INTRODUCTION
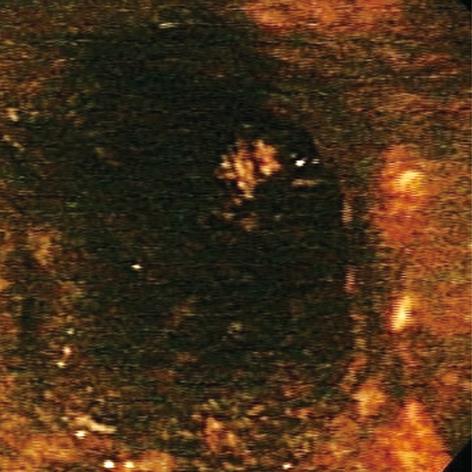
Acute esophageal necrosis (AEN), commonly referred to as “black esophagus” or “acute necrotizing esophagitis”, is a rare clinical disorder classically characterized by a striking endoscopic image of diffuse, circumferential, black-appearing, distal esophageal mucosa on esophagogastroduodenoscopy (EGD) (Figure 1) that stops abruptly at the gastroesophageal junction (GEJ)[1,2]. Proximal esophageal extension is common. Biopsy is recommended although not required to make the diagnosis. First described in 1990 by Goldenberg et al[3], this medical phenomenon was again brought into the spotlight by our large review series in 2007, in which we analyzed all cases reported in the English literature to date and formulated the concept of a distinct clinical syndrome[2]. Its etiology is likely multifactorial. AEN is thought to arise from a combination of an ischemic insult to the esophagus, impaired mucosal barrier systems, and a backflow injury from chemical contents of gastric secretions[2,4-6]. Male sex, older age, chronic medical conditions, including diabetes mellitus, hematologic and solid organ malignancy, malnutrition, renal insufficiency, cardiovascular compromise, trauma, and thromboembolic phenomena place a patient at a higher risk for developing AEN[4,7-13]. Clinical presentation is almost universally related to upper gastrointestinal bleeding. Complications may include stenosis or stricture formation in the distal esophagus, perforation, mediastinitis, and death. Overall mortality is largely related to the underlying medical condition and approaches 32%[2].
Figure 1 Black esophagus: acute esophageal necrosis.
In this latest review, we discuss the epidemiology of AEN syndrome, its pathophysiological and clinical features, etiology, presentation, distinction from other mimickers of “black esophagus”, natural course of the disease, staging, complications, suggested treatment options, and prognosis.
EPIDEMIOLOGY
Literature analysis shows that the estimated prevalence of AEN is low. Two large autopsy series from the United States and France have reported zero cases in a series of 1000 adult autopsies[14] and 0.2% in 3000 autopsies, respectively[15]. Two large retrospective series that have reviewed the findings in > 100 000 endoscopies have estimated the incidence at approximately 0.01% (12 patients)[5,12], and another retrospective analysis of 10 295 endoscopies has shown an incidence of 0.28% (29 patients)[13]. A 1-year prospective study of EGD findings in 3900 patients has identified eight cases of AEN (0.2%)[6]. However, these numbers are likely underestimated and the true prevalence of AEN remains unknown, largely due to the potential of subclinical presentation of the disease and the early healing of the mucosa that can be seen with transient ischemic or chemical injury. An intriguing paper by Japanese authors has reported AEN as the fourth leading cause of coffee ground emesis, hematemesis, or melena in a series of 239 hospital admissions for upper gastrointestinal bleeding, which accounted for approximately 6% of the cases[16].
AEN clearly shows sex and age predilection. Men are four times more commonly affected than women, and although the disease has been documented in every age group, the peak incidence occurs in the sixth decade of life with an average age of 67 years[2]. General debilitation and poor clinical condition with multiple medical co-morbidities are associated with increased likelihood of development of AEN. Personal history of diabetes mellitus (24%), malignancy (20%), hypertension (20%), alcohol abuse (10%), and coronary artery disease (9%) places a patient at risk of developing AEN[2].
ANATOMY AND PATHOPHYSIOLOGY
The esophagus is an expandable muscular organ lined by a stratified squamous epithelium. Characteristically, esophageal wall is composed of four distinct layers: mucosa, submucosa, muscularis propria, and adventitia. Unlike the rest of gastrointestinal tract, the esophagus lacks serosa and is separated from the adjacent organs by few millimeters of connective tissue, an important factor in potential development of extrinsic necrotic injury to the organ and spread of the infectious processes.
The esophagus receives an intricate segmental vascular supply that separates this organ into three parts: upper, middle, and distal esophagus. The arterial network of the upper esophagus is derived from the descending branches of the inferior thyroid arteries. Additional variable supply may come from branches of the subclavian, thyroidea ima, common carotid, or superior thyroid arteries. Mid-esophageal blood supply is derived from the bronchial arteries, right third or fourth intercostal arteries, and numerous small esophageal arteries off the descending aorta. Distal esophagus receives its blood flow from branches off the left gastric artery or left inferior phrenic artery, but variations are common and small branches off the celiac, splenic, short gastric, or left hepatic arteries may provide additional or alternative blood supplies[17,18]. Relative to the densely vascularized proximal and middle parts of the organ, the distal esophagus is slightly more “watershed” and therefore the first signs of the ischemic injury to the esophagus typically appear there. Overall, the complex anastomotic vascular network that is present in the esophageal submucosa generally makes esophageal infarction a rare clinical disease[18].
An important physiological property of the esophagus is the ability to use intricate protective barrier systems to shield itself from the injurious chemical reflux of the stomach contents. A delicate microarray of the epithelial tight junctions, zonula adherens, intercellular glycoconjugated material, intrinsic cellular buffering systems and anion exchangers, and extensive desmosomal network, coupled with bicarbonate, lubricant, mucus, and epidermal growth factor production by submucosal glands account for comprehensive defense mechanisms and restorative function of the organ[18]. Compromise or inadequacy of these barriers may lead to cell damage, and ultimately, death. Local blood supply to the lamina propria and submucosa, which is essential in maintaining effective buffering systems, is influenced by a variety of factors, including autoregulation in response to the intraluminal acidic pH of gastric contents[19,20]. Initial augmentation in blood delivery needed for maintenance of protective and reparative processes may be significantly downregulated by an overwhelming back diffusion of hydrogen ions[19,21]; an important factor in pathogenesis of AEN, particularly in the setting of an already compromised blood flow. At the cellular and molecular level, the mechanism of the esophageal injury is similar to the development of ischemic changes in the small and large bowel. Reactive oxygen metabolites formed as a result of reperfusion injury and leukocytic migration may directly and indirectly damage important mechanisms of cellular viability and function, which results in cellular lysis and death[18,22].
Finally, anti-reflux barriers and luminal clearance are important factors in preventing significant esophageal injury. Integrity of the lower esophageal sphincter mechanism, gravity and esophageal peristalsis are vital. Compromise or ineffectiveness of any one of these may deeply potentiate necrotic injury[23].
ETIOLOGY
Tissue insult seen in AEN is likely multifactorial and usually results from a combination of tissue hypoperfusion, impaired local defense barriers, and massive influx of gastric contents that acutely overwhelm the already vulnerable esophageal mucosa[2]. Perfusion injury in the development of AEN is best demonstrated by an avascular distal esophagus reported by Goldenberg et al[24]. Significant vasculopathy that is typically associated with diabetes mellitus, atherosclerosis, cardiovascular and renal diseases is an important risk factor that may predispose some patients to rapid tissue injury, as well as general susceptibility to hemodynamic instability and compromise. Low-flow state related to sepsis, cardiac arrhythmias, congestive heart failure, severe third spacing, systemic inflammatory response syndrome due to severe pancreatitis, lactic- and ketoacidosis, acute blood loss, hypothermia, trauma and shock may lead to ischemic compromise of the esophagus[2,12,16,25-31]. Thromboembolic phenomena and underlying coagulopathy seen in patients with solid tumor or hematological malignancies, anticardiolipin antibody syndrome, and atherosclerosis have also been implicated in the development of AEN[8,9,11,16,29]. Striking predilection to the distal esophagus is likely explained by its lesser degree of vascularization relative to the proximal and middle portions of the organ[12].
Vascular compromise to the distal esophageal tissue can also explain some of the duodenal pathology commonly seen in association with AEN, namely, duodenal bulb ulcers, erosions, inflammation, and edema[3,6,24]. The common blood supply from the branches off the celiac axis makes distal esophageal and duodenal pathologies less of a coincidence, but rather possibly related entities. Characteristic relative sparing of the gastric mucosa can be explained by the acidic impact on the ischemic esophageal and duodenal surface[7], as well as typically rapid repair of the injured gastric mucosa (within hours) compared to the esophagus (may take days)[18]. The duodenal bulb ulcers and edema may result in gastric outlet obstruction that, in turn, potentiates the development of mucosal necrosis of the distal esophagus[5,32]. A transient form of non-obstructive gastropathy can be seen in acute alcohol intoxication and diabetic ketoacidosis, two medical conditions that are linked to the development of AEN[25,26,33,34]. Back flow injury from the acid, pepsin, and other gastric contents is augmented by the increased transient lower esophageal sphincter relaxation, decreased resting lower esophageal pressure, prolonged recumbence, decreased esophageal peristalsis, and increased gastroesophageal reflux that may be related to the multiple medical conditions seen in the patient with AEN (debilitated condition, significant hiatal hernia, alcohol use, diabetes mellitus, postoperative state, etc.).
Alteration in physiological processes is an important co-factor in the development of AEN. Critical illness, poor nutritional status, and general deconditioning may contribute to the diminished mucosal buffering, compromised local protective barriers, and impaired defensive mechanisms that may potentiate ischemic and chemical injury to the esophagus[6]. Such risk factors include malignancy, cirrhosis, chronic pulmonary disease, neutropenia, renal insufficiency or failure, diabetes mellitus, hypoalbuminemia, immunosupression after solid organ transplantation, acquired immune deficiency syndrome (AIDS), postoperative status, and sepsis[2,10,12,13,27,35-39].
Diffuse AEN may be associated with local infection and tissue biopsy is essential for proper management, which includes antiviral or antimicrobial agents. Exact distribution is variable and probably depends on mode of transmission of the infectious agent, from external spread reported from a superior mediastinitis that affects cervical and thoracic esophagus[40], to primary esophageal intraluminal infection seen in viral and fungal disease in immunocompromised patients[35,36,41]. Reported pathogens include Klebsiella pneumoniae[40] and cytomegalovirus[36,41], herpes simplex virus[37], Penicillium chrysogenum[35], Candida[7], and other fungal species.
Other diseases and medical conditions associated with the development of AEN include erythema multiforme or Stevens-Johnson syndrome, gastric volvulus, hematoma from traumatic transection of thoracic aorta, hypersensitivity to antibiotics, acute fatty liver of pregnancy, drug-induced hepatitis, photodynamic therapy, polyarteritis nodosa, pulmonary lobectomy with paraesophageal lymph node dissection, severe infectious mediastinitis, emphysematous gastritis, hypoxia, and pancreatitis[2,7,12,39,40,42-49]. A single case of isolated proximal black esophagus has been reported in a patient who underwent cardiac catheterization, an event that was possibly related to the procedure[50,51].
PRESENTATION
A typical patient with AEN is an elderly male with multiple medical comorbidities, who manifests with signs of upper gastrointestinal bleeding. Clinical presentation of AEN ranges from hematemesis, coffee ground emesis, and melena (overall, accounting for nearly 90% of the cases) to asymptomatic black esophagus that was noted during a percutaneous gastrostomy tube placement[2,52]. Review of systems may be remarkable for epigastric/abdominal pain, vomiting, dysphagia, nausea, low-grade fever, lightheadedness, and syncope. Other associated clinical conditions or endoscopic findings may include multiorgan failure[53], cardiac, pulmonary, and renal disease, diabetes mellitus and ketoacidosis, vasculopathy, coagulopathy, peptic ulcer disease, general debilitation and malnourishment, cirrhosis, acute alcoholic hepatitis, acute fatty liver of pregnancy, acute pancreatitis, sepsis, ischemic processes (stroke, ischemic bowel, ischemic gangrene), and trauma. Physical findings in a patient with AEN are usually confounded by the underlying medical conditions but may be notable for cachectia, fever, hypoxia, hemodynamic instability or compromise, including arrhythmia and hypotension, pallor, abdominal tenderness, and guaiac-positive stools. Laboratory analysis may show leukocytosis and anemia and computed tomography (CT) may reveal thickened distal esophagus, hiatal hernia, distended fluid-filled stomach with possible gastric outlet obstruction, and rarely, external compression of the esophagus by a large mediastinal process.
DIFFERENTIAL DIAGNOSIS
Classic AEN presents as a circumferential, black-appearing, diffusely necrotic esophageal mucosa, preferentially affecting the distal esophagus, of variable length, that stops abruptly at the GEJ. Proximal extension of the mucosal injury is common and the entire esophagus can appear black. Differential diagnosis includes malignant melanoma, acanthosis nigricans, coal dust deposition, pseudomelanosis, and melanocytosis of the esophagus[54-63]. Detailed history and physical examination provide essential clues to the diagnosis. Classic endoscopic appearance may be supported by brush cytology or biopsy specimen that will confirm the diagnosis. This is especially important in rare cases of isolated involvement of proximal and middle esophagus. Additionally, a history of corrosive ingestion[64] should be sought for and diligently excluded.
CLINICAL DIAGNOSIS
The characteristic endoscopic appearance described above makes the diagnosis of AEN. Almost universal predilection to the distal esophagus (97%) is a remarkable feature of this disorder[2]. Additional endoscopic findings may include duodenal peptic ulcer disease, erosions, edema, and sings of gastric outlet obstruction[23]. Early evaluation may also be notable for signs of bleeding, including active oozing from the distal esophagus and blood clots and “coffee grounds” material in the stomach. Esophageal biopsy or brushings of the affected esophageal tissue are supportive but not required to make the proper diagnosis. Histological findings include absence of viable epithelium, abundant necrotic debris, and necrotic changes in the mucosa, possibly extending into the submucosa and even muscularis propria. Full-thickness necrosis has been described in the surgical specimens. Associated findings may include heavy leukocytic infiltrate, visible vascular thrombi, deranged muscle fibers, and severe inflammatory changes[2]. Biopsy specimens should be sent for bacterial, fungal, and viral cultures to exclude infectious etiologies associated with AEN or a superimposed infection[7,26]. Special care should be taken to evaluate for the presence of multinucleated giant cells or viral inclusion bodies, particularly in immunocompromised patients. At this time, the role of angiography for diagnosis of AEN is not clearly defined, likely related to its potential risks, lack of effective therapeutic intervention in the absence of a recognizable lesion, and tendency for a spontaneous recovery with correction of the underlying medical illness.
Upper gastrointestinal bleeding in a patient with multiple medical conditions, vasculopathy, hemodynamic compromise, general debilitation, malignancy, ischemic disease, and hypercoagulable state should raise the possibility of AEN among other diagnoses. A strong association between diabetic ketoacidosis and AEN has been suggested by some authors, with four cases of black esophagus seen in 29 hospital admissions (14%)[16].
DISEASE COURSE AND STAGING
Generally, uncomplicated AEN follows an indolent clinical course and has a predictable endoscopic and histopathological trajectory. The recently proposed staging system attempts to classify disease progression and detail the distinct phases of AEN[2]. Stage 0 designates a pre-necrotic viable esophagus. Stage 1 refers to an acutely diseased organ with an endoscopic picture dominated by a striking diffuse, circumferential, black-appearing esophageal mucosa with occasional yellow exudates and signs of friability, loss of light reflex, rigidity and under-distension of the lumen. These endoscopic findings nearly universally start at the GEJ, involve the distal esophagus, and variably extend proximally, potentially covering the entire organ. Histological appearance is notable for lack of viable squamous epithelium, presence of necrotic debris, and pronounced necrosis of the mucosa with possible extension into the submucosa and muscularis propria. Associated findings may include heavy leukocytic infiltrate with severe inflammatory changes, visible vascular thrombi, deranged muscle fibers, and mucosal infection with viral, fungal, and bacterial pathogens. Stage 2 describes the healing phase of the AEN dominated by residual black areas in the esophagus and thick white exudates composed of necrotic debris that cover friable pink mucosa. This “chess-board” appearance can sometimes be the presenting endoscopic picture of AEN on delayed endoscopy. The exact timing of this change is unknown and it likely parallels the underlying general condition of the patient. However, it has been observed as late as 1 mo after the diagnosis. Stage 2 is also notable for some improvement in histological appearance of the esophageal tissue, with scattered areas of necrosis among underlying cellular regeneration, granulation tissue, and inflammatory changes. As early as 1-2 wk after diagnosis, the esophageal mucosa acquires its normal endoscopic appearance in stage 3 of the AEN with only microscopically present granulation tissue, a sequelae of recent injury.
COMPLICATIONS
The most serious complication of AEN is perforation, which can occur in severe cases that result in full-thickness necrosis of the esophageal tissue. Its reported incidence is just below 7%[2], and should be suspected in rapidly decompensating patients. Traumatic or spontaneous rupture of the thoracic aorta resulting in extrinsic esophageal compression by hematoma, photodynamic therapy for esophageal cancer, herniated gastric volvulus, and severe thromboembolic disease place a patient at a higher risk for this complication. Perforation can be seen in stage 1 of the disease, and it may lead to rapid clinical deterioration, mediastinitis, mediastinal abscess formation, empyema, and generalized sepsis. Prompt recognition, intravenous antibiotics, and surgical intervention are life-saving. Mortality may be especially high given a generally poor underlying medical condition and a limited reserve in many patients with AEN.
Another possible sequela of AEN is the formation of stenotic areas or strictures, which can be seen in > 10% of patients. These findings typically occur in stages 2 and 3 of the disease and likely result from protective scar formation during the acute, reparative, and healing phase of AEN. Acid suppression followed by possible endoscopic dilatation, if needed, typically produces resolution of reported symptoms.
Microbial superinfection of necrotic media is an important consideration in treating patients with AEN[26]. Patients should be diligently monitored for early signs of clinical decompensation and sepsis with prompt institution of intravenous antimicrobial therapy.
MANAGEMENT
Development of AEN carries a generally poor prognosis and the goal of therapy should be directed at treating the coexisting medical diseases. Timely systemic resuscitation and patient stabilization play pivotal roles in the management of this disease. Intravenous hydration, correcting anemia with packed red blood cell transfusion, and keeping the patient nil-per-os should be instituted. Parenteral support may be needed in malnourished patients to improve their nutritional status and expedite healing. Passage of the nasogastric tube should not be undertaken, to avoid perforation.
Medical management of AEN includes aggressive intravenous proton pump inhibitor or histamine receptor blocker therapy until improvement in clinical status, at which time, a change to an oral formulation is appropriate. Probably, oral therapy should be continued for a few months after the resolution of symptoms to aid prevention of stricture formation, in the absence of underlying reflux disease. Recent reports of esophageal manometry and pH monitoring in patients with AEN have shown a lack of significant acid reflux and normal motility at 5-7 mo after recovery[23,65]. Oral suspension of sucralfate may be used as a single therapy or as a supplement to the intravenous acid-reducing agents, excluding the cases with concomitant renal failure.
Antimicrobial therapy is important in the setting of positive esophageal cultures, stains for fungal agents, or visualization of multinucleated giant cells or inclusion bodies on histological evaluation of the biopsy specimen. Empirical antibiotics should be initiated in cases of suspected esophageal perforation, rapid clinical decompensation, unexplained fevers, and immune compromise in AIDS, cirrhosis, transplant recipient, and dialysis patients. Prophylactic use of antibiotics in sterile necrosis is probably not necessary and few reports have potentially linked the antibiotic use itself to the development of AEN[49].
Surgical intervention in patients with AEN is reserved for perforated esophagus with resultant mediastinitis and abscess formation. Esophagectomy, decortication, lavage, and delayed reconstruction may be performed in addition to standard surgical approaches in the setting of gastric volvulus or transected thoracic aorta. Primary closure of the perforated esophageal tissue should not be attempted. Subtotal esophagectomy and esophagogastrostomy have been reported in AEN-induced esophageal stricture that was refractory to repeated dilatations[3,26].
PROGNOSIS AND ROLE OF REPEAT ENDOSCOPY
Prognosis of AEN largely depends on coexisting medical conditions and ordinarily parallels the general state of health of a patient. As a rule, overall prognosis is poor with nearly one third of patients succumbing to the underlying critical illness. However, mortality specific to the AEN is much lower, at around 6%[2]. Important risk factors include esophageal perforation, diabetic ketoacidosis, and compromised immune system. Overall, reversible phenomena of the disease and its tendency for spontaneous healing are favorable factors in predicting complete recovery in otherwise healthy individuals. Relapsing AEN has been reported only once[66] but may be an important diagnosis to consider in the appropriate clinical setting. It may also explain some of the observed color changes of black esophagus reported in the literature[4]. Repeat endoscopic evaluation should be done to assess mucosal healing and document resolution of AEN, as well as to determine the duration of antacid therapy. Endoscopic sessions with balloon dilatation may be necessary in patients suffering from dysphasia in the setting of esophageal stenosis or stricture formation, which are well-known complications of AEN.
CONCLUSION
AEN is a rare syndrome that arises in the characteristic clinical setting of combined ischemia from hemodynamic compromise, gastric outlet obstruction with backflow chemical injury, and inadequate protective barriers and physiological reserve due to critical illness and general deconditioning. It presents with signs of upper gastrointestinal bleeding and has a characteristic appearance on upper endoscopy of circumferential, diffuse, black-appearing, friable esophageal mucosa, almost universally affecting the distal part of the organ and abruptly ending at the GEJ. Histological tissue necrosis confirms the diagnosis. Treatment relies on aggressive resuscitation, correction of underlying medical conditions, institution of therapy with proton pump inhibitors and sucralfate, and monitoring for signs of infection or perforation. Mortality is high and is largely related to the principal medical conditions and the gravity of the overall state of health.
Peer reviewers: Volker F Eckardt, MD, Professor, Chief, Department of Gastroenterology, Deutsche Klinik für Diagnostik, Aukammallee 33, 65191 Wiesbaden, Germany; Akihito Nagahara, Associate Professor, Department of Gastroenterology, Juntendo University School of Medicine, 2-1-1 Hongo Bunkyo-ku, Tokyo 113-8421, Japan
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH