5, 5 and 10 μg/mL) and OSM (0, 1.25, 2.5 and 5 μg/mL) (R&D Systems, Abingdon, UK) were added back to this medium to rescue its liver-inducing activity. The hepatic differentiation was examined based on the hepatic lineage gene and protein expressions and functional characterizations as described above.
To further investigate the role of FGF-4, HGF and OSM in hepatic differentiation, an in vitro hepatic induction assay was conducted using different combinations of FGF-4, HGF and OSM. The mBM-MSCs were inoculated in medium A with different combinations of 10 ng/mL FGF-4, 20 ng/mL HGF and 10 ng/mL OSM[21] at 5 × 104 cells/cm2 on culture flasks. As a negative control, the mBM-MSCs were cultured in medium A without FGF-4, HGF and OSM. Cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37°C. After 72 h, non-adherent cells and debris were removed and the adherent cells were cultured continuously. Cultures were maintained by medium exchange every 3 d. The hepatic differentiation was examined based on the hepatic lineage gene and protein expressions and functional characterizations as described above.
Statistical analysis was performed using the SPSS version 16.0, and data were expressed as mean ± SD. Differences between the values were determined by paired-samples t test. A value of P < 0.05 was considered statistically significant.
To evaluate the possible cytokines that participate in the hepatic differentiation of BM-MSCs under liver-injury conditions, the gene expression levels of cytokines/proteins in injured liver were initially examined at different injury time-points (12, 24 and 48 h) by microarray analysis. The results showed that more than 1200 genes were significantly up- or down-regulated during these time periods, and most of them were closely related to hepatocyte detoxification and metabolisms (data not shown). Totally, 40 cytokines/chemokines or their corresponding receptors closely associated with cellular growth, differentiation and migration were found to be significantly up-regulated (2-62 folds), among which 18 cytokines, including FGF-3, FGF-4, FGF-10, FGF-12, FGF-13, FGF-14, FGF-15, FGF-17, FGF-18, FGF-20, FGF-21, HGF, OSM, b-NGF, IGF-2, TGFβ-1, TGFβ-2 and TGFβ-3, were largely contributed to the hepatic growth and development. Therefore, these cytokines were considered to be potentially involved in the hepatic differentiation of mBM-MSCs under liver-injury conditions, and to be the candidates for further identification.
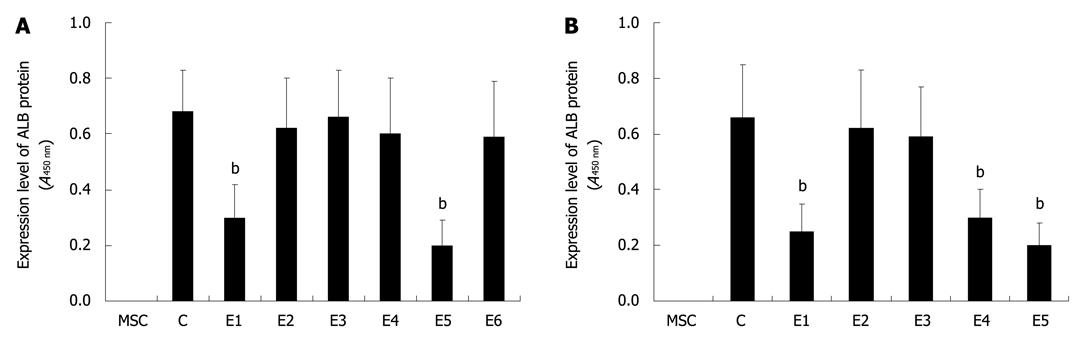
Based on the microarray analysis as described above, the cytokine candidates were removed from the conditioned medium by a co-immunoprecipitation strategy using different combinations of specific antibodies which fall into several groups, designated as E1 (anti-FGF-3 and -FGF-4), E2 (anti-FGF-10, -FGF-12 and -FGF-13), E3 (anti-FGF-14, -FGF-15 and -FGF-17), E4 (anti-FGF-18, -FGF-20 and -FGF-21), E5 (anti-HGF, -OSM and -bNGF) and E6 (anti-IGF-1, -TGF-β1, -TGF-β2 and -TGF-β3). The conditioned medium from each group was used for further hepatic differentiation. The results showed that, after being induced for 20 d, the ALB in differentiated cells in E1 and E5 groups was more significantly down-regulated (P < 0.01) than that in the control group. However, no significant down-regulations of ALB could be observed in other experimental groups (E2, E3, E4 and E6), suggesting that FGF-3, FGF-4, HGF, OSM and bNGF in the conditioned medium examined in E1 and E5 groups may play essential roles in the hepatic differentiation of mBM-MSCs (Figure 1A). In an attempt to verify this observation, the FGF-3, FGF-4, HGF, OSM and bNGF were removed selectively: E1, anti-FGF-4; E2, anti-FGF-3; E3, anti-bNGF; E4, anti-HGF; and E5, anti-OSM. The hepatic differentiation of mBM-MSCs in each group was detected and the results showed that the ALB in differentiated cells in E1, E4 and E5 groups was significantly down-regulated (P < 0.01) compared with that in the control group. However, no significant down-regulations of ALB could be observed in E2 and E3 groups (Figure 1B). These results indicated that FGF-4, HGF and OSM are key factors involved in the hepatic differentiation of mBM-MSCs. This observation seems in accordance with some previous reports showing that FGF-4, HGF and OSM played important roles in liver healing, regeneration and initiation of development[23-26].
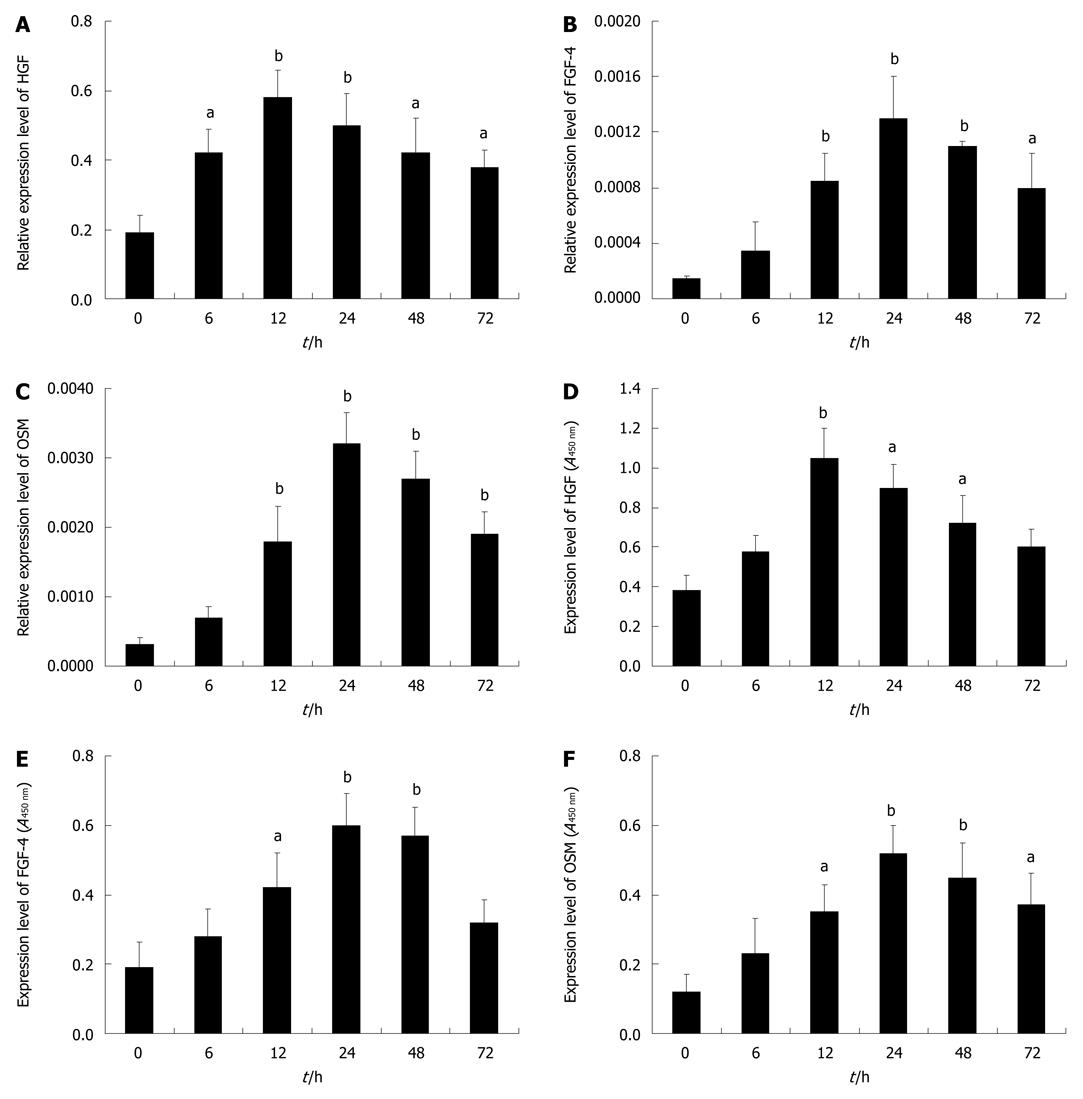
To obtain a deep insight into the role of FGF-4, HGF and OSM in liver injury, a kinetic expression analysis of these cytokines during liver injury was performed. The results showed that the expression of HGF mRNA was significantly up-regulated at 6 h after injury (P < 0.05), peaked at 12 h (P < 0.01) and kept in high level up to 72 h (Figure 2A). Similarly, the expressions of FGF-4 and OSM mRNAs were importantly up-regulated at 12 h (P < 0.01) after injury, peaked in 24-48 h (P < 0.01) and also kept in high levels up to 72 h (Figure 2B and C). Accordingly, the expression changes of HGF, FGF-4 and OSM proteins were generally identical with their mRNA expressions (Figure 2D-F).
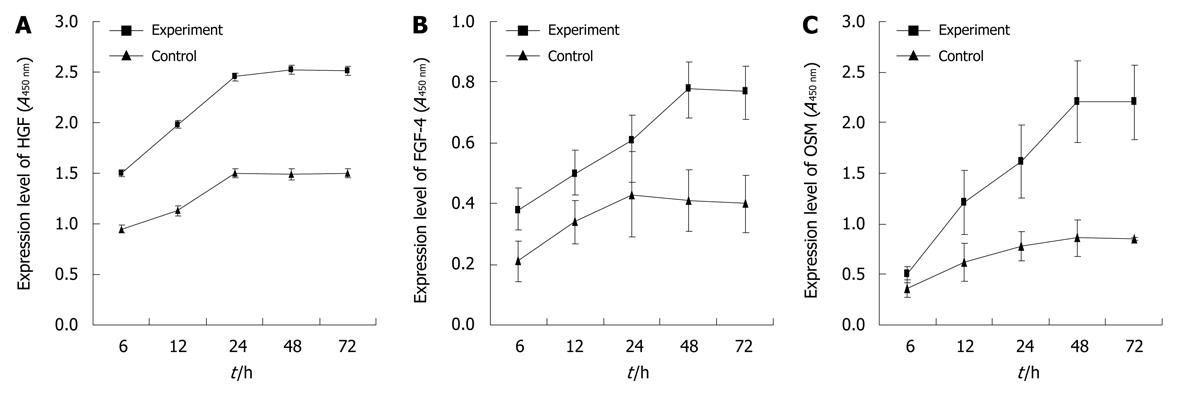
To further investigate the existence and occurrence of FGF-4, HGF and OSM in liver-injury conditioned medium, the kinetic secretion of FGF-4, HGF and OSM into the cultured medium was determined. The results showed that the concentrations of HGF and OSM proteins were dramatically up-regulated at 12 h after culture, peaked at 24 or 48 h and kept in high levels up to 72 h (Figure 3A and C); while the FGF-4 was significantly up-regulated at 24 h and peaked at 48 h (Figure 3B). Notably, it showed that FGF-4, HGF and OSM protein expressions could also be detected in the cell culture without undergoing liver injury (Figure 3), possibly due to the fact that isolation of cells from liver itself is a tissue “anatomy” which may result in the “injured signal” to stimulate the cells to secrete cytokines.
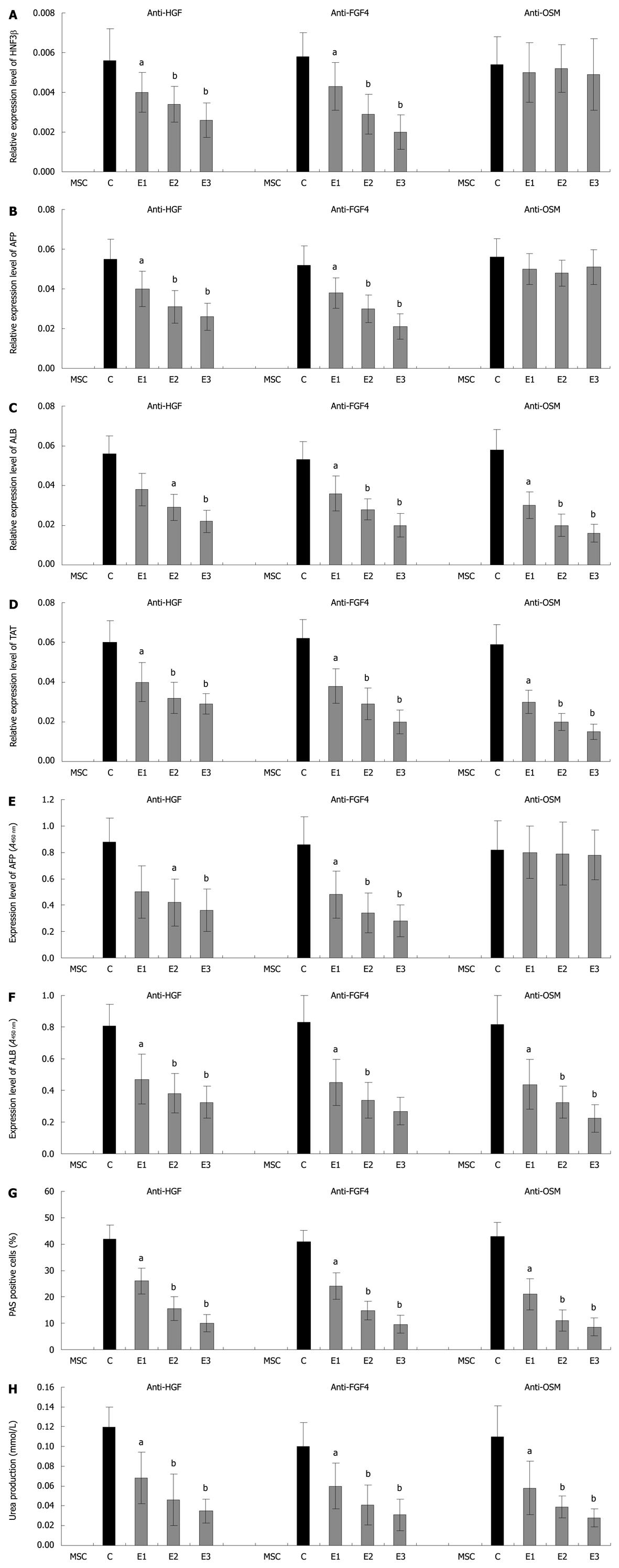
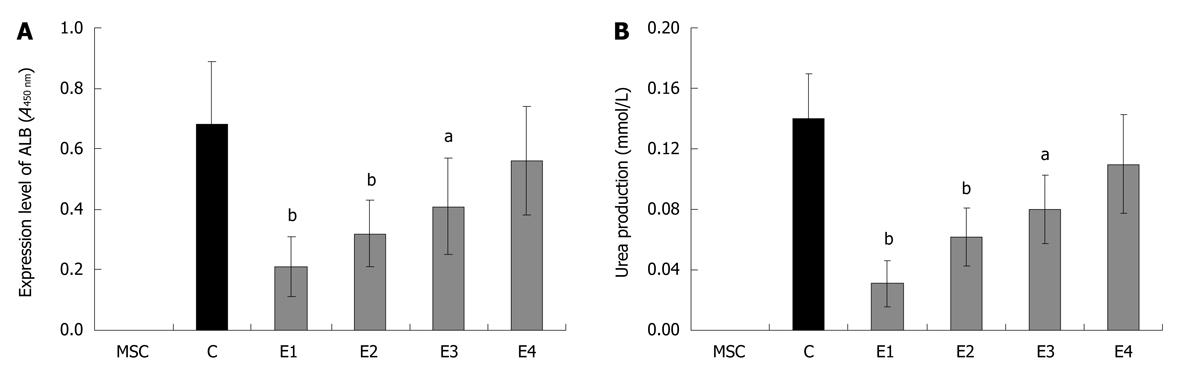
To investigate the effects of each cytokine in the hepatic differentiation, a number of depletion experiments were conducted using different combinations of antibodies. The results showed that after treatment with anti-FGF-4 and anti-HGF independently, the hepatic differentiation induced by the conditioned medium was significantly (P < 0.01) down-regulated with the increased use of antibody (E1: 1:500, E2: 1:200, E3: 1:100) as determined by the expression of both the early liver-specific marker (HNF3β and AFP) and the late liver-specific marker (ALB and TAT) at mRNA or protein levels, and by the functional [Periodic acid-Schiff (PAS) and urea production] analyses (Figure 4A-H). However, after treatment with anti-OSM alone, the expressions of HNF3β and AFP in cells were not significantly decreased (Figure 4A and B), while the expressions of ALB and TAT were dramatically (P < 0.01) restrained at both mRNA and protein levels (Figure 4C, D and F). Accordingly, the PAS and urea production were also dramatically (P < 0.01) decreased in anti-OSM treatment groups (Figure 4G and H). These results suggested that FGF-4, HGF and OSM may play different roles in the hepatic differentiation of mBM-MSCs. Among these factors, FGF-4 and HGF may be essential for the initiation of early hepatic differentiation, while OSM may be critical for the maturation of hepatocytes.
In order to find further evidence on the role of FGF-4, HGF and OSM in the conditioned medium in hepatic differentiation, we performed a rescue experiment in which three recombinant cytokines were added back to the cytokine-removed medium treated with anti-FGF-4 (1:100), anti-HGF (1:100) and anti-OSM (1:100). The results showed that administration of FGF-4, HGF and OSM into this cytokine-removed medium could significantly rescue the hepatic differentiation with the increased concentrations of the cytokines as determined by the expression of ALB protein and urea synthesis (E1: 0 ng/mL FGF-4 + 0 ng/mL HGF + 0 ng/mL OSM; E2: 1.25 ng/mL FGF-4 + 2.5 ng/mL HGF + 1.25 ng/mL OSM; E3: 2.5 ng/mL FGF-4 + 5 ng/mL HGF + 2.5 ng/mL OSM; E4: 5 ng/mL FGF-4 + 10 ng/mL HGF + 5 ng/mL OSM) (Figure 5A and B). These results provided solid support that FGF-4, HGF and OSM are the key cytokines that contribute to the induction of hepatic differentiation in the liver-injury conditioned medium.
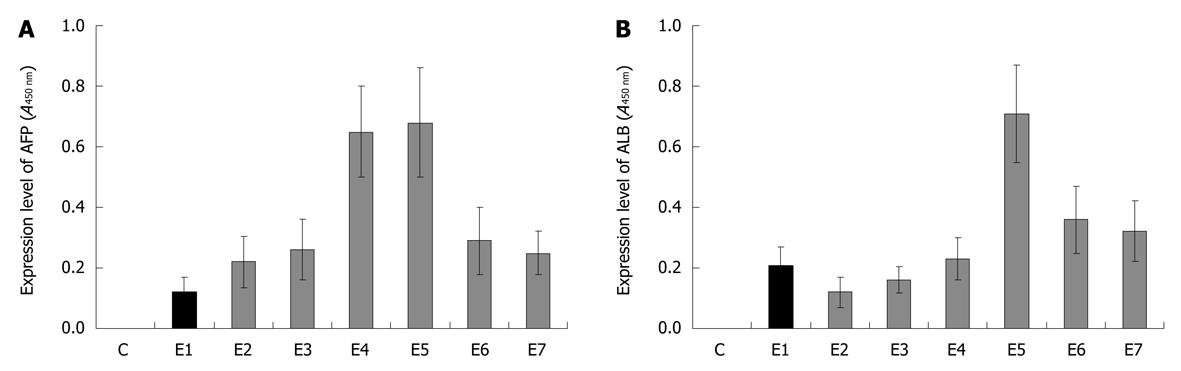
Based on the observations from the above experiments that FGF-4, HGF and OSM are crucial for hepatic differentiation in the conditioned medium, we performed a further hepatic differentiation experiment induced directly by FGF-4, HGF and OSM in different combinations. The results showed that FGF-4, HGF and OSM may have synergistic effects on the hepatic differentiation of mBM-MSCs, indicating that the three cytokines may play different roles in the induction of hepatic differentiation. As shown in Figure 6, after induced with a factor alone or combination of the two, the hepatic differentiation of mBM-MSCs was lowly detectable as determined by the synthesis of both AFP (early hepatic differentiation marker) and ALB (late hepatic differentiation marker) in differentiated cells. In the group induced with FGF-4 (10 ng/mL) and HGF (20 ng/mL, E4), the concentration of AFP was highly detectable, while ALB was lowly detectable, suggesting that FGF-4 and HGF have a synergistic effect on the initiation of early hepatic differentiation. In contrast, both of the AFP and ALB proteins could be highly detected in group E5 in which the mBM-MSCs were induced by the combination of these three factors. This indicated that FGF-4, HGF and OSM had a synergistic effect in the differentiation of functional hapatocytes from mBM-MSCs. Furthermore, FGF-4 and HGF exhibited a cooperative effect on the early hepatic differentiation of mBM-MSCs, while OSM is essential to the maturation of hepatocytes in the late hepatic differentiation.
Mesenchymal stem cells (MSCs) have emerged as a promising resource of functional hepatocytes for treatment of liver diseases because of its plasticity of multiple cell lineages. To date, many inducing systems for hepatic differentiation from MSCs have been developed[22,27,28]. However, the rate of hepatocyte-like cells differentiated from MSCs is still very low and the mechanisms are also not well known. It is undoubted that exposition of MSCs to the inducing systems, resembling the conditions in liver development, injury and regeneration, could acquire a more efficient differentiation[29]. Our previous studies have shown that mBM-MSCs could be induced to differentiate into hepatic cells by conditioned culture medium of hepatocytes[4]. Thus identification of the exact cytokines involved in liver-injury conditions for the mBM-MSCs differentiation needed further studies. This study provides such investigation. Eighteen cytokines closely related to liver growth, repair and development were chosen as candidates from numerous up-regulated cytokine genes by microarray. It was found that three cytokines (FGF-4, HGF and OSM) may play a crucial role in the conditioned medium-induced hepatic differentiation, since hepatic differentiation was dramatically decreased after removing FGF-4, HGF and OSM from the conditioned medium. Therefore, the present study provided a direct basis on the selection of cytokines for hepatic differentiation. However, besides these three key cytokines, some other factors involved in the liver injury need to be identified for improving the cytokine-based inducing system.
FGF-4, HGF and OSM play important roles in liver regeneration, healing, initiation and development. FGF-4 was considered to be one of the most important fibroblast growth factor family members that can irritate the proliferation of mesodermal and endodermal cells and improve development of fetal liver[30]. HGF was found to be essential for the development of several epithelial organs and was one of the most well characterized cytokine for the stimulation of DNA synthesis in primary hepatocyte cultures, and for liver development[31]. The OSM, however, is a member of the interleukin-6 family produced by hematopoietic cells and induces differentiation of fetal hepatic cells, conferring various metabolic activities of adult liver[32]. These three factors participate in different liver developmental stages. Thus, we further examined the exact roles of FGF-4, HGF and OSM in the hepatic differentiation from mBM-MSCs. It clearly showed that after removing FGF-4 and HGF from the conditioned medium by their antibodies, either the early or the late hepatic differentiation induced by the conditioned medium could be significantly down-regulated, while after removing OSM from the conditioned medium, only the late hepatic differentiation was down-regulated. It suggested that FGF-4, HGF and OSM also play different roles in the hepatic differentiation of mBM-MSCs, and FGF-4 and HGF are essential for the initiation of hepatic differentiation, while OSM is critical for the maturation of hepatocytes.
In conclusion, the present study analyzed the potential factors in injured liver for hepatic differentiation from mBM-MSCs. It was found that FGF-4, HGF and OSM might be the key cytokines. They played different roles during hepatic differentiation, which is similar to their functions in liver development. Hopefully, our study would not only provide evidence of cytokine selection for hepatic differentiation, but also benefit the exploration of the molecular mechanisms underlying the differentiation of BM-MSCs into hepatocytes.
Acute liver failure is a severe liver disease with a mortality of 60%-90%. The only therapeutic option, orthotopic liver transplantation, is limited because of the shortage of suitable donor organs. Mesenchymal stem cells (MSCs), known for their capacity to proliferate indefinitely and differentiate into almost all types of cells, including hepatocytes, have provided the hope of cellular replacement therapy for liver failure.
Mouse liver-injury conditioned culture medium dramatically facilitated the differentiation of mouse bone marrow MSCs (mBM-MSCs) into functional hepatic cells. However, which cytokines direct hepatic fate specification of mBM-MSCs still remains unclear. In this study, the authors demonstrate that fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF) and oncostatin M (OSM) may play crucial roles in the differentiation of mBM-MSCs in the liver-injury conditioned medium.
In the present study, the authors reported the identification of cytokines involved in hepatic fate specification of mBM-MSCs in the liver-injury conditioned medium. By removing cytokines from conditioned medium and adding back cytokines into the “cytokine-removed” conditioned medium, it was demonstrated that FGF-4, HGF and OSM may play crucial roles in the conditioned medium-induced hepatic differentiation. Furthermore, different combinations of FGF-4, HGF and OSM were used to induce hepatic differentiation, and the result showed that FGF-4 and HGF had a cooperative effect on the early hepatic differentiation of mBM-MSCs, while OSM was essential to the maturation of hepatocytes in the late hepatic differentiation. This is the first study to report that FGF-4, HGF and OSM FGF-4, HGF and OSM not only play crucial roles in the hepatic differentiation of mBM-MSCs, but also have profound synergistic effect on the hepatic differentiation of mBM-MSCs pro and con. This in vitro study would contribute to the improvement of hepatic cell resource for cell-based therapies for acute and chronic end-stage liver diseases and provide a model for more detailed characterization on the molecular mechanisms underlying the differentiation of BM-MSCs into hepatocytes.
This study may benefit not only the better understanding of novel mechanisms underlying BM-MSCs involved in liver repair and regeneration, but also the improvement of cytokine-based hepatic inducing strategy, in which a rich cellular resource for cytotherapy of acute liver diseases with BM-MSCs would be provided.
FGF-4 is one of the most important fibroblast growth factor family members that can irritate the proliferation of mesodermal and endodermal cells and improve development of fetal liver; HGF is one of the most well characterized cytokines for the stimulation of DNA synthesis in primary hepatocyte cultures and liver development; OSM is a member of the interleukin-6 family which is produced by hematopoietic cells and induces differentiation of fetal hepatic cells, conferring various metabolic activities of adult liver.
The authors corroborate that 3 cytokines (HGF, FGF4 and OSM) are fundamental for directing the differentiation of BM-MSCs towards hepatocytes. The work is interesting and could be helpful for developing effective inducing systems of hepatic differentiation from BM-MSCs.
Peer reviewers: Belén Beltrán, MD, PhD, Gastroenterology Department (Medicina Digestiva), Hospital Universitari La Fe, Avda Campanar 21, 46009 Valencia, Spain; Catherine Greene, PhD, Senior Lecturer, Department of Medicine, Royal College of Surgeons in Ireland, Education and Research Centre, Beaumont Hospital, Dublin 9, Ireland
S- Editor Wang YR L- Editor Ma JY E- Editor Ma WH
| 1. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. |
| 2. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. |
| 3. | Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180-192. |
| 4. | Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102:52-63. |
| 5. | Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815-820. |
| 6. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. |
| 7. | Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis. 2001;27:632-636. |
| 8. | Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411-4422. |
| 9. | Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571-579. |
| 10. | Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104-112. |
| 11. | Zhao J, Zhang N, Prestwich GD, Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol Biosci. 2008;8:836-842. |
| 12. | Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281-292. |
| 13. | Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581-2587. |
| 14. | Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075-2082. |
| 15. | Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648-2659. |
| 16. | Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. |
| 17. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. |
| 18. | Wang Y, Johnsen HE, Mortensen S, Bindslev L, Ripa RS, Haack-Sørensen M, Jørgensen E, Fang W, Kastrup J. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768-774. |
| 19. | Jin HK, Carter JE, Huntley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest. 2002;109:1183-1191. |
| 20. | Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, Zhang GR. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2009;Epub ahead of print. |
| 21. | Chen Y, Pan RL, Zhang XL, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Induction of hepatic differentiation of mouse bone marrow stromal stem cells by the histone deacetylase inhibitor VPA. J Cell Mol Med. 2009;13:2582-2592. |
| 22. | Pan RL, Chen Y, Xiang LX, Shao JZ, Dong XJ, Zhang GR. Fetal liver-conditioned medium induces hepatic specification from mouse bone marrow mesenchymal stromal cells: a novel strategy for hepatic transdifferentiation. Cytotherapy. 2008;10:668-675. |
| 23. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. |
| 24. | Kang XQ, Zang WJ, Song TS, Xu XL, Yu XJ, Li DL, Meng KW, Wu SL, Zhao ZY. Rat bone marrow mesenchymal stem cells differentiate into hepatocytes in vitro. World J Gastroenterol. 2005;11:3479-3484. |
| 25. | Ong SY, Dai H, Leong KW. Hepatic differentiation potential of commercially available human mesenchymal stem cells. Tissue Eng. 2006;12:3477-3485. |
| 26. | Ong SY, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials. 2006;27:4087-4097. |
| 27. | Snykers S, Vanhaecke T, De Becker A, Papeleu P, Vinken M, Van Riet I, Rogiers V. Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol. 2007;7:24. |
| 28. | Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570-581. |
| 29. | Snykers S, Vanhaecke T, Papeleu P, Luttun A, Jiang Y, Vander Heyden Y, Verfaillie C, Rogiers V. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94:330-341; discussion 235-239. |
| 30. | Rappolee DA, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120:2259-2269. |
| 31. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. |
| 32. | Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11:177-183. |