Published online Jun 7, 2010. doi: 10.3748/wjg.v16.i21.2638
Revised: March 14, 2010
Accepted: March 21, 2010
Published online: June 7, 2010
AIM: To investigate the efficacy of Viusid, a nutritional supplement, as an antioxidant and an immunomodulator in patients with chronic hepatitis C.
METHODS: Sixty patients with chronic hepatitis C who were non-responders to standard antiviral treatment were randomly assigned to receive Viusid (3 sachets daily, n = 30) or placebo (n = 30) for 24 wk. The primary outcome was the change in serum malondialdehyde and 4-hydroxyalkenals (lipid peroxidation products). Secondary outcomes were changes in serum tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-10 (IL-10).
RESULTS: Statistically significant reductions in serum 4-hydroxyalkenals and malondialdehyde levels were observed in both groups in comparison with pretreatment values, but the patients who received Viusid showed a more marked reduction as compared with the control group (P = 0.001). TNF-α levels significantly increased from 6.9 to 16.2 pg/mL (P < 0.01) in the patients who received placebo in comparison with almost unchanged levels, from 6.6 to 7.1 pg/mL (P = 0.26), in the patients treated with Viusid (P = 0.001). In addition, IL-10 levels were markedly increased in the patients treated with Viusid (from 2.6 to 8.3 pg/mL, P = 0.04) in contrast to the patients assigned to placebo (from 2.8 to 4.1 pg/mL, P = 0.09) (P = 0.01). Likewise, the administration of Viusid markedly increased mean IFN-γ levels from 1.92 to 2.89 pg/mL (P < 0.001) in comparison with a reduction in mean levels from 1.80 to 1.68 pg/mL (P = 0.70) in the placebo group (P < 0.0001). Viusid administration was well tolerated.
CONCLUSION: Our results indicate that treatment with Viusid leads to a notable improvement of oxidative stress and immunological parameters in patients with chronic hepatitis C.
- Citation: Gomez EV, Perez YM, Sanchez HV, Forment GR, Soler EA, Bertot LC, Garcia AY, del Rosario Abreu Vazquez M, Fabian LG. Antioxidant and immunomodulatory effects of Viusid in patients with chronic hepatitis C. World J Gastroenterol 2010; 16(21): 2638-2647
- URL: https://www.wjgnet.com/1007-9327/full/v16/i21/2638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i21.2638
Hepatitis C virus (HCV) infection is one of the most important causes of chronic liver disease leading to cirrhosis and hepatocellular carcinoma (HCC)[1]. Peginterferon plus ribavirin is the only therapeutic alternative with proven efficacy in patients with chronic hepatitis C (CHC)[2]. Unfortunately, sustained virological response is achieved in only approximately 50% of the patients. HCV protease and polymerase inhibitors have been evaluated recently, but the increased rate of adverse events and discontinuations reported during the trials may possibly preclude their use in clinical practice[3,4].
The exact pathogenesis of liver injury and fibrosis has not been fully elucidated. Previous studies have found increased oxidative damage in patients chronically infected with HCV[5,6]. These findings have been supported by increased levels of lipid peroxidation (LPO) products (such as malondialdehyde and hydroxynonenal), an overabundance of reactive oxygen species, elevated superoxide dismutase (SOD) activity and reduced levels of glutathione (GSH) in serum or erythrocytes of patients with CHC compared with healthy controls[5-9].
Similarly, cytokine dysregulation is thought to play a crucial role in the persistence of viral infection and as a key mediator in the inflammatory and fibrogenic processes of patients with HCV infection[10].
Studies have shown that increased production of interferon-γ (IFN-γ) is strongly associated with spontaneous resolution of HCV infection compared with scant production in patients with persistent infection[11,12]. Likewise, it has been reported that patients with chronic HCV infection show enhanced serum interleukin (IL)-10 concentration[13,14]; however, long-term IL-10 therapy to treat chronically HCV-infected patients leads to a significant improvement of inflammation and fibrosis[15].
Several studies have suggested a positive association between increased serum tumor necrosis factor-α (TNF-α), IL-1 and -6 levels, and the degree of hepatic inflammation and fibrosis in patients with CHC[16,17].
This provides a rationale for studies using antioxidant and immunomodulatory agents; however, trials involving these agents have not clearly demonstrated their potential benefits, in part due to the limitations of non-randomized studies with small sample size, lack of dependable end points, and poor understanding of the role of different antioxidants in CHC[18-23].
There is an obvious need for the continuous development of new treatment strategies for CHC. Thus, the administration of compounds with antioxidant and immunomodulatory properties could be a plausible strategy to halt the natural course of the disease, particularly in patients with poor response to antiviral therapy. Viusid, a nutritional supplement, contains different molecules (ascorbic acid, zinc, and glycyrrhizic acid) with recognized antioxidant properties (Table 1)[24-26]. Its different chemical compounds are activated through a molecular activation principle that strongly increases their biological activity without modifying their physical structure. Recently, Vilar Gomez et al[27] reported that the addition of Viusid to the conventional interferon/ribavirin therapy was associated with significant histologic and biochemical improvements, especially in patients without sustained virological response. They suggested that the Viusid-related effect on histologic features, especially fibrosis, appears to be associated with antioxidant and/or immunomodulatory properties. All of these effects could modulate the histologic pattern of CHC, especially inflammation and fibrosis, in an attempt to halt disease progression. Therefore, we conducted a randomized double-blind and placebo-controlled study to evaluate whether Viusid may have a beneficial effect on oxidative stress and cytokine parameters in patients with chronic hepatitis C.
| Malic acid | 0.666 g | Ascorbic acid | 0.020 g |
| Glycyrrhizic acid | 0.033 g | Folic acid | 66 mcg |
| Glucosamine | 0.666 g | Cyanocobalamine | 0.3 mcg |
| Arginine | 0.666 g | Zinc sulfate | 0.005 g |
| Glycine | 0.333 g | Pyridoxal | 0.6 mg |
| Calcium pantothenate | 0.002 g |
We recruited 60 patients with chronic hepatitis C diagnosis at a tertiary care center (National Institute of Gastroenterology, Havana, Cuba).
Inclusion criteria included male and female patients of 18 to 65 years of age who had a positive test for anti-HCV antibody, HCV RNA detectable in serum by polymerase chain reaction (PCR), persistently elevated ALT at least on two occasions, liver biopsy consistent with chronic hepatitis, non-responders to previous treatment with peginterferon plus ribavirin, and absence of significant alcohol ingestion (weekly ethanol consumption of more than 40 g).
Exclusion criteria included presence of any other form of liver disease, positive screening for viral hepatitis A and B and human immunodeficiency virus (HIV), pregnancy or lactation, decompensated cirrhosis, concomitant disease with reduced life expectancy, severe psychiatric conditions, drug dependence, evidence of liver cancer at entry into the study on the basis of ultrasonography and α-fetoprotein levels less than 50 ng/L, and refusal to participate in the study.
The study was conducted in compliance with the Declaration of Helsinki and approved by the ethics committee and the institutional review board of the National Institute of Gastroenterology. All patients provided written informed consent for participation. The trial had been registered at ClinicalTrials.gov (NCT00778843).
After initial evaluation, all patients who met the eligibility criteria were consecutively enrolled in the study. They were randomly assigned to receive: Viusid (3 sachets daily, n = 30) or placebo (3 sachets daily, n = 30) for 24 wk. Viusid was provided by Catalysis, S.L. (Madrid, Spain).
Randomization was conducted by allocation into blocks of 4 (block randomization). It was performed by a health worker experienced in randomization techniques who was not involved in the evaluation or treatment of the participants. The physicians, study coordinators, and patients did not have access to the randomization scheme.
The patients, investigators, and study coordinators were blinded as to the treatment administered. Catalysis, Spain provided Viusid and placebo sachets. There was no difference in appearance, smell or flavor between Viusid and placebo.
All patients were evaluated at baseline and at monthly intervals by an experienced hepatologist who strongly encouraged the subjects to accomplish the treatment.
Clinical assessment, including body weight, liver tests, glucose, creatinine, and hemoglobin determinations, along with compliance with the study medication (verified through sachet count) and adverse events, was determined at baseline and/or at monthly intervals during the 24 wk of the study.
The HCV-RNA level was quantified by PCR assay (Amplicor Monitor HCV v. 2.0; Roche Molecular Systems; lower limit of detection, 600 IU/mL). HCV genotyping was performed by reverse hybridization (Inno-LiPA HCV; Innogenetics, Ghent, Belgium). Liver biopsy was performed at least 6 mo before the inclusion in the study and histological results were classified by a single local pathologist according to Ishak score. Liver biopsies were also performed after conclusion of the antiviral treatment.
Venous blood samples were collected after an overnight fast; 7 mL of blood was drawn into tubes containing EDTA. Plasma was obtained by centrifugation (4000 g for 15 min at 4°C) and frozen at -70°C until assayed. After separation of the plasma, the buffy coat was removed and the packed cells washed three times with cold isotonic saline. Then, a known volume of erythrocytes was lysed with cold distilled water (1:4) and stored in a refrigerator at 4°C for 15 min, and the cell debris was removed by centrifugation. The erythrocyte lysates were stored at -70°C until assayed. In general, all samples were analyzed in duplicate.
Oxidative stress determination: Blood for oxidative stress determination was drawn at baseline and every two months up to study conclusion.
The blood oxidative stress status was evaluated by assaying nine parameters: plasma lipid peroxidation products (malondialdehyde and 4-hydroxyalkenals), advanced oxidation protein products, and antioxidant defense systems (glutathione peroxidase, Cu/Zn superoxide dismutase, catalase, glutathione reductase, glutathione, and myeloperoxidase).
Erythrocyte catalase, glutathione reductase, and glutathione peroxidase activities were determined with BIOXYTECH® CAT-520™, BIOXYTECH® GR-340™ and BIOXYTECH® GPx-340™ (OXIS Research, Portland, USA), respectively. Their values were expressed as units per milligram of hemoglobin.
SOD in erythrocytes was measured by the Fulbert method[28], based on the capacity of SOD to inhibit the reduction of ferricytochrome C by the xanthine/xanthine oxidase system. A standard curve was prepared using commercially available SOD (Sigma-Aldrich). Reaction development was monitored spectrophotometrically at 405 nm. One SOD unit is defined as the amount of enzyme that inhibits cytochrome C reduction by 50% under the respective conditions; activity is expressed per hemoglobin.
LPO measurement was performed using the BIOXYTECH® LPO-586™ kit (OXIS Research, Portland, USA) and was based on the reaction of a chromogenic reagent (N-methyl-2-phenylindole) with malondialdehyde (MDA) and 4-hydroxyalkenals at 45°C.
Determination of advanced oxidation protein products (AOPP) was based on spectrophotometric detection according to the method described by Witko-Sarsat[29]. Briefly, 200 μL of plasma [diluted 1:5 with phosphate buffered saline (PBS)], 200 μL of chloramine-T (0-100 μmol/L) for calibration, and 200 μL of PBS as blank were applied on a microtiter plate. 10 μL of 1.16 mol/L potassium iodide and 20 μL of acetic acid were added to each well, and absorbance at 340 nm was measured immediately. Concentration of AOPP was expressed in chloramine units (μmol/L).
GSH concentration was measured by the method of Sedlak and Lindsay, in which 5,5'-dithiobis-(2-nitrobenzoic acid) reacting with sulfhydryl groups yields 2-nitro-5-mercaptobenzoic acid with maximal absorbance at 412 nm[30]. Glutathione (Sigma-Aldrich) was used to generate the standard curves.
Serum myeloperoxidase (MPO) concentration was measured using a high-sensitivity, quantitative sandwich enzyme immunoassay (OXIS Research, Portland, USA). In this assay, the lowest detection limit of MPO is 1.02 ng/mL.
Serum cytokine determinations: Blood for cytokine measurement was drawn at baseline and every two months up to study conclusion.
Serum cytokine levels (IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12; TNF-α, TNF-β, IFN-γ, cathepsin L, and GM-CSF) were measured using commercial sandwich-type ELISA kits following the procedure recommended by the manufacturers (Bender MedSystem, Vienna, Austria). Detection limits were as follows: 1.1 pg/mL for IL-1α, 0.3 pg/mL for IL-1β, 9.9 pg/mL for IL-2, 0.92 pg/mL for IL-6, 1.0 pg/mL for IL-10, 2.1 pg/mL for IL-12; 2.3 pg/mL for TNF-α, 4.6 pg/mL for TNF-β, 0.99 pg/mL for IFN-γ, 1.71 ng/mL for cathepsin L, and 2.9 pg/mL for GM-CSF.
The primary outcome was the change in serum MDA and 4-hydroxyalkenals (lipid peroxidation products). Secondary outcomes were changes in serum TNF-α, IL-10 and IFN-γ. Change was the difference between measurements obtained at baseline and at the end of treatment (24 wk).
The baseline characteristics were summarized as percentages for categorical variables and as mean ± SE for continuous variables. The χ2 test was applied to categorical variables. The two-sample t-test was used to compare means, and the Mann-Whitney U-test if they were not normally distributed. Outcome measurements included all patients who were randomized and received at least one dose of study medication (intention-to-treat analysis). The Wilcoxon signed-rank tests were used to compare changes between the baseline and post-treatment measurements, and the Wilcoxon rank-sum tests were used for treatment group comparisons. The safety analysis included all treated patients who had at least one safety evaluation after baseline.
The study was designed to have a statistical power of 80% to detect an absolute difference of 28% in the changes of serum MDA and 4-hydroxyalkenals (28% in the group with Viusid versus 0% in the placebo group). Considering a type I error of 0.05 and a type II error of 0.20, 30 patients per arm were needed to reach statistical significance.
All confidence intervals, significance tests, and resulting P values were two-sided, with an alpha level of 0.05.
Statistical analyses were performed using SPSS Inc. for Windows, release 15, Chicago, IL.
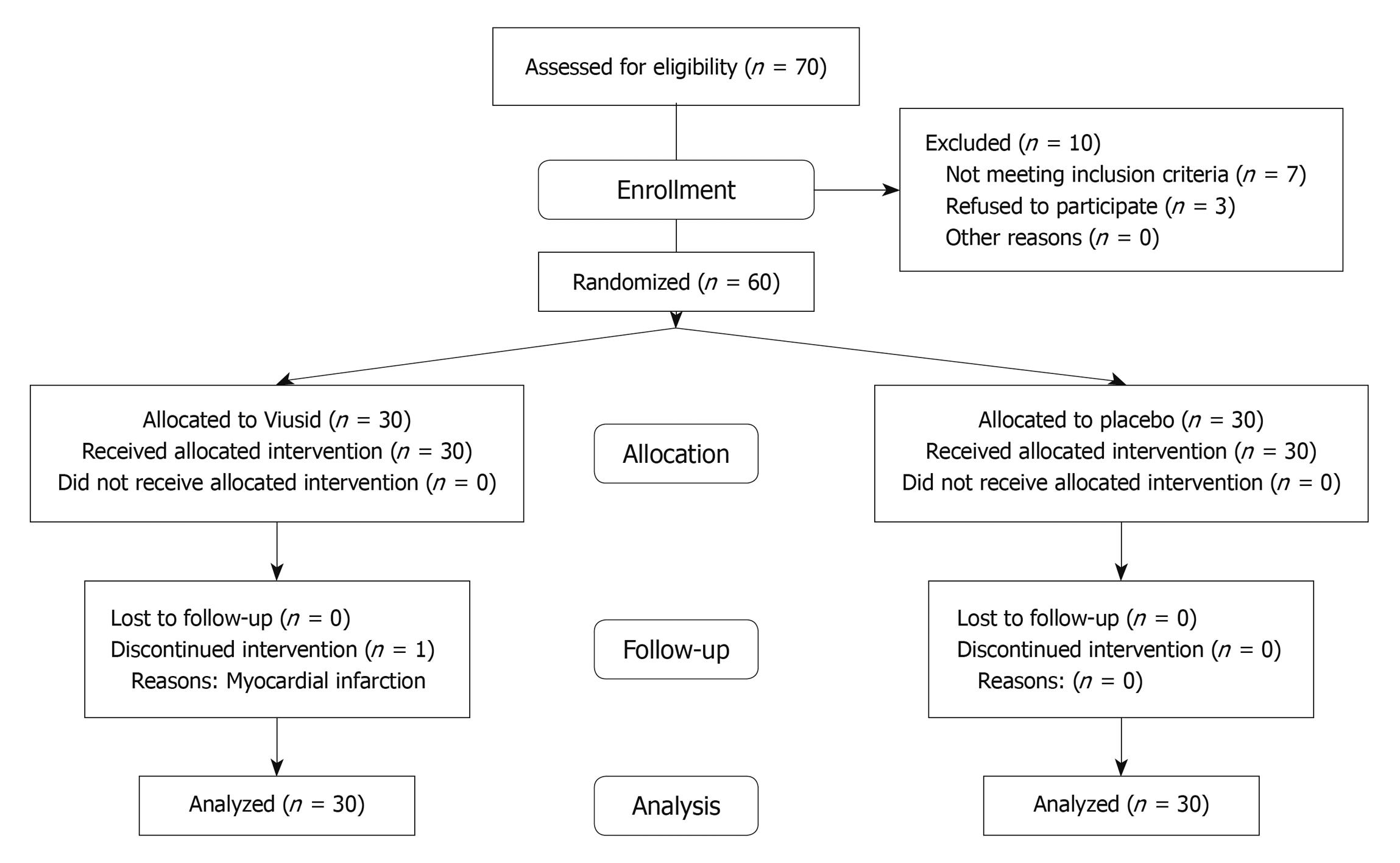
A total of 60 patients were recruited at a tertiary center (National Institute of Gastroenterology, Havana, Cuba) between October 2008 and May 2009. The flow of participants through the trial is presented in Figure 1. All randomized patients received at least one dose of the study medication; 1 subject assigned to Viusid did not complete the study and the reason for discontinuation was a myocardial infarction in week 16 of the study. No patient was lost during the follow-up period and no patient received co-interventions during the trial that could have affected the outcomes.
All patients who underwent random allocation were analyzed for outcomes according to original group assignment (intention-to-treat analysis).
Baseline characteristics were comparable across the two groups (Table 2). The majority of the patients in the Viusid group were female; however, there was no statistical difference in comparison with the placebo group. All patients were non-responders to previous antiviral therapy with peginterferon plus ribavirin and infected with genotype 1, and more than half of the patients had a viral load of over 600 000 IU/mL (75%).
| Variable | Viusid (n = 30) | Placebo (n = 30) | P value1 |
| Age (yr) | 49.3 ± 2.1 | 49.7 ± 2.2 | 0.90 |
| Sex, n (%) | |||
| Male | 10 (33) | 16 (53) | 0.19 |
| Female | 20 (67) | 14 (47) | |
| Body mass index (kg/m2) | 25.3 ± 0.7 | 26.4 ± 0.7 | 0.28 |
| HCV RNA > 600 000 IU/mL, n (%) | 24 (80) | 21 (70) | 0.36 |
| Genotype 1, n (%) | 30 (100) | 30 (100) | 1.00 |
| Ishak inflammation score | 5.98 ± 3 | 5.88 ± 3 | 0.55 |
| Ishak fibrosis score | 1.88 ± 0.91 | 2.06 ± 1.8 | 0.68 |
| ALT (IU/L) | 66.9 ± 7.6 | 75.4 ± 8.4 | 0.45 |
| AST (IU/L) | 55.9 ± 7 | 65.8 ± 7.6 | 0.34 |
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.14 | 5.8 ± 0.4 | 0.09 |
| Alkaline phosphatase (mmol/L) | 217.8 ± 12.8 | 221.1 ± 13.7 | 0.86 |
| Creatinine (mmol/L) | 80.4 ± 2.8 | 82.8 ± 3.6 | 0.59 |
| Hemoglobin (g/L) | 126.4 ± 1.4 | 131.3 ± 2.7 | 0.11 |
| γ-glutamyltransferase (IU/L) | 72.7 ± 13.5 | 84.1 ± 13.8 | 0.55 |
| Bilirubin (mmol/L) | 11.4 ± 1.4 | 14.1 ± 1.3 | 0.16 |
| Albumin (g/L) | 47.8 ± 0.5 | 47.6 ± 0.9 | 0.80 |
| Uric acid (mmol/L) | 257 ± 16.8 | 276 ± 13.4 | 0.37 |
| IL-1α (pg/mL) | 3.26 ± 0.7 | 3.09 ± 0.5 | 0.89 |
| IL-1β (pg/mL) | 3.7 ± 0.1 | 4 ± 0.1 | 0.29 |
| IL-2 (pg/mL) | 17.9 ± 2.3 | 19.9 ± 4.7 | 0.93 |
| IL-6 (pg/mL) | 2.5 ± 0.2 | 2.1 ± 0.3 | 0.35 |
| IL-10 (pg/mL) | 2.6 ± 0.6 | 2.8 ± 4.1 | 0.67 |
| IL-12 (pg/mL) | 7.5 ± 1.4 | 7.6 ± 1.2 | 0.92 |
| IFN-γ (pg/mL) | 1.92 ± 0.2 | 1.80 ± 0.1 | 0.63 |
| TNF-α (pg/mL) | 6.6 ± 0.2 | 6.9 ± 0.5 | 0.45 |
| TNF-β (pg/mL) | 8.5 ± 0.4 | 9.1 ± 0.6 | 0.58 |
| Cathepsin L (ng/mL) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.99 |
| GM-CSF (pg/mL) | 4.4 ± 0.1 | 4.3 ± 0.1 | 0.97 |
| MDA (μmol/L) | 0.97 ± 0.05 | 1.12 ± 0.09 | 0.17 |
| 4-hydroxyalkenals (μmol/L) | 1.75 ± 0.09 | 1.92 ± 0.14 | 0.30 |
| Cu/Zn SOD (U/mg Hb) | 17.6 ± 1.1 | 18.7 ± 0.9 | 0.46 |
| CAT (U/mg Hb) | 235 ± 23.7 | 289 ± 21.8 | 0.09 |
| GR (mU/mg Hb) | 6.1 ± 0.7 | 6.9 ± 1.1 | 0.73 |
| GPx (mU/mg Hb) | 169 ± 11.6 | 176 ± 11.2 | 0.66 |
| GSH (μmol/L) | 26.3 ± 2.3 | 31.8 ± 3.2 | 0.16 |
| MPO (ng/mL) | 221 ± 6.6 | 221 ± 6.7 | 0.91 |
| AOPP (μmol/L) | 36.1 ± 5.4 | 26.2 ± 5.5 | 0.20 |
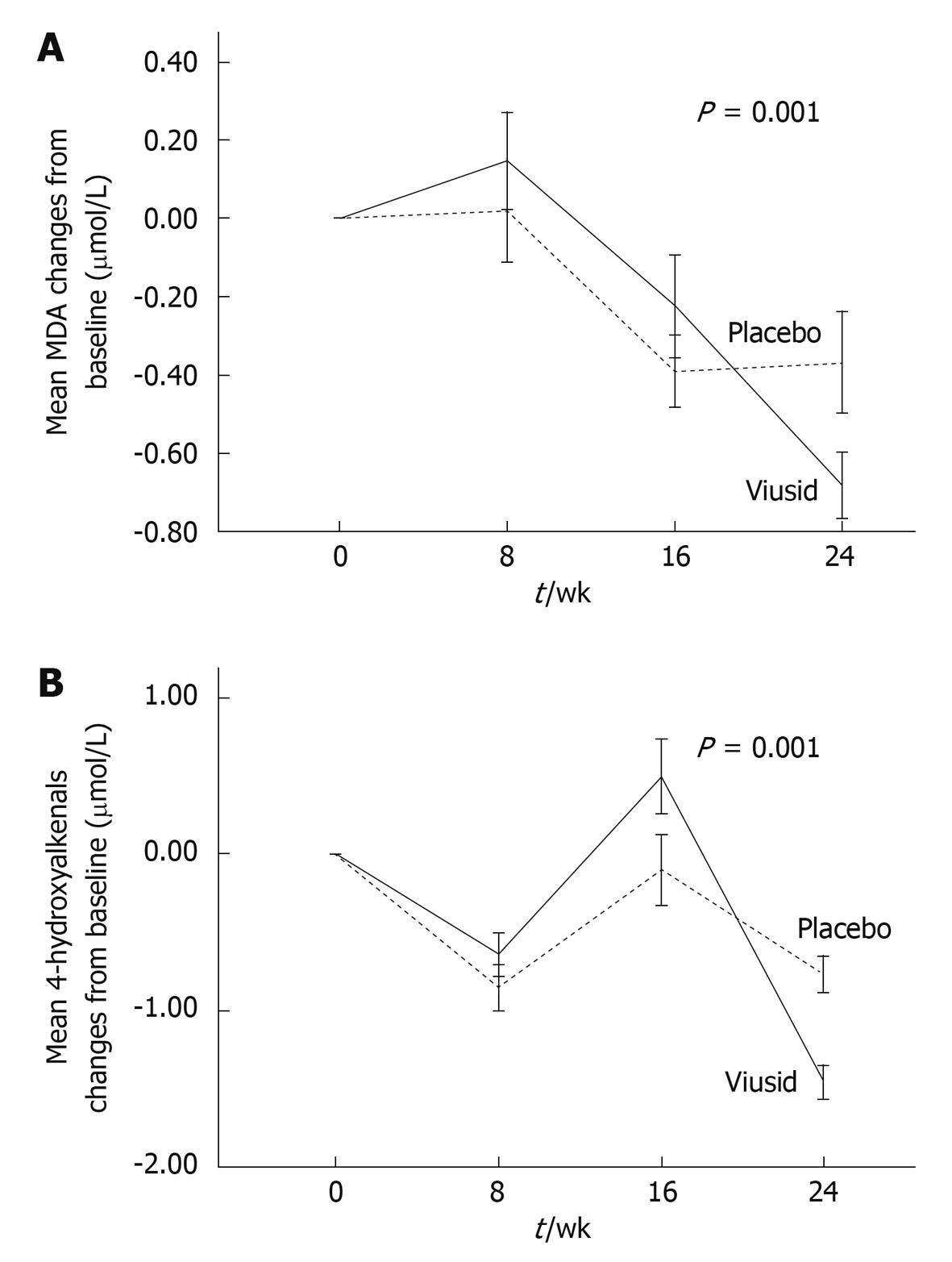
Lipid peroxidation products (primary end point): There were significant mean reductions from baseline to 6 mo in MDA and 4-hydroxyalkenals in each group of treatment (Table 3). However, the administration of Viusid markedly reduced the mean MDA level from 0.97 to 0.29 μmol/L with a mean improvement of 0.68 (95% CI: 0.50-0.84) vs 1.12 to 0.76 μmol/L with a mean improvement of 0.36 (95% CI: 0.11-0.62) for placebo (P = 0.001) in comparison with baseline values (Figure 2A). Similarly, significant changes in the mean of serum 4-hydroxyalkenals occurred in the patients assigned to Viusid (1.76 to 0.53 μmol/L), with a mean difference of 1.22 (95% CI: 0.97-1.45), as compared with the patients treated with placebo (1.92 to 1.39 μmol/L), with a mean difference of 0.53 (95% CI: 0.43-1.17) (Figure 2B).
| Variable | Viusid (n = 30) | Placebo (n = 30) | P value1 | ||||||
| Before treatment | After treatment | Change | P value | Before treatment | After treatment | Change | P value | ||
| MDA (μmol/L) | 0.97 ± 0.05 | 0.29 ± 0.05 | -0.68 ± 0.08 | < 0.001 | 1.12 ± 0.09 | 0.76 ± 0.08 | -0.36 ± 0.12 | 0.002 | 0.001 |
| 4-hydroxyalkenals (μmol/L) | 1.75 ± 0.09 | 0.53 ± 0.06 | -1.22 ± 0.1 | < 0.001 | 1.92 ± 0.14 | 1.39 ± 0.13 | -0.53 ± 0.2 | 0.01 | 0.001 |
| Cu/Zn SOD (U/mg Hb) | 17.6 ± 1.1 | 3.26 ± 0.4 | -14.3 ± 1.2 | < 0.001 | 18.7 ± 0.9 | 3.44 ± 0.39 | -15.3 ± 1.04 | < 0.001 | 0.71 |
| CAT (U/mg Hb) | 235 ± 23.7 | 17.6 ± 2.3 | -217.4 ± 24 | < 0.001 | 289 ± 21.8 | 21 ± 2.7 | -268 ± 22 | < 0.001 | 0.13 |
| GR (mU/mg Hb) | 6.1 ± 0.7 | 11 ± 1.1 | 4.9 ± 1.3 | < 0.001 | 6.9 ± 1.1 | 12 ± 1.2 | 5.1 ± 1.6 | 0.001 | 0.97 |
| GPx (mU/mg Hb) | 169 ± 11.6 | 147 ± 12 | -22 ± 17 | 0.20 | 176 ± 11.2 | 136 ± 16 | -40 ± 19 | 0.05 | 0.87 |
| GSH (μmol/L) | 26.3 ± 2.3 | 16.4 ± 1.9 | -9.9 ± 3 | 0.002 | 31.8 ± 3.2 | 19.8 ± 2.5 | -12 ± 4.1 | 0.005 | 0.69 |
| MPO (ng/mL) | 221 ± 6.6 | 123 ± 12.9 | -98 ± 14.2 | < 0.001 | 221 ± 6.7 | 115 ± 22 | -106 ± 23.2 | < 0.001 | 0.61 |
| AOPP (μmol/L) | 36.1 ± 5.4 | 48.4 ± 13.7 | 12.3 ± 11.2 | 0.39 | 26.2 ± 5.5 | 35.9 ± 7.6 | 9.7 ± 9.3 | 0.30 | 0.57 |
Antioxidant defense system markers: Surprisingly, significant reductions in erythrocyte Cu/Zn SOD, CAT, GR, and serum GSH and MPO were observed in both groups of treatment in comparison with pretreatment values; however, no significant difference was observed between groups (Table 3).
Advanced oxidation protein products: Serum AOPP concentrations remained unchanged for the patients who received Viusid or placebo as compared with baseline values (Table 3).
Serum cytokine results are summarized in Table 4.
| Variable | Viusid (n = 30) | Placebo (n = 30) | P value1 | ||||||
| Before treatment | After treatment | Change | P value | Before treatment | After treatment | Change | P value | ||
| IL-1α (pg/mL) | 3.26 ± 0.7 | 2.61 ± 0.3 | -0.60 ± 0.8 | 0.04 | 3.09 ± 0.5 | 6.62 ± 2.3 | 3.53 ± 2.4 | 0.04 | 0.04 |
| IL-1β (pg/mL) | 3.7 ± 0.1 | 2.9 ± 0.1 | -0.8 ± 0.1 | < 0.001 | 4 ± 0.1 | 3 ± 0.1 | -1 ± 0.2 | < 0.001 | 0.24 |
| IL-2 (pg/mL) | 17.9 ± 2.3 | 11.3 ± 0.2 | -6.5 ± 2.2 | < 0.01 | 19.9 ± 4.7 | 12.1 ± 0.8 | -7.8 ± 3.9 | 0.04 | 0.39 |
| IL-6 (pg/mL) | 2.5 ± 0.2 | 2.1 ± 0.2 | -0.4 ± 0.3 | 0.14 | 2.1 ± 0.3 | 2 ± 0.2 | -0.1 ± 0.3 | 0.71 | 0.24 |
| IL-10 (pg/mL) | 2.6 ± 0.6 | 8.3 ± 4.1 | 5.7 ± 4 | 0.04 | 2.8 ± 4.1 | 4.1 ± 0.1 | 1.3 ± 0.2 | 0.09 | 0.01 |
| IL-12 (pg/mL) | 7.5 ± 1.4 | 5.1 ± 0.9 | -2.4 ± 1.1 | 0.04 | 7.6 ± 1.2 | 6.2 ± 1.5 | -1.4 ± 1.4 | 0.34 | 0.29 |
| IFN-γ (pg/mL) | 1.92 ± 0.2 | 2.89 ± 0.4 | 0.97 ± 0.5 | < 0.001 | 1.80 ± 0.1 | 1.68 ± 0.1 | -0.11 ± 0.2 | 0.70 | < 0.0001 |
| TNF-α (pg/mL) | 6.6 ± 0.2 | 7.1 ± 0.4 | 0.45 ± 0.4 | 0.26 | 6.9 ± 0.5 | 16.2 ± 3.4 | 9.3 ± 3.1 | < 0.01 | 0.001 |
| TNF-β (pg/mL) | 8.5 ± 0.4 | 5.4 ± 0.1 | -3.1 ± 0.5 | < 0.001 | 9.1 ± 0.6 | 6.3 ± 1.2 | -2.8 ± 1.2 | 0.03 | 0.21 |
| Cathepsin L (ng/mL) | 1.7 ± 0.1 | 2.9 ± 0.2 | 1.2 ± 0.2 | < 0.001 | 1.7 ± 0.1 | 3.1 ± 0.2 | 1.4 ± 0.2 | < 0.001 | 0.16 |
| GM-CSF (pg/mL) | 4.4 ± 0.1 | 4.6 ± 0.1 | 0.2 ± 0.2 | 0.24 | 4.3 ± 0.1 | 4.6 ± 0.1 | 0.3 ± 0.2 | 0.11 | 0.60 |
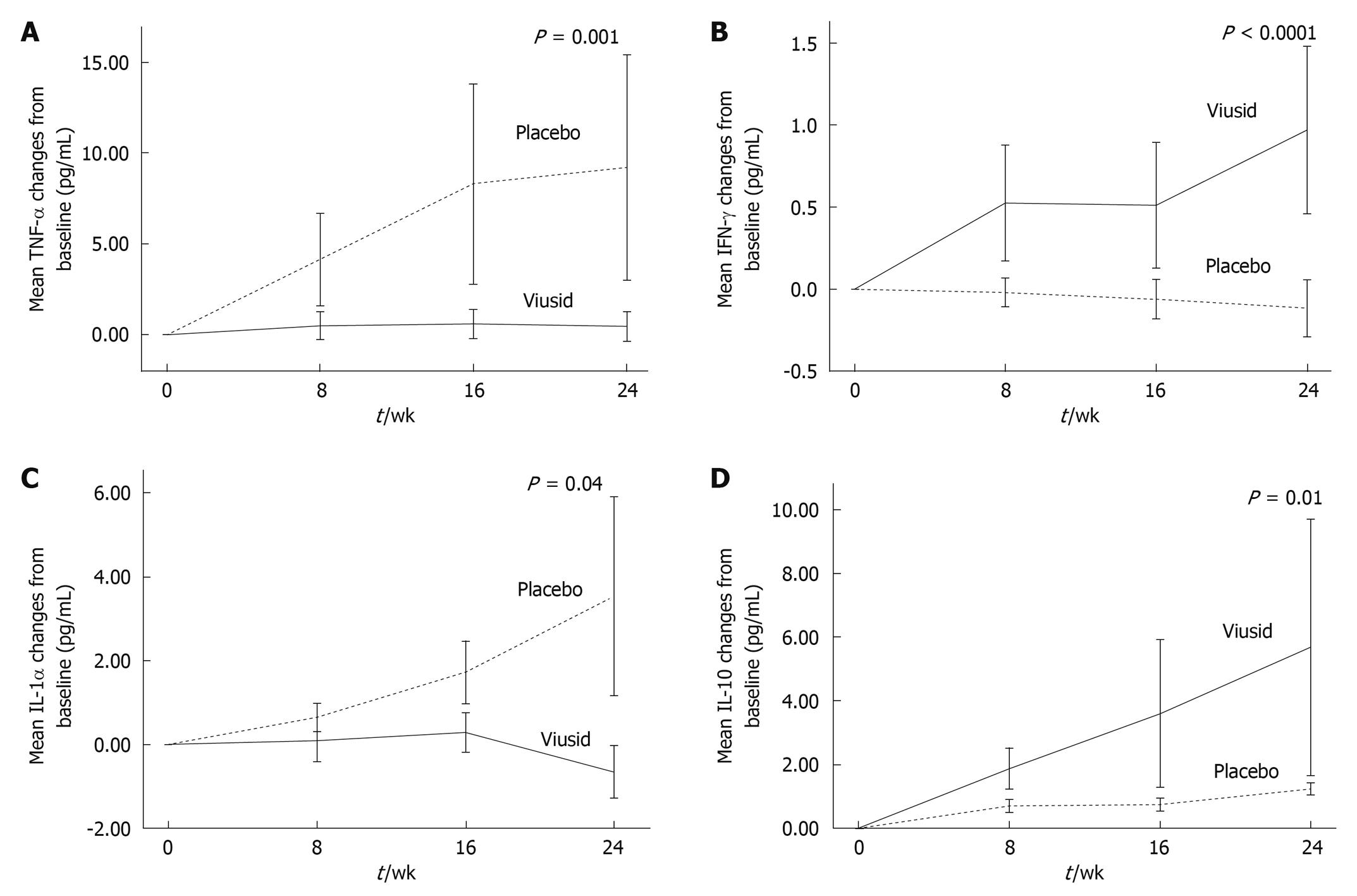
TNF-α levels (secondary outcome) significantly increased from 6.9 to 16.2 pg/mL (P < 0.01) in the patients treated with placebo in comparison with almost unchanged levels (6.6 to 7.1 pg/mL, P = 0.26) in the patients treated with Viusid (P = 0.001) (Figure 3A). Similarly, the administration of Viusid markedly increased the mean IFN-γ level (secondary outcome) from 1.92 to 2.89 pg/mL (P < 0.001) in comparison with reduced mean levels from 1.80 to 1.68 pg/mL (P = 0.70) in the placebo group (P < 0.0001) (Figure 3B).
Likewise, IL-1α levels slightly decreased from 3.26 to 2.61 pg/mL (P = 0.04) in the patients who received Viusid, as compared with a significant increase from 3.09 to 6.62 pg/mL (P = 0.04) in subjects assigned to placebo (Figure 3C). In addition, IL-10 levels were markedly increased in the patients treated with Viusid (from 2.6 to 8.3 pg/mL, P = 0.04) in contrast to the patients assigned to placebo (from 2.8 to 4.1 pg/mL, P = 0.09) (P = 0.01) (Figure 3D).
Unexpectedly, significant reductions in the mean serum level of IL-1β, IL-2, TNF-β, and cathepsin L were recorded in all patients at 24 wk of treatment in comparison with baseline; however, no significant difference was observed between the groups.
Serum IL-6, IL-12, and GM-CSF concentrations remained unchanged in the patients who received Viusid or placebo as compared with baseline values.
At 24 wk of treatment, γ-glutamyltransferase (GGT) levels were considerably reduced in comparison with baseline values (Table 5). GGT levels were reduced by 44% (from 72.7 to 40.8 IU/L, P = 0.001) in patients receiving Viusid, as compared with 12% (from 84.1 to 74.4 IU/L, P = 0.56) in the patients assigned to placebo (P = 0.002). Uric acid levels slightly decreased by 23% (from 257 to 198 mmol/L, P = 0.01) in patients receiving Viusid, as compared with 3% (from 276 to 267 mmol/L, P = 0.66) in subjects assigned to placebo.
| Variable | Viusid (n = 30) | Placebo (n = 30) | P value1 | ||||
| Before treatment | After treatment | P value | Before treatment | After treatment | P value | ||
| Body mass index (kg/m2) | 25.6 ± 0.7 | 25.7 ± 0.7 | 0.92 | 26 ± 0.7 | 27.2 ± 0.6 | 0.08 | 0.12 |
| Hemoglobin (g/L) | 125.8 ± 1.4 | 125.2 ± 2.1 | 0.74 | 132.2 ± 2.7 | 135.6 ± 3.1 | 0.09 | 0.13 |
| Alkaline phosphatase (mmol/L) | 217.2 ± 12 | 206.1 ± 15 | 0.49 | 217.1 ± 13 | 211.4 ± 13 | 0.69 | 0.80 |
| ALT (IU/L) | 67.6 ± 7.6 | 65.1 ± 9.9 | 0.68 | 67.7 ± 8.4 | 63.5 ± 6 | 0.38 | 0.82 |
| AST (IU/L) | 56 ± 7 | 57.7 ± 9.1 | 0.81 | 62.7 ± 7.6 | 53.9 ± 6.5 | 0.11 | 0.24 |
| Fasting plasma glucose (mmol/L) | 5 ± 0.1 | 4.8 ± 0.2 | 0.17 | 5.6 ± 0.4 | 5.4 ± 0.5 | 0.69 | 0.91 |
| Creatinine (mmol/L) | 79.4 ± 2.8 | 75.7 ± 2.4 | 0.06 | 83.3 ± 3.6 | 77.7 ± 3 | 0.17 | 0.67 |
| γ-glutamyltransferase (IU/L) | 72.7 ± 13 | 40.8 ± 6.1 | 0.001 | 84.1 ± 14 | 74.4 ± 10 | 0.56 | 0.002 |
| Albumin (g/L) | 47.4 ± 0.5 | 46.6 ± 0.8 | 0.43 | 46.5 ± 0.9 | 46.1 ± 1 | 0.72 | 0.79 |
| Bilirubin (mmol/L) | 12.1 ± 0.08 | 11.9 ± 0.07 | 0.91 | 13.8 ± 0.07 | 14.3 ± 0.08 | 0.79 | 0.67 |
| Uric acid (mmol/L) | 257 ± 16.8 | 198 ± 13.8 | 0.01 | 276 ± 13.4 | 267 ± 18 | 0.66 | 0.04 |
In both groups, aminotransferase levels were unaffected in comparison with pretreatment values (Table 5).
Headache and diarrhea were reported in one subject who received Viusid. No laboratory adverse event was reported with the use of Viusid. There was no incidence of discontinuation or dose modification of Viusid secondary to adverse events.
Encouraging effects of Viusid on liver histology have been reported in patients with chronic hepatitis C and nonalcoholic fatty liver disease[27,31]; however, pathophysiologic mechanisms for explaining these effects remain unknown. There is a potential hepatoprotective mechanism in the chemical composition of Viusid that could be explained by the anti-inflammatory and antioxidant properties of its different molecules, such as zinc, glycyrrhizin acid, and ascorbic acid[24-26]. Thus, the aim of this study was to investigate the role of Viusid as an antioxidant and an immunomodulator in patients with CHC who have failed previous antiviral treatment.
Several studies have demonstrated increased production of MDA in CHC, which is an indirect marker for oxidative stress (lipid peroxidation), and its levels have been associated with moderate-to-severe inflammation and fibrosis compared with those patients who had milder disease, suggesting higher oxidative stress with advanced stage of the disease[32,33].
We found that MDA and 4-hydroxyalkenal levels were significantly reduced in the serum of the patients treated with Viusid as compared with placebo, indicating an important effect on lipid peroxidation products. Recent evidence shows that increased lipid peroxidation products, such as 4-hydroxynonenal, play a decisive role in stellate cell activation and fibrosis progression[34]. Increased 4-hydroxynonenal levels upregulate procollagen and tissue inhibitor of metalloproteinase-1 gene expression. Matrix metalloproteinase-1 plays an important role in degrading collagen; therefore, the inhibition of this enzyme could increase fibrosis. Thus, this provides a putative mechanism to explain in part the effect of Viusid on fibrosis reduction in patients with CHC[27]. Therefore, these results support the rationale for further studies in other forms of liver disease such as alcoholic liver disease and nonalcoholic fatty liver disease.
Encouraging antioxidant effects of ascorbic acid and zinc have been reported recently. Ascorbic acid and zinc supplementation have been associated with a significant reduction of LPO products[35,36].
Surprisingly, a marked reduction of serum antioxidant defense system markers was seen in the whole group during treatment, maybe reflecting the “regression towards the mean” phenomenon, a placebo-induced effect, or poor maintenance of the redox homeostasis status.
Imbalanced Th1- and Th2-type response has been postulated to play an important role in influencing both the persistence of HCV infection and the extent of liver damage[10,37,38]. In this context, IFN-γ and IL-12 are Th1-type cytokines, which are critical in the development of T-cell-mediated immunity, paramount for viral clearance. Thus, an increased production of serum IFN-γ has been strongly linked to self-limited HCV infection[11,12]. Our data suggest an increased production of IFN-γ in the patients treated with Viusid when compared with baseline, maybe suggesting a reestablishment of the T-cell-mediated immunity via Th1-type response; however, the underlying mechanisms by which Viusid is able to induce IFN-γ are still poorly understood. On the other hand, IL-10 is a Th2-type response cytokine with a suppressive effect on the generation of Th1-type response cytokines (IFN-γ and IL-12), which have recognized anti-inflammatory and slight proviral effects[13-15]. Our data show that serum IL-10 levels were markedly increased with the administration of Viusid in spite of simultaneously increasing serum IFN-γ levels. IL-10 is known to suppress the secretion of pro-inflammatory cytokines, such as TNF-α, IFN-γ, and IL-12[15,39,40]. In contrast, administration of neutralizing monoclonal antibodies to anti-IL-10 enhances in vitro IFN-γ production in HCV patients[41], suggesting that IL-10 levels differ widely between individuals, possibly due to polymorphisms in the promoter region of the IL-10 gene[42].
Recent data support the hypothesis that long-term IL-10 therapy to treat chronically HCV-infected patients leads to significant histological improvement of inflammation and fibrosis[15].
Our data suggest that increased serum IL-10 levels could be a significant finding to explain the effect of Viusid on histological features (inflammation and fibrosis) in patients with CHC and non-alcoholic fatty liver disease (NAFLD)[27,31].
Recent studies have indicated that glycyrrhizin, an aqueous extract of licorice root and the main ingredient of Viusid, can induce a significant production of IL-10 by liver dendritic cells in concanavalin A-induced hepatitis in mice[43].
Our findings also show that a short course of Viusid slightly reduces IL-1α and stabilizes TNF-α concentrations in patients with CHC. These are markers of inflammation and fibrosis that are elevated in HCV-infected subjects compared with healthy controls[16,17,38]; therefore, their reduction or stabilization could be associated with histological improvement, in particular inflammation and fibrosis.
Viusid has chemical ingredients with recognized immune regulatory properties that would explain in part the effect on cytokine secretion. Zinc supplementation has demonstrated that it can significantly increase IFN-γ and IL-10 secretion and reduce IL-1 and TNF-α secretion in patients with chronic inflammatory diseases[44].
GGT has been regarded as a biomarker of hepatobiliary disease and alcohol consumption/abuse[45]. In addition to liver disease, GGT has been associated with high all-cause mortality, cardiovascular disease incidence and death, and cancer incidence and death[46,47]. Accumulating experimental evidence suggests that GGT is a key mediator in the mechanisms of oxidative stress. GGT-mediated oxidative stress has been reported capable of inducing lipid oxidation, oxidation of protein thiols, alterations of the normal protein phosphorylation patterns, and biological effects such as the activation of transcription factors[48].
In the current study, we found that serum GGT levels were significantly reduced in the patients treated with Viusid in comparison with unchanged levels in the patients assigned to placebo. The mechanism that explains the contribution of Viusid to GGT reduction has not been fully elucidated and further studies are needed to evaluate the clinical impact of long-term Viusid administration in patients with other forms of chronic liver disease, cardiovascular disease, metabolic syndrome, and cancer.
On the other hand, the administration of Viusid did not improve aminotransferase levels during the 24 wk of treatment. In contrast to what has been suggested in two recently published papers[33,37], Viusid without supportive therapy such as antiviral therapy or lifestyle modification for CHC and NAFLD, respectively, is unable to induce improvement in aminotransferase levels, regardless of its proven and significant antioxidant and immunomodulatory capability. Further studies must evaluate higher doses and long-term administration of Viusid to determine its effect on aminotransferase levels in patients with chronic liver diseases.
The main strength of this study is the presence of a concurrent placebo control group allowing adequate comparison between control and experimental groups for outcome measures. A possible weakness of our study is that patients were treated for 24 wk only, and it remains unclear whether some parameters related to oxidative stress and immunological status can be improved beyond 24 wk.
In conclusion, our data suggest that Viusid improves oxidative stress through reduction of lipid peroxidation products and that it has an immunomodulatory effect on cytokine secretion via increased production of IFN-γ and IL-10, decreased production of IL-1α, and stabilized TNF-α secretion in patients with CHC who have failed previous antiviral treatment. Our findings also highlight the limitations in our understanding of the complex mechanism of the host immune response and its interaction with pro-oxidant/antioxidant status.
The pathogenesis of chronic hepatitis C (CHC) is associated with severe oxidative stress and non-selective immunological disturbance that lead to necroinflammation and the progression of fibrosis. Several trials have suggested that antioxidant and immunostimulant therapies may have a beneficial effect. Two previous clinical studies have reported that the Viusid-related effect on histologic features, especially fibrosis, appears to be associated with antioxidant and/or immunomodulatory properties. However, the putative mechanism of action of Viusid is unknown.
Peginterferon plus ribavirin is the only therapeutic alternative with proven efficacy in patients with CHC. Unfortunately, sustained virological response is achieved in only approximately 50% of patients. Viusid is a nutritional supplement with documented effects on histological parameters (steatosis, inflammation and fibrosis) in patients with CHC and nonalcoholic fatty liver diseases. It contains different molecules (ascorbic acid, zinc, and glycyrrhizic acid) with recognized antioxidant and immunomodulatory properties. However, is necessary to confirm whether Viusid may have a beneficial effect on oxidative stress and cytokine parameters in patients with CHC.
Recent studies have suggested that the Viusid-related effect on histologic features, especially fibrosis, appears to be associated with antioxidant and/or immunomodulatory properties. All of these effects could modulate the histologic pattern of CHC, especially inflammation and fibrosis, in an attempt to halt disease progression. The identification of the molecular mechanisms of action of Viusid as an immunomodulatory and/or antioxidant product in patients with CHC could support a new strategy of treatment in patients who have failed previous antiviral treatment.
In current clinical practice, there are no available strategies of treatment for patients with CHC who have failed previous antiviral treatment. Therefore, there is an obvious need for the continuous development of new treatment strategies for CHC. Thus, the administration of compounds with antioxidant and immunomodulatory properties such as Viusid could be a plausible strategy to halt the natural course of the disease, particularly in patients with poor response to antiviral therapy. Further studies are needed to evaluate the clinical impact of the administration of Viusid in patients with end-stage liver disease secondary to CHC.
Viusid is a nutritional supplement. It contains different molecules (ascorbic acid, zinc, and glycyrrhizic acid) with recognized antioxidant and immunomodulatory properties. The different compounds in the supplement are activated through a molecular activation principle which strongly increases their biological activity without modifying their structure. With this method the molecules are treated under a determined electric field during a time span calculated beforehand and under certain specific conditions for each kind of molecule.
In this report, the authors reported the results of a randomized double-blind and placebo-controlled study to evaluate the effect of Viusid on oxidative stress and cytokine parameters in patients with chronic hepatitis C who had been non-responders to previous antiviral therapy with peginterferon plus ribavirin and infected with genotype 1.
Peer reviewers: Dr. BS Anand, Professor, Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030, United States; Dr. Yoshiaki Iwasaki, Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, 2-5-1, Shikata-cho, Okayama 700-8558, Japan
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Williams R. Global challenges in liver disease. Hepatology. 2006;44:521-526. [Cited in This Article: ] |
| 2. | Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444-2451. [Cited in This Article: ] |
| 3. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [Cited in This Article: ] |
| 4. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [Cited in This Article: ] |
| 5. | De Maria N, Colantoni A, Fagiuoli S, Liu GJ, Rogers BK, Farinati F, Van Thiel DH, Floyd RA. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic Biol Med. 1996;21:291-295. [Cited in This Article: ] |
| 6. | Boya P, de la Peña A, Beloqui O, Larrea E, Conchillo M, Castelruiz Y, Civeira MP, Prieto J. Antioxidant status and glutathione metabolism in peripheral blood mononuclear cells from patients with chronic hepatitis C. J Hepatol. 1999;31:808-814. [Cited in This Article: ] |
| 7. | Swietek K, Juszczyk J. Reduced glutathione concentration in erythrocytes of patients with acute and chronic viral hepatitis. J Viral Hepat. 1997;4:139-141. [Cited in This Article: ] |
| 8. | Ko WS, Guo CH, Yeh MS, Lin LY, Hsu GS, Chen PC, Luo MC, Lin CY. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:4697-4702. [Cited in This Article: ] |
| 9. | Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, Warnes TW. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol. 2002;36:805-811. [Cited in This Article: ] |
| 10. | Gramenzi A, Andreone P, Loggi E, Foschi FG, Cursaro C, Margotti M, Biselli M, Bernardi M. Cytokine profile of peripheral blood mononuclear cells from patients with different outcomes of hepatitis C virus infection. J Viral Hepat. 2005;12:525-530. [Cited in This Article: ] |
| 11. | Neumann-Haefelin C, Timm J, Spangenberg HC, Wischniowski N, Nazarova N, Kersting N, Roggendorf M, Allen TM, Blum HE, Thimme R. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47:1824-1836. [Cited in This Article: ] |
| 12. | Sarih M, Bouchrit N, Benslimane A. Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunol Lett. 2000;74:117-120. [Cited in This Article: ] |
| 13. | Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301-1309. [Cited in This Article: ] |
| 14. | Pham TN, Mercer SE, Michalak TI. Chronic hepatitis C and persistent occult hepatitis C virus infection are characterized by distinct immune cell cytokine expression profiles. J Viral Hepat. 2009;16:547-556. [Cited in This Article: ] |
| 15. | Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, Cabrera R, Liu C, Davis GL. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859-868. [Cited in This Article: ] |
| 16. | Farinati F, Cardin R, Bortolami M, Guido M, Rugge M. Oxidative damage, pro-inflammatory cytokines, TGF-alpha and c-myc in chronic HCV-related hepatitis and cirrhosis. World J Gastroenterol. 2006;12:2065-2069. [Cited in This Article: ] |
| 17. | Zekri AR, Ashour MS, Hassan A, Alam El-Din HM, El-Shehaby AM, Abu-Shady MA. Cytokine profile in Egyptian hepatitis C virus genotype-4 in relation to liver disease progression. World J Gastroenterol. 2005;11:6624-6630. [Cited in This Article: ] |
| 18. | Groenbaek K, Friis H, Hansen M, Ring-Larsen H, Krarup HB. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: a randomized trial among chronic hepatitis C virus-infected patients. Eur J Gastroenterol Hepatol. 2006;18:985-989. [Cited in This Article: ] |
| 19. | Marotta F, Yoshida C, Barreto R, Naito Y, Packer L. Oxidative-inflammatory damage in cirrhosis: effect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol. 2007;22:697-703. [Cited in This Article: ] |
| 20. | Gabbay E, Zigmond E, Pappo O, Hemed N, Rowe M, Zabrecky G, Cohen R, Ilan Y. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol. 2007;13:5317-5323. [Cited in This Article: ] |
| 21. | Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605-612. [Cited in This Article: ] |
| 22. | Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925-1936. [Cited in This Article: ] |
| 23. | Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. J Hepatol. 2007;46:734-742. [Cited in This Article: ] |
| 24. | Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39:671-686. [Cited in This Article: ] |
| 25. | Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26:391-404. [Cited in This Article: ] |
| 26. | Lee CH, Park SW, Kim YS, Kang SS, Kim JA, Lee SH, Lee SM. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull. 2007;30:1898-1904. [Cited in This Article: ] |
| 27. | Vilar Gomez E, Gra Oramas B, Soler E, Llanio Navarro R, Ruenes Domech C. Viusid, a nutritional supplement, in combination with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Liver Int. 2007;27:247-259. [Cited in This Article: ] |
| 28. | Fulbert JC, Succari M, Cals MJ. Semi-automated assay of erythrocyte Cu-Zn superoxide dismutase activity. Clin Biochem. 1992;25:115-119. [Cited in This Article: ] |
| 29. | Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524-2532. [Cited in This Article: ] |
| 30. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. [Cited in This Article: ] |
| 31. | Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L, Yasells Garcia A, Del Rosario Abreu Vazquez M. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;30:999-1009. [Cited in This Article: ] |
| 32. | Yadav D, Hertan HI, Schweitzer P, Norkus EP, Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2634-2639. [Cited in This Article: ] |
| 33. | Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Charlotte F, Piton A, Opolon P, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation in chronic hepatitis C: correlation with pathological features. J Clin Pathol. 1997;50:401-406. [Cited in This Article: ] |
| 34. | Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60-68. [Cited in This Article: ] |
| 35. | Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837-844. [Cited in This Article: ] |
| 36. | Huang HY, Appel LJ, Croft KD, Miller ER 3rd, Mori TA, Puddey IB. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am J Clin Nutr. 2002;76:549-555. [Cited in This Article: ] |
| 37. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [Cited in This Article: ] |
| 38. | Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157-169. [Cited in This Article: ] |
| 39. | Neuman MG, Benhamou JP, Marcellin P, Valla D, Malkiewicz IM, Katz GG, Trepo C, Bourliere M, Cameron RG, Cohen L. Cytokine--chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149:126-136. [Cited in This Article: ] |
| 40. | Trinchieri G. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J Exp Med. 2001;194:F53-F57. [Cited in This Article: ] |
| 41. | Piazzolla G, Tortorella C, Schiraldi O, Antonaci S. Relationship between interferon-gamma, interleukin-10, and interleukin-12 production in chronic hepatitis C and in vitro effects of interferon-alpha. J Clin Immunol. 2000;20:54-61. [Cited in This Article: ] |
| 42. | Eskdale J, Keijsers V, Huizinga T, Gallagher G. Microsatellite alleles and single nucleotide polymorphisms (SNP) combine to form four major haplotype families at the human interleukin-10 (IL-10) locus. Genes Immun. 1999;1:151-155. [Cited in This Article: ] |
| 43. | Abe M, Akbar F, Hasebe A, Horiike N, Onji M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J Gastroenterol. 2003;38:962-967. [Cited in This Article: ] |
| 44. | Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353-357. [Cited in This Article: ] |
| 45. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [Cited in This Article: ] |
| 46. | Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D'Agostino RB, Vasan RS. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127-133. [Cited in This Article: ] |
| 47. | Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477-485.e11. [Cited in This Article: ] |
| 48. | Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535-539. [Cited in This Article: ] |