Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2812
Revised: March 22, 2010
Accepted: March 29, 2010
Published online: June 14, 2010
AIM: To investigate gastrointestinal (GI) symptoms in peritoneal dialysis (PD) patients and to explore related factors contributing to GI symptoms.
METHODS: One hundred and twelve patients undergoing PD participated in the study. The gastrointestinal symptom rating scale was used for measuring GI symptoms. Information on age, height, weight, body mass index, disease leading to chronic renal failure, history of corticosteroid therapy, presence of predialytic GI symptoms, daily dosage of pills, and duration, type and daily dialysate volume of PD was obtained by interviewing patients and/or reviewing the medical records. Hemoglobin, albumin and Kt/V data were obtained from follow-up database. We used multiple regression analysis with stepwise backward variable selection to test for factors predicting GSRS scores with significance level of selection entry at 0.05 and selection of stay at 0.10.
RESULTS: The prevalence of eating dysfunction, reflux and indigestion in the PD patients was 44.2%, 32.7%, 32.7%, respectively. A history of corticosteroid therapy (b = 8.93, P < 0.001) and all pills daily intake (b = 0.16, P = 0.007) were positively correlated to GI symptoms, while residual renal Kt/V (b = -3.47, P = 0.009) was negatively correlated to GI symptoms. Other factors were proven to be not associated with GI symptoms, with P > 0.05.
CONCLUSION: Eating dysfunction, reflux and indigestion were common in PD patients. Daily dosage of pills and corticosteroid history predicted GI symptoms, while residual renal function prevented them.
- Citation: Dong R, Guo ZY. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: Multivariate analysis of correlated factors. World J Gastroenterol 2010; 16(22): 2812-2817
- URL: https://www.wjgnet.com/1007-9327/full/v16/i22/2812.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i22.2812
Gastrointestinal (GI) symptoms are common in patients with chronic renal failure (CRF)[1-3], especially in patients having continuous ambulatory peritoneal dialysis (CAPD)[4]. A variety of GI symptoms in CAPD patients have been reported[4,5], of which, gastroesophageal reflux symptoms (GERS), dyspepsia and eating dysfunction seem to be the most common ones[4,5].
Anderson et al[6] reported that 44.7% of PD patients had frequent gastroesophageal reflux disease (GERD) and that age < 60 years, smoking, and body mass index (BMI) ≥ 27 predicted GERD; in contrast, sex, race, diabetes, PD, non steroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers (CCBs), and coffee and alcohol use did not. Stojakowska et al[7] have proven a negative correlation between GERD symptom score index and normalized protein catabolic rate (nPCR), and a positive correlation between GERD symptom score and the time from onset of CAPD, through observation in 43 patients. It is not clear whether PD per se is a risk factor for GERD[6].
It is the high prevalence of GI symptoms in PD patients that raises questions about contributing factors and other possible factors, but previous studies obtained controversial results with a relatively small sample size. Whether the onset of these GI symptoms is related to the chronic renal failure itself, its treatment, or, alternatively, other factors, is still unknown.
The aim of this study was to investigate GI symptoms in CAPD patients and to explore the possible correlated factors contributing to these symptoms.
Patients gave informed consent and the study was approved by the Ethics Committee of Changhai Hospital, Shanghai, China.
The patients on active PD were recruited from the PD unit in Changhai Hospital. They consisted of in-patients and out-patients who maintained PD for at least three months. Patients with dementia, severe infectious illness, hepatocholecystopathy, peritonitis in the last three months, unstable blood pressure or glucose levels, and unwillingness to participate in the study were excluded.
To evaluate the presence of GI symptoms in PD patients, they were asked to complete the gastrointestinal symptom rating scale (GSRS) measuring GI symptoms in general.
The GSRS, a self-administered questionnaire, includes 15 items and uses a 7-grade Likert scale defined by descriptive anchors such that 1 = none, 2 = minor, 3 = mild, 4 = moderate, 5 = moderately severe, 6 = severe, and 7 = very severe discomfort. The questionnaire was originally constructed as an interview-based rating scale designed to evaluate a wide range of GI symptoms[8] and was later modified to become a self-administered questionnaire[9]. The items can be grouped into five dimensions: abdominal pain syndrome (three items), reflux syndrome (two items), indigestion syndrome (four items), diarrhea syndrome (three items), and constipation syndrome (three items). One dimension, eating dysfunction, which was developed in a manner analogous to the GSRS[10], was also considered relevant for the study and added to the original GSRS. Eating dysfunction dimension includes questions concerning early satiety, difficulties in eating normal portions, and postprandial pain. The questions concern symptom severity during the previous two weeks. A dimension score was calculated as the mean value of the items belonging to the specific syndrome with a minimum value of 1 and a maximum value of 7.
By interviewing patients and/or reviewing the medical records we obtained information on age, height, weight, BMI, disease leading to CRF, history of corticosteroid therapy, presence of predialytic GI symptoms, daily dosage of pills and duration, type and daily dialysate volume of PD. The latest serum hemoglobin (HGB), albumin (ALB), and Kt/V urea, as an index of dialysis adequacy, were obtained from the follow-up database. Kt/V were calculated by Daugirdas Formula[11].
Data were presented as mean (SD) for continuous variables that were approximately normally distributed, as median and interquartile range for skewed continuous variables, and as percentage for categorical variables. We used multiple regression analysis with stepwise variable selection to test for factors that predicted the GSRS scores with significance level of selection entry at 0.05 and selection of stay at 0.10. Results were considered significant when P < 0.05. All analyses were performed with SPSS for Windows, version 16.0.
In total, one hundred and twelve PD patients were approached to participate in the study and completed the questionnaires. Table 1 presents characteristics of the included PD patients. The sex distribution among PD patients was 61 men and 51 women. The mean age among PD patients was 59.67 (14.18) years and the mean BMI was 23.26 (4.27) kg/m2. Patients with diabetes mellitus (DM) made up 24.1% of the study population in total and a majority (87.5%) of PD patients had no GI symptoms before the start of PD. The median duration of PD was 15.00 (8.00-33.00) mo and 53.6% of the patients underwent CAPD.
| Clinical features | Patient (n = 112) |
| Age (mean ± SD) (yr) | 59.67 ± 14.18 |
| Sex | |
| Male | 61 (54.5) |
| Female | 51 (45.5) |
| BMI, mean (SD) (kg/m2) | 23.26 (4.27) |
| DM status | |
| DM | 27 (24.1) |
| Non-DM | 85 (75.9) |
| Disease leading to chronic renal failure | |
| Chronic glomerulonephritis | 40 (35.7) |
| Primary hypertension | 31 (27.7) |
| Diabetes mellitus | 22 (19.6) |
| Polycystic kidney disease | 7 (6.2) |
| Gout | 3 (2.7) |
| Primary hypertension combined with gout | 3 (2.7) |
| Obstructive nephropathy | 2 (1.8) |
| Chronic interstitial nephritis | 1 (0.9) |
| Nephrotic syndrome | 1 (0.9) |
| Ischemic renal disease | 1 (0.9) |
| Microscopic Polyarteritis | 1 (0.9) |
| Steroid history | |
| Yes | 8 (7.1) |
| No | 104 (92.9) |
| Daily dosage of pills, median (interquartile ranges)1 | 12.00 (6.00-21.25) |
| Predialytic GI symptoms | |
| Yes | 14 (12.5) |
| No | 98 (87.5) |
| PD Duration, median (interquartile ranges) (mo)2 | 15.00 (8.00-33.00) |
| Type of PD | |
| CAPD | 60 (53.6) |
| IPD | 50 (44.6) |
| Daily peritoneal dialysate volume (mean ± SD) (L)3 | 7.83 ± 1.28 |
| Albumin (mean ± SD) (g/L)4 | 33.96 ± 4.77 |
| Hemoglobin (mean ± SD) (g/L)5 | 101.24 ± 20.06 |
| Residual renal Kt/V, median (interquartile ranges)6 | 0.39 (0.00-0.82) |
| Peritoneal Kt/V (mean ± SD)6 | 1.51 ± 0.39 |
| Total Kt/V (mean ± SD)6 | 2.00 ± 0.51 |
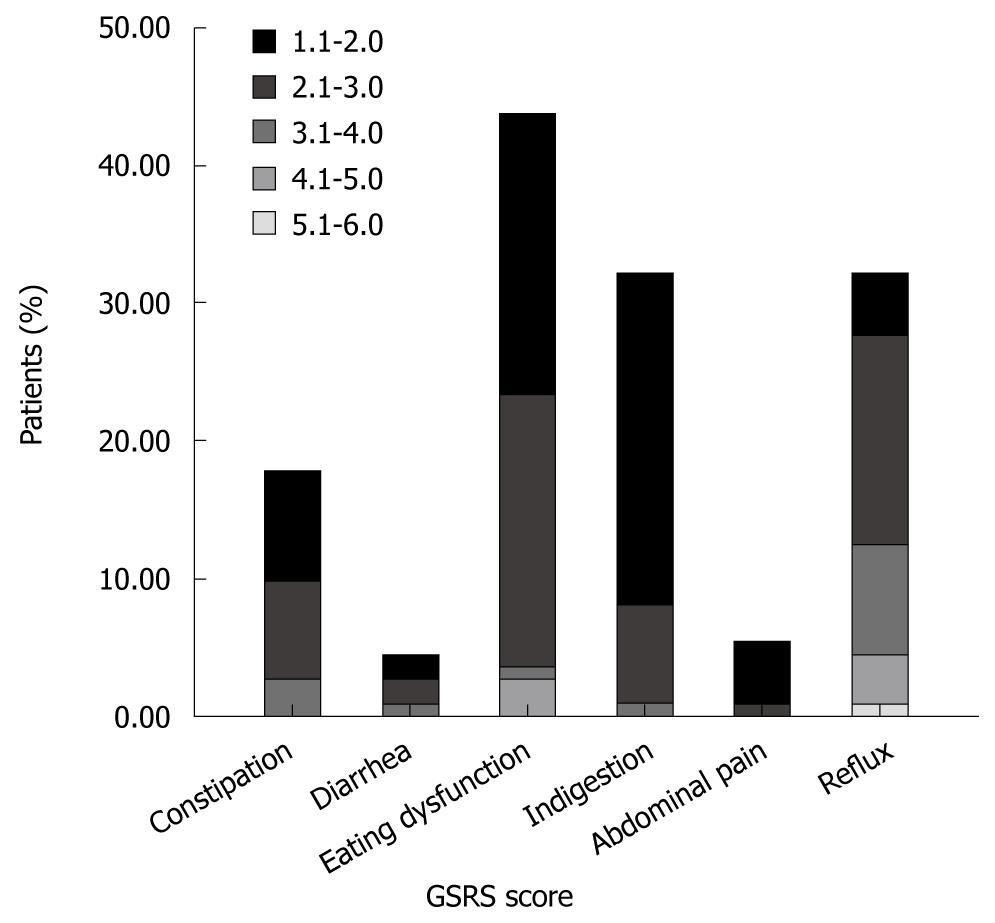
The prevalence of troublesome GI symptoms (GSRS > 1) was 61.6% for any dimension, 44.2% for eating dysfunction, 32.7% for reflux, 32.7% for indigestion, 18.6% for constipation, 6.2% for abdominal pain, and 5.3% for diarrhea (Figure 1). The mean GSRS scores for eating dysfunction were 1.57 (0.84), for reflux 1.71 (1.15), for indigestion 1.32 (0.56), for constipation 1.23 (0.58), for diarrhea 1.07 (0.35), for abdominal pain 1.04 (0.19).
There was no significant relationship between the GSRS scores and age, BMI, hemoglobin, albumin, presence of diabetes, presence of predialytic GI symptoms, peritoneal Kt/V, and duration, type and daily dialysate volume of PD, with P > 0.05 (Table 2).
| Variables | t | P-value |
| Age | 1.96 | 0.54 |
| DM status | 1.01 | 0.31 |
| BMI | -0.50 | 0.62 |
| Hemoglobin | 0.82 | 0.42 |
| Albumin | 0.68 | 0.50 |
| Predialytic GI symptoms | 0.49 | 0.62 |
| PD type | -0.89 | 0.38 |
| Duration of PD | -0.58 | 0.57 |
| Daily dialysate volume | -1.15 | 0.26 |
| Peritoneal Kt/V | -0.21 | 0.84 |
| Total Kt/V | -0.24 | 0.81 |
There were three statistically significant predictors for the GSRS scores (Table 3). A history of corticosteroid therapy (b = 8.93, P < 0.001) was significantly related to the GSRS scores and its coefficient was positive, indicating that if a patient had a history of corticosteroid therapy, he/she would attain higher GSRS scores than a patient who had not. Next, the daily dosage of pills as patients daily intake (b = 0.16, P = 0.007) was significant and its coefficient was also positive, indicating that the more pills patients took daily, the higher the GSRS scores. Finally, the residual renal Kt/V (b = -3.47, P = 0.009) was significant and its coefficient was negative which would indicate that higher residual renal Kt/V was related to lower GSRS scores (Table 3).
| Variables | Unstandardized coefficients | Standardized coefficients | t | P value | |
| B | SD | β | |||
| Constant | 21.89 | 1.25 | - | 17.58 | < 0.001 |
| History of corticosteroids | 8.93 | 2.13 | 0.39 | 4.20 | < 0.001 |
| Daily dosage of pills | 0.16 | 0.06 | 0.26 | 2.80 | 0.007 |
| Residual renal Kt/V | -3.47 | 1.30 | -0.25 | -2.68 | 0.009 |
The present study shows that gastrointestinal symptoms are common in PD patients, especially eating dysfunction (44.2%), reflux and indigestion (both 32.7%). A history of corticosteroid therapy (b = 8.93, P < 0.001) and the dosage of pills patients took daily (b = 0.16, P = 0.007) were positively related to GSRS scores, whereas residual renal Kt/V (b = -3.47, P = 0.009) was negatively correlated to GSRS scores. Other suspected factors were not statistically related to GSRS scores, including age, BMI, hemoglobin, albumin, presence of diabetes, presence of predialytic GI symptoms, peritoneal Kt/V, total Kt/V and duration, type and daily dialysate volume of PD.
More than half of the PD patients had various gastrointestinal complaints and patients who had a history of corticosteroid therapy seemed to be susceptible to gastrointestinal symptoms. The more pills patients took daily, the more complaints of GI symptoms. Otherwise, residual renal function seemed to be a protective factor for GI symptoms in PD patients since the higher the residual renal Kt/V, the less GI symptom complaints. In this study, PD specific factors including peritoneal Kt/V, total Kt/V, duration, type and daily dialysate volume of PD, were proven to contribute little to these symptoms.
GI symptoms are common in PD patients with a prevalence ranging from 43% to 58%[5-7,12]. Our results with a high prevalence of eating dysfunction, indigestion and reflux symptoms are in line with these previous studies. Furthermore, these three symptoms were also shown to have the highest prevalence of all GI symptoms in PD patients in a recent study[5]. The underlying pathophysiological mechanisms might be (1) Delayed gastric emptying, which is common in CRF patients. Strid et al[13] found that PD patients had longer gastric emptying time than predialytic state, but other studies found no obvious effect of dialysate on gastric emptying[14-16]; (2) Increased intraperitoneal pressure (IPP). Dejardin et al[17] found the occurrence of GERS was not different for patients with elevated day and night IPP; (3) Decreased lower esophageal sphincter pressure (LESP). Kim et al[18] demonstrated that CAPD patients with upper GI symptoms had lower LESP at 2000 mL of infused dialysate than patients without. In contrast, Hylander et al[19] found no systematic changes in intragastric or LESPs at any time of CAPD; and (4) Other factors. Aguilera et al[20] discovered GI abnormalities were negatively associated with nutrition. Van et al[21] have demonstrated a glucose-based dialysate in the abdomen of PD patients with delayed gastric emptying.
As we know, PD patients are in a complicated clinical condition and clinical manifestations are affected by many factors, among which drug taking is an important one. However, drugs have some adverse effects. In general, patients who have a history of corticosteroid therapy are more susceptible to GI symptoms than patients who haven’t, which also applies to PD patients. Not unexpectedly, we found corticosteroid taking history alone accounted for 18.9% of GSRS scores, which is probably due to injury of the digestive system caused by corticosteroids. High prevalence of GI symptoms was also found in a transplant population and a rheumatism population[22,23], who both had a large consumption of corticosteroids.
Besides drug history, the daily dosage of pills patients take also plays an important role in causing GI symptoms. To our knowledge, this is the first study counting pills of patients’ daily intake and looking for an association between the amount of pills and GI symptoms. We did find that the more pills taken, the more severe the GI symptoms. In our study, the average amount of pills a PD patient took daily was almost 15, and the maximum amount was 51, which consisted of CCBs, angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), statins, active vitamin D, iron agents, ketoacids, anti-platelet drugs and varieties of Chinese medicine, which were especially common in China. The underlying mechanisms are not fully understood.
Compared to hemodialysis (HD), PD is thought to provide better preservation of residual renal function (RRF), which in many studies partly accounts for reduction of mortality risk, decreasing inflammation factors, declining oxidative and carbonyl stress[24-27]. In addition, in our study, another highlight of RRF we discovered was that it can prevent PD patients from developing GI symptoms. Apart from residual renal Kt/V, peritoneal Kt/V and total Kt/V were not proven to be correlated to GI symptom scores. These results indicated that the beneficial effect of residual renal clearance and peritoneal clearance were not equivalent with regard to patient outcome, and residual renal function may play a much more important role. Previous study has also shown that for each mL/min per 1.73 m2 increase in residual renal glomerular filtration rate (GFR), a 12% reduction in mortality rate was found[26]. In contrast, no significant effect of peritoneal clearance on patient survival was established[26]. Regarding this, GI symptoms in PD patients were more associated with the uremia itself, therefore PD specific factors were not involved.
Previous study has concluded that age < 60 and BMI ≥ 27 predicted GERD in the general population, while diabetes and PD did not[6]. However, we found neither age, BMI nor diabetes with PD predicted GI symptoms in PD patients. This may be due to the limitation in sample size. Furthermore, albumin, as an index of nutrition, and duration of PD were not related to GI symptoms in our study. However, other studies obtained controversial results. A negative correlation between GERD symptom score and nPCR, and a positive correlation between GERD symptom score and the duration of CAPD, were found through observation in 43 patients[7]. Moreover, analyses of 99 dialytic patients revealed that gastrointestinal symptom scores were not different in hypoalbuminemic and normoalbuminemic patients[28]. In addition, gastrointestinal life quality was found not to be correlated with the duration of PD treatment[29]. Factors which contributed to these conflicting results might be the sample size and the different ways used in evaluating the GI symptoms.
Our study included only CAPD and intermittent peritoneal dialysis (IPD) patients and neither of these groups was independently associated with GI symptom scores. Further evaluation in a larger population with different types of PD is needed for finding out the relation between PD type and GI symptoms.
Volume of dialysis fluid used was also a non-predictor. Another study recruited 61 PD patients and showed a strong linear correlation between IPP and intraperitoneal volume, but failed to find any influence of IPP on the occurrence of GERS except that patients with GERS had a higher BMI[17]. The increased IPP was still not a rational explanation for the high prevalence of GI symptoms in the PD population.
One limitation of the present study is the relatively small number of samples, which may influence the power of statistical tests. Some factors, such as age, daily dosage and PD type showed a tendency to be correlated with GI symptoms, but correlation did not reach statistical significance because of the fact that 31 of 112 samples were excluded from the multiple regression model for the missing value of Kt/V and we only recruited patients with CAPD and IPD. Another limitation refers to the probable subjective bias in answering the questionnaire. Only one self-administered questionnaire was used in the study. Still another limitation refers to the lack of a control group consisting of patients undergoing HD.
In conclusion, the present study demonstrates a high prevalence of troublesome GI symptoms in PD patients, a positive correlation between history of corticosteroid use, the amount of pills patients take daily, and GI symptoms, and a negative correlation between residual renal function and GI symptoms. GI symptoms are more common in PD than in HD patients as well as compared to the predialytic population[4] which strongly suggests that PD treatment is a putative cause of GI symptoms. However, the present study failed to prove a relationship between PD specific factors and GI symptoms. Therefore, further evaluation in a larger population of PD patients with a control group is needed.
Gastrointestinal (GI) symptoms are more common in patients undergoing peritoneal dialysis (PD) than in patients with chronic renal failure undergoing hemodialysis, though the cause and the correlated factors are largely unknown. Uremia itself and the impaired digestive system caused by PD are the main suspected causes.
PD patients are proven to have a higher prevalence of GI symptoms. Many studies have focused on the impact of dialysate on the gastrointestinal tract but obtained controversial results. In this study, the authors demonstrate that gastrointestinal complaints in PD patients are more related to a history of corticosteroid therapy, the number of pills taken daily and residual renal function rather than effects of the dialysate.
Recent reports have highlighted the high prevalence of GI symptoms in PD patients and their poor treatment. This is the first study exploring possible correlated factors from almost all details in the life of PD patients. In addition, this is the first study demonstrating that dialysate is not the main cause of GI symptoms in PD patients. Furthermore, this study suggests that GI symptoms in PD patients may be affected by residual renal function and drug history.
By understanding what factors are related to GI symptoms in PD patients, this study may represent a future strategy for prevention and intervention in the treatment of PD patients with GI symptoms.
GSRS, Gastrointestinal Symptom Rating Scale, a self-administered questionnaire, includes 15 items and uses a 7-grade Likert scale defined by descriptive anchors such that 1 = none, 2 = minor, 3 = mild, 4 = moderate, 5 = moderately severe, 6 = severe, and 7 = very severe discomfort. The items can be grouped into five dimensions: abdominal pain syndrome (three items), reflux syndrome (two items), indigestion syndrome (four items), diarrhea syndrome (three items), and constipation syndrome (three items). One dimension, eating dysfunction, which was developed in a manner analogous to the GSRS, was also considered relevant for the study and added to the original GSRS.
This is a straightforward, well done study with straightforward conclusions. I hope the authors will take the next step and perform a more rigorous study with 2 control arms (chronic renal failure and HD) in a prospective fashion.
Peer reviewer: Eric S Hungness, MD, FACS, Assistant Professor, Division of Gastrointestinal and Oncologic Surgery, Northwestern University Feinberg School of Medicine, 676 N. St. Clair St., Suite 650, Chicago, IL 60611-2908, United States
S- Editor Wang YR L- Editor Logan S E- Editor Ma WH
| 1. | Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, Hartley I, Maxwell D. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102:1990-1997. [Cited in This Article: ] |
| 2. | Hammer J, Oesterreicher C, Hammer K, Koch U, Traindl O, Kovarik J. Chronic gastrointestinal symptoms in hemodialysis patients. Wien Klin Wochenschr. 1998;110:287-291. [Cited in This Article: ] |
| 3. | Fallone CA, Mayrand S. Gastroesophageal reflux and hyperacidity in chronic renal failure. Perit Dial Int. 2001;21 Suppl 3:S295-S299. [Cited in This Article: ] |
| 4. | Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17:1434-1439. [Cited in This Article: ] |
| 5. | Strid H, Fjell A, Simrén M, Björnsson ES. Impact of dialysis on gastroesophageal reflux, dyspepsia, and proton pump inhibitor treatment in patients with chronic renal failure. Eur J Gastroenterol Hepatol. 2009;21:137-142. [Cited in This Article: ] |
| 6. | Anderson JE, Yim KB, Crowell MD. Prevalence of gastroesophageal reflux disease in peritoneal dialysis and hemodialysis patients. Adv Perit Dial. 1999;15:75-78. [Cited in This Article: ] |
| 7. | Stojakowska M, Błaut U, Smoleńiski O, Thor PJ. [Gastroesophageal reflux disease and its’ influence on nutritional status in patients treated with peritoneal dialysis]. Folia Med Cracov. 2005;46:59-66. [Cited in This Article: ] |
| 8. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [Cited in This Article: ] |
| 9. | Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681-687. [Cited in This Article: ] |
| 10. | Svedlund J, Sullivan M, Liedman B, Lundell L. Long term consequences of gastrectomy for patient’s quality of life: the impact of reconstructive techniques. Am J Gastroenterol. 1999;94:438-445. [Cited in This Article: ] |
| 11. | Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205-1213. [Cited in This Article: ] |
| 12. | Lee SW, Song JH, Kim GA, Yang HJ, Lee KJ, Kim MJ. Effect of dialysis modalities on gastric myoelectrical activity in end-stage renal disease patients. Am J Kidney Dis. 2000;36:566-573. [Cited in This Article: ] |
| 13. | Strid H, Simrén M, Stotzer PO, Abrahamsson H, Björnsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39:516-520. [Cited in This Article: ] |
| 14. | Hubalewska A, Stompór T, Płaczkiewicz E, Staszczak A, Huszno B, Sułowicz W, Szybiński Z. Evaluation of gastric emptying in patients with chronic renal failure on continuous ambulatory peritoneal dialysis using 99mTc-solid meal. Nucl Med Rev Cent East Eur. 2004;7:27-30. [Cited in This Article: ] |
| 15. | Fernström A, Hylander B, Grybäck P, Jacobsson H, Hellström PM. Gastric emptying and electrogastrography in patients on CAPD. Perit Dial Int. 1999;19:429-437. [Cited in This Article: ] |
| 16. | Guz G, Bali M, Poyraz NY, Bagdatoglu O, Yeğin ZA, Doğan I, Atasever T, Sert S, Sindel S. Gastric emptying in patients on renal replacement therapy. Ren Fail. 2004;26:619-624. [Cited in This Article: ] |
| 17. | Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant. 2007;22:1437-1444. [Cited in This Article: ] |
| 18. | Kim MJ, Kwon KH, Lee SW. Gastroesophageal reflux disease in CAPD patients. Adv Perit Dial. 1998;14:98-101. [Cited in This Article: ] |
| 19. | Hylander BI, Dalton CB, Castell DO, Burkart J, Rössner S. Effect of intraperitoneal fluid volume changes on esophageal pressures: studies in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1991;17:307-310. [Cited in This Article: ] |
| 20. | Aguilera A, Bajo MA, Espinoza M, Olveira A, Paiva AM, Codoceo R, Garca P, Sánchez S, Celadilla O, Castro MJ. Gastrointestinal and pancreatic function in peritoneal dialysis patients: their relationship with malnutrition and peritoneal membrane abnormalities. Am J Kidney Dis. 2003;42:787-796. [Cited in This Article: ] |
| 21. | Van V, Schoonjans RS, Struijk DG, Verbanck JJ, Vanholder RC, Van B, Lefebvre RA, De V, Lameire NH. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int. 2002;22:32-38. [Cited in This Article: ] |
| 22. | Herrero JI, Benlloch S, Bernardos A, Bilbao I, Castells L, Castroagudin JF, González L, Irastorza I, Navasa M, Otero A. Gastrointestinal complications in liver transplant recipients: MITOS study. Transplant Proc. 2007;39:2311-2313. [Cited in This Article: ] |
| 23. | Karateev AE, Nasonova VA, Murav’ev IuV. [The assessment of the effect of glucocorticosteroid and nonsteroidal anti-inflammatory preparations on the development of an erosive-ulcerative lesion of the gastrointestinal tract in patients with rheumatic diseases]. Ter Arkh. 1999;71:26-30. [Cited in This Article: ] |
| 24. | Sanabria M, Muñoz J, Trillos C, Hernández G, Latorre C, Díaz CS, Murad S, Rodríguez K, Rivera A, Amador A. Dialysis outcomes in Colombia (DOC) study: a comparison of patient survival on peritoneal dialysis vs hemodialysis in Colombia. Kidney Int Suppl. 2008;S165-S172. [Cited in This Article: ] |
| 25. | Furuya R, Kumagai H, Odamaki M, Takahashi M, Miyaki A, Hishida A. Impact of residual renal function on plasma levels of advanced oxidation protein products and pentosidine in peritoneal dialysis patients. Nephron Clin Pract. 2009;112:c255-c261. [Cited in This Article: ] |
| 26. | Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293-1302. [Cited in This Article: ] |
| 27. | Cueto-Manzano AM, Rojas-Campos E, Martínez-Ramírez HR, Valera-González I, Medina M, Monteón F, Ruiz N, Becerra M, Palomeque MA, Cortés-Sanabria L. Can the inflammation markers of patients with high peritoneal permeability on continuous ambulatory peritoneal dialysis be reduced on nocturnal intermittent peritoneal dialysis? Perit Dial Int. 2006;26:341-348. [Cited in This Article: ] |
| 28. | Silang R, Regalado M, Cheng TH, Wesson DE. Prokinetic agents increase plasma albumin in hypoalbuminemic chronic dialysis patients with delayed gastric emptying. Am J Kidney Dis. 2001;37:287-293. [Cited in This Article: ] |
| 29. | Heine GH, Kastner CY, Jahnke T, Köhler H, Kuhlmann MK. Does a history of peritoneal dialysis result in an impaired gastrointestinal life quality? Hemodial Int. 2007;11:461-467. [Cited in This Article: ] |