Published online Jan 14, 2010. doi: 10.3748/wjg.v16.i2.264
Revised: September 21, 2009
Accepted: September 28, 2009
Published online: January 14, 2010
AIM: To identify prognostic factors from pretreatment variables of the initial transarterial chemoembolization (TACE) procedure in unresectable hepatocellular carcinoma (HCC).
METHODS: One thousand and five hundred and sixty-nine patients with unresectable HCC underwent TACE as initial treatment were retrospectively studied. Pretreatment variables of the initial TACE procedure with a P value less than 0.05 by univariate analysis were subjected to Cox proportional hazards model.
RESULTS: The median overall survival time and 1-, 5-, 10-year survival rates were 10.37 mo, 47%, 10%, and 7%, respectively. A Cox proportional hazard model showed that 8 pretreatment factors of regional lymph nodes metastasis, Child-Pugh class, macrovascular invasion, greatest dimension, α-fetoprotein (AFP), Hepatitis virus B, tumor capsule, and nodules were independent prognostic factors. Patients with multimodality therapy have better survival than those with TACE treatment only.
CONCLUSION: Tumor status, hepatic function reserve, AFP, and hepatitis virus B status were independent prognostic factors for unresectable HCC. Distant metastasis might not be a contraindication to TACE. Multimodality therapy might improve survival.
- Citation: Shi M, Chen JA, Lin XJ, Guo RP, Yuan YF, Chen MS, Zhang YQ, Li JQ. Transarterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World J Gastroenterol 2010; 16(2): 264-269
- URL: https://www.wjgnet.com/1007-9327/full/v16/i2/264.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i2.264
Hepatocellular carcinoma (HCC) constitutes the majority of liver cancers which is the sixth most common cancer and the third most common cause of cancer death worldwide[1]. While curative therapies include surgical resection, transplantation and percutaneous ablation[2], these are not suitable for use in a great number of HCC patients due either to advanced stage of disease or to poor liver function at the time of diagnosis[3,4]. Transarterial chemoembolization (TACE) has become the most popular modality for palliative treatment among these patients. Two randomized trials from Europe and Asia have confirmed better survival associated with TACE as compared with conservative treatment in selected patients[5,6].
However, considerable controversies remain surrounding definition of suitable candidates for TACE. Many published studies attempted to delineate the survival and identify the prognostic factors related to TACE[7-14] and have shown varying patient baseline characteristics and outcomes across a wide range of geographical distribution, probably because of different inclusion criteria, epidemiological variations in hepatitis B and C infections, and local surveillance for HCC.
Globally, more than half of new HCC cases occur in China[15]. However, to the best of our knowledge, the baseline characteristics, outcomes and prognostic factors among TACE-treated HCC patients in China have not been fully described. In the present study, we conducted a 13 year retrospective analysis of patients with unresectable HCC who underwent TACE as initial treatment and to identify prognostic factors from variables prior to the initial TACE procedure. We hypothesized that findings of this study should help to select patients who would benefit most from TACE, and help to design and estimate samples for further prospective randomized studies.
This study was approved by the Ethical Committee of the Cancer Center of Sun Yat-Sen University and it followed the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from every patient before treatment.
From September 1991 to December 2004, 1830 patients with unresectable HCC underwent TACE at the Cancer Center of Sun Yat-sen University. All patients received routine preoperative investigations, including liver function tests, α-fetoprotein (AFP), hepatitis serology, chest radiography, abdominal ultrasonography, and abdominal CT scanning with contrast-enhancement of images in the arterial and portal venous phases. AFP > 200 ng/mL (normal, < 20 ng/mL) refers to an abnormal elevation of the tumor marker. Further investigations were performed in those with suspected extrahepatic metastases. Only patients who met the following inclusion criteria were enrolled: (1) the hepatic lesion was clinicopathologically diagnosed as HCC based on the diagnostic criteria used by the European Association for the Study of the Liver[16]; (2) there was not any treatment prior to TACE; and (3) the HCC was considered to be unresectable by our leading investigator (Professor Jin-Qing Li). Unresectable disease was defined as extensive bilobular involvement of the liver by a large solitary tumor or by multiple tumors, or invasion of major blood vessels including the main portal vein, hepatic veins, inferior vena cava, and main hepatic artery. Patients with any of the following were excluded: (1) obstructive jaundice; (2) hepatic encephalopathy; (3) Child-Pugh score > 11; and (4) poor data integrity.
Finally, 1569 patients were recruited, comprising 1423 (90.7%) males and 146 (9.3%) females with a mean age of 49.36 ± 11.62 (mean ± SD) years. Diagnosis of HCC was based on biopsy in 131 (8.3%) patients, AFP plus radiology in 1167 (74.4%), and radiology alone in 271 (17.3%), respectively.
There were 1338 (86.0%) patients who tested positive for Hepatitis virus B surface antigen (HbsAg) and 48 (3.1%) positive for hepatitis virus C antibody. The mean tumor size was 10.53 ± 4.09 cm (range, 1.0-28.0 cm) and macrovascular invasion was seen in 293 (18.7%) patients. Tumor(s) with direct invasion of adjacent organs was seen in 30 (1.9%) patients. The rate of regional lymph node involvement was 3.2% (n = 50) according to the CT-revealed threshold size (larger than 1.0 cm). In total, 269 (17.1%) patients were found with distant metastases to lung [221 (82.3%)], bone [24 (9.0%)], adrenal gland [17 (6.4%)], pelvic cavity [3 (1.1%)], kidney [2 (0.7%)], respectively. According to the TNM classification[17], 502 (32.0%) patients were classified as Stage I, 91 (5.8%) as Stage II, 659(42.0%) as Stage IIIa, 14 (0.9%) as Stage IIIb, 34 (2.2%) as Stage IIIc, and 269 (17.1%) as Stage IV. According to the Child-Pugh classification, 1413 (90.8%) patients were Child-Pugh class A, 144 (9.2%) were class B, and none were class C.
All patients gave informed consent before any procedure. TACE was performed using the Seldinger technique. A selective 5 French catheter was introduced and visceral angiography was carried out to assess the arterial blood supply to the liver and to confirm patency of the portal vein. Depending on the size, location and arterial supply of the tumor, the tip of the catheter was advanced into the right or left hepatic artery, or tumor-feeding branches (as there was a need for super selective catheterization in some cases).
Hepatic artery infusion chemotherapy was performed using one drug or combinations of mitomycin C, carboplatin, and fluorouracil. After that, chemoembolization was performed using doxorubicin mixed with 5 mL of lipiodol (Lipiodol Ultra-Fluide; Andre Guerbet Laboratories, Aulnay-Sous-Bois, France). Pure lipiodol or gelatin sponge particles were then injected if the chemoembolized artery territory didnot show stagnant flow. The dose of anticancer agent-lipiodol emulsion and the pieces of embolic materials used for TACE were determined based on the tumor size, extension of the lesions and tumor blood supply.
After the initial TACE treatment, patients were followed up by CT scan every 1 to 2 mo to evaluate their tumor status. The survival of TACE-treated patients was calculated from the date of procedure through to the end of follow-up on March 17, 2008. All patient deaths, irrespective of causes, were deemed as the end point. The mean length of follow-up was 16.62 mo (range, 0.07-133.27). TACE-related death was defined as death within 1 mo of the initial therapy.
New TACEs were performed every 4-10 wk until CT scans and AFP levels suggested the stabilization of the tumor, and unless the patient rejected further treatment or it was not technically feasible either because of hepatic artery occlusion or impaired liver function. The patients were started on multimodality therapy (including surgery, local ablation, radiotherapy, and systemic chemotherapy) when treatment options other than TACE were indicated.
There were 838 (53.4%) patients treated with only one session of TACE, 447 (28.5%) with two sessions, 156 (9.9%) with three sessions, and 128 (8.2%) with more than three sessions. On average, 1.79 sessions (range, 1 to 11) of TACE were given to each patient. There were 359 patients on multimodality therapy, among which 107 patients underwent exploratory laparotomy. Surgical resection was performed in 97 (6.2%), 7 underwent liver transplantation, 3 underwent lung resection, 217 (13.8%) underwent local ablation (including alcohol injection, radiofrequency, microwave), 40 (2.5%) underwent radiotherapy and 60 (3.8%) underwent systemic chemotherapy.
Pretreatment variables with possible prognostic significance were analyzed. Each variable was stratified into 2-4 strata. The survival rates were obtained by the Life Table method. Median survival lengths were obtained by the Kaplan-Meier method and compared by the log-rank test. Multivariate analysis was performed with the Cox proportional hazards model. All variables with a P value less than 0.05 by univariate analysis were subjected to multivariate analysis. All significance tests were rendered 2-tailed, and a P value less than 0.05 was considered statistically significant. All statistical processing was performed using the Statistical Package for Social Science V8.0 (SPSS, Inc., Chicago, IL, USA).
During a 13 years follow-up, the total numbers of patient deaths, patients lost to follow-up (in our institute the follow-up is once a year; if a patient cannot be followed up after 1 year, they are considered to be lost to follow-up) and alive cases were 1301 (82.9%), 203 patients (12.9%) and 65 (4.1%), respectively. The annual distribution of deaths was 798 in year 1, 328 in year 2, 85 in year 3, 56 in year 4, 17 in year 5, 8 in year 6, 5 in year 7, 1 in year 9, and 3 in year 12, respectively. The number of patients lost to follow-up was 106 in year 1, 18 in year 2, 27 in year 3, 12 in year 4, 8 in year 5, 4 in year 6, 11 in year 7, 11 in year 8, 3 in year 9, and 1 each in years 10 through 12, respectively.
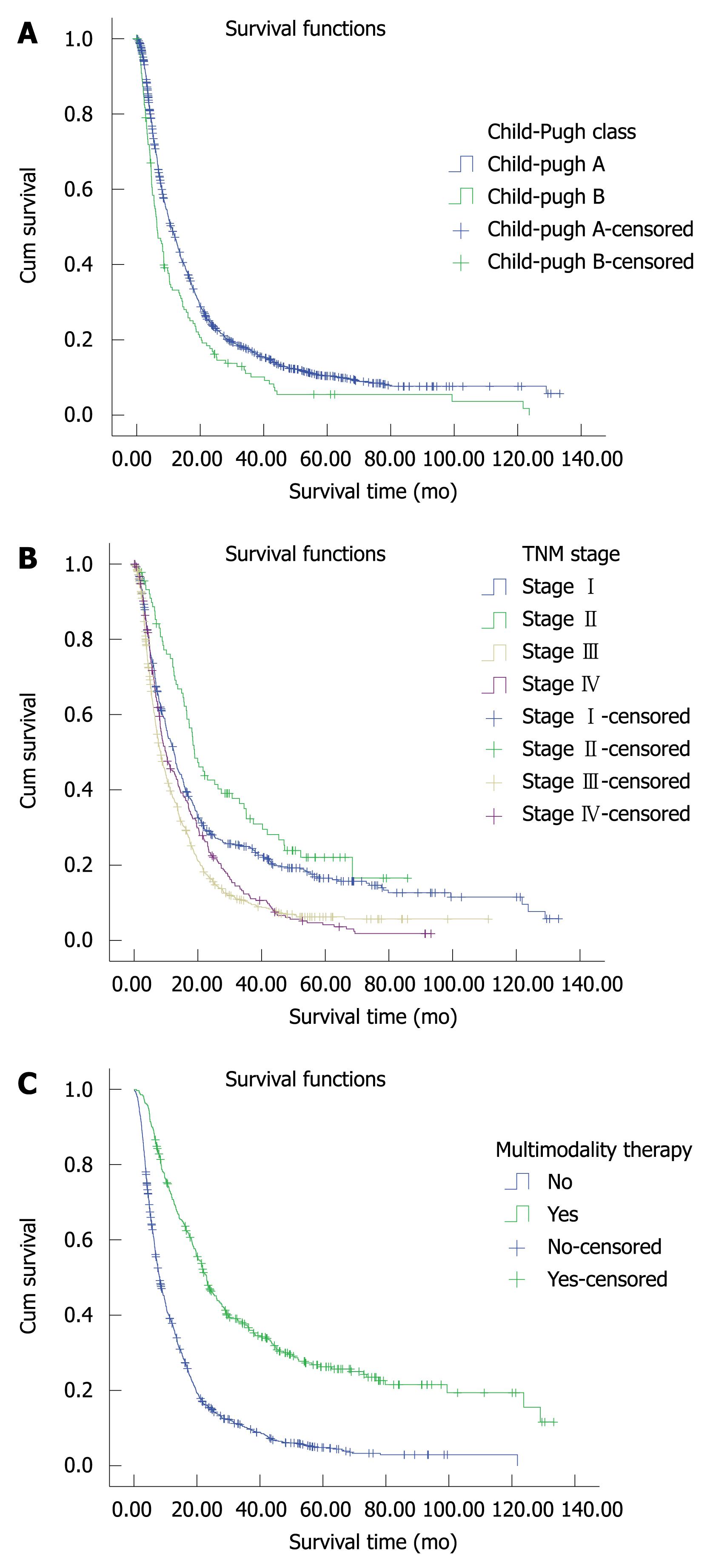
Of the 1569 patients who underwent TACE as initial treatment, the overall median length of survival and 1-, 2-, 3-, 4-, 5-, 10-, and 12-year survival rates were 10.37 mo, 47%, 24%, 17%, 12%, 10%, 7%, and 4%, respectively. Kaplan-Meier analysis showed a significant difference in median survival length between patients with Child-Pugh class A and those with class B (Figure 1A) but not among patients with different TNM stages. Patients with Stage II disease showed the best survival rate (Figure 1B).
The median length of survival and 1-, 2-, 3-, 4-, 5-, 10-, 12-year survival rates among patients on multimodality therapy were 23.13 mo, 72%, 47%, 37%, 30%, 26%, 19%, and 12%, compared with 8.23 mo, 40%, 16%, 11%, 7%, 5%, 3%, and 0, respectively, among those treated with TACE alone. There was a statistical difference in survival between these two subsets of patients (P = 0.000) (Figure 1C).
Univariate analysis revealed 9 pretreatment factors as prognostic variables: age, hepatitis B virus (HBV), AFP, Child-Pugh class, nodules, greatest dimension, tumor capsule, macrovascular invasion, and regional lymph node metastasis (Table 1). Multivariate analysis showed the following as independent prognostic factors: regional lymph nodes metastasis, Child-Pugh class, macrovascular invasion, greatest dimension, AFP, HBV, tumor capsule, and nodules with hazard ratios ranging from 1.164 to 1.736 (Table 2).
| Variable | Patients | Survival time (%) | Median survival length (mo) | P value | ||
| n (%) | 1 yr | 5 yr | 10 yr | |||
| Age (yr) | ||||||
| ≤ 50 | 848 (54.0) | 46 | 9 | 7 | 9.63 | 0.003 |
| > 50 | 721 (46.0) | 49 | 11 | 7 | 11.60 | - |
| HBV | ||||||
| HBsAg(-)HBeAg(-) | 215 (13.8) | 55 | 15 | 12 | 13.70 | 0.000 |
| HBsAg(+)HBeAg(-) | 811 (52.2) | 50 | 11 | 9 | 11.43 | - |
| HBsAg(+)HBeAg(+) | 529 (34.0) | 40 | 7 | 3 | 8.27 | - |
| AFP (ng/mL) | ||||||
| ≤ 20 | 292 (19.0) | 61 | 18 | - | 15.80 | 0.000 |
| 21-200 | 296 (19.3) | 56 | 13 | 9 | 13.50 | - |
| > 200 | 946 (61.7) | 41 | 7 | 6 | 8.53 | - |
| Child-Pugh grade | ||||||
| A | 1413 (90.8) | 49 | 11 | 8 | 10.97 | 0.000 |
| B | 144 (9.2) | 34 | 6 | 4 | 6.40 | - |
| Nodules | ||||||
| ≤ 3 | 855 (54.5) | 52 | 12 | 8 | 12.30 | 0.000 |
| > 3 | 714 (45.5) | 42 | 8 | - | 8.57 | - |
| Greatest dimension (cm) | ||||||
| ≤ 5.0 | 164 (10.5) | 71 | 25 | 16 | 19.03 | 0.000 |
| 5.1-10 | 624 (39.8) | 50 | 11 | 6 | 11.77 | - |
| 10.1-15 | 606 (38.6) | 43 | 8 | 7 | 9.10 | - |
| > 15 | 175 (11.2) | 29 | 3 | - | 6.10 | - |
| Tumor capsule | ||||||
| No | 818 (52.8) | 41 | 9 | 5 | 8.40 | 0.000 |
| Yes | 732 (47.2) | 55 | 11 | 9 | 12.50 | - |
| Macrovascular invasion | ||||||
| No | 1276 (81.3) | 50 | 12 | 8 | 11.70 | 0.000 |
| Yes | 293 (18.7) | 34 | 3 | - | 7.10 | - |
| Regional lymph node metastasis | ||||||
| No | 1519 (96.8) | 48 | 10 | 7 | 10.50 | 0.032 |
| Yes | 50 (3.2) | 31 | 8 | - | 7.10 | - |
| Distant metastasis | ||||||
| No | 1302 (82.9) | 48 | 12 | 8 | 10.40 | 0.183 |
| Yes | 269 (17.1) | 46 | 4 | - | 10.13 | - |
| Variable | P value | Hazard ratio (95% CI) |
| Regional lymph nodes metastasis | 0.001 | 1.736 (1.272-2.370) |
| Child-Pugh class | 0.017 | 1.254 (1.041-1.510) |
| Macrovascular invasion | 0.002 | 1.253 (1.083-1.450) |
| Greatest dimension | 0.000 | 1.250 (1.167-1.340) |
| AFP | 0.000 | 1.237 (1.149-1.333) |
| HBV | 0.000 | 1.232 (1.130-1.343) |
| Tumor capsule | 0.004 | 1.192 (1.059-1.343) |
| Nodules | 0.012 | 1.164 (1.034-1.310) |
Severe complications occurred in 353 (22.5%) patients after the initial TACE, including abnormal embolization in 9 patients, ascites in 235, pleural effusion in 32, acute renal failure in 4, hepatic failure in 38, jaundice in 197, upper gastrointestinal bleeding in 12, respiratory tract infection in 16, and abdominal infection in 3. Among these, there were 22 (1.4%) cases of TACE-related death with 20 caused by hepatic failure and 2 by upper gastrointestinal bleeding.
For patients of unresectable HCC, the overall median survival was 8.7 wk[18] and 3 mo[19] in two untreated populations. The goal of TACE as a palliative treatment is to prolong survival and to enhance quality of life. Unlike neoplasms of other organs, the survival of HCC depends not only on tumor status, but also on hepatic functional reserve, performance status, and response to treatment[14,20,21].
In the current study, performance status was not assessed because of data unavailability. Given that all pre-TACE HCCs were unresectable in our cohort, the eligibility for other treatment modalities after initial TACE may indirectly reflect patient response to treatment. In our effort to screen for prognosis-related factors using univariate analysis of pretreatment factors indicated as significant, subsequent multivariate analysis stratified by multimodality therapy showed 8 factors as independently prognostic: regional lymph nodes metastasis, Child-Pugh class, macrovascular invasion, greatest dimension, AFP, HBV, tumor capsule, and nodules. These factors could be classified into 3 groups in nature: tumor status, hepatic function reserve, AFP and HBV. While AFP level has been shown to be an independent prognostic factor in many studies[7,10,13,14,20,22,23], the cutoff values of AFP level varied widely. In the current study, survival rates appeared statistically different among patients with AFP being less than 20, 21-200, and greater than 200 ng/mL. As an important causal factor of HCC in the current study, HBV infection was seen in most of the patients with a prevalence (86.2%) similar to data from Hong Kong[5] (80.0%), but higher than those from Japan[7] (11.0%), Spain[6] (6.3%), and America[24] (49.0%). However, HBV status as an independent prognostic factor has seldom been reported[25]. As shown by Kaplan-Meier analysis in our study, the survival rates in HBsAg-negative and HBsAg-positive HBeAg-negative individuals didnot differ from each other (P = 0.062), but were statistically higher compared with HBeAg-positive patients (P = 0.000). These findings suggested the potential benefits of antiviral therapy in HBeAg-positive patients. As with almost all reports related to TACE, in the current study Child-Pugh class was an independent prognostic factor. As to the tumor status, macrovascular invasion, greatest dimension, and nodules were proven by a large prospective cohort study[7]. Tumor capsule has been rarely noted as a prognostic factor[23]. Regional lymph node metastasis as independent factor was not assessed fully due to lack of data in most literature.
Surprisingly, adjacent organs invasion and distant metastasis, two of major components in TNM staging system, were not independent prognostic factors. Zhang et al[26] showed that 67.5% Stage IV HCC patients died of liver failure caused by the progressive intrahepatic lesions and only 20% of those died of respiratory failure due to metastatic lesions. These results may explain why the survival rates of distant metastasis patients were not different from that of local unresectable HCC patients who underwent TACE. The progressive treatment for intrahepatic lesions may be of importance for Stage IV HCC. Our data supported the concept that the validity of the TNM stage system had only been assessed in patients who underwent resection[27].
In the current study, tumor status and liver function were main prognostic factors. Since only 144 (9.2%) patients were Child-Pugh class B, the overall survival rates were largely determined by tumor status. Overall, the median survival length and survival rates in our study appeared somewhat dissatisfactory as compared with results from a meta-analysis[28], which revealed survival rates of 62% ± 20%, 30% ± 15%, and 19% ± 16% at 1, 3, and 5 years, along with a mean survival time of 18 ± 9 mo. This may be explained by a higher percentage of patients with locally advanced lesions included in our study than in others. To the best of our knowledge, the mean greatest dimension (10.53 ± 4.09 cm) in the current study may be greater than any lesions reported before. However, the 10-year survival rate up to 7% in the whole group and 3% in TACE group supported the concept of heterogeneity of HCC in terms of survival.
In the current study, 353 (22.5%) patients developed severe complications after the initial TACE, which was mostly correlated to liver function. TACE-related death occurred in 22 (1.4%) patients, in which 20 deaths were caused by hepatic failure and 2 by upper gastrointestinal bleeding. While acute liver function impairment may arise from TACE treatment, Caturelli et al[29] showed that TACE didnot induce significant long term worsening of liver function in patients with class A or B cirrhosis. Hence, the relations among TACE variables, interval of TACE treatment, survival, and complications need to be further explored.
In conclusion, tumor status, hepatic function reserve, AFP, and HBV status were independent prognostic factors for unresectable HCC treated by TACE. Distant metastasis might not be a contraindication for TACE treatment. For a better survival, once the tumor status allows treatments other than TACE, multimodality therapy should be recommended.
Hepatocellular carcinoma (HCC) constitutes the majority of liver cancers, which is the sixth most common cancer and the third most common cause of cancer death worldwide. A great number of HCC patients are suitable for curative therapy due either to advanced stage of disease or to poor liver function at the time of diagnosis. Transarterial chemoembolization (TACE) has become the most popular modality for palliative treatment among these patients. However, suitable candidates for TACE are still difficult to determine.
Two randomized trials from Europe and Asia have confirmed better survival associated with TACE compared with conservative treatment in selected patients. But the survival and prognostic factors of different studies varied due to the difference in patient baseline characteristics, treatment procedure, and regimens used. Hence, it is important to identify the prognostic factors associated with different geographical distributions of HCC.
In the current study, the pretreatment prognostic factors of regional lymph node metastasis, Child-Pugh class, macrovascular invasion, greatest dimension, α-fetoprotein, Hepatitis virus B, tumor capsule, and nodules were similar to other studies. However, three important items were indicated: (1) distant metastasis might not be a contraindication to TACE; (2) antiviral therapy in HBeAg-positive patients might benefit survival; (3) multimodality therapy might improve survival.
The finding of this study might help to select patients who would benefit most from TACE, and help to design and estimate samples for further prospective randomized studies.
In this manuscript, the authors retrospectively reviewed their experience with TACE in unresectable HCC. This experience is quite extensive, as it concerns 1569 patients over a 13-year period.
Peer reviewer: Dr. Olivier Detry, Department of Abdominal Surgery and Transplantation, University of Liège, CHU Sart Tilman B35, B-4000 Liège, Belgium
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [Cited in This Article: ] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [Cited in This Article: ] |
| 3. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [Cited in This Article: ] |
| 4. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. [Cited in This Article: ] |
| 5. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [Cited in This Article: ] |
| 6. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [Cited in This Article: ] |
| 7. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [Cited in This Article: ] |
| 8. | Dvorchik I, Carr BI. A simple prognostic scoring system for patients with unresectable hepatocellular carcinoma treated by chemo-embolization. Cancer Detect Prev. 2007;31:154-160. [Cited in This Article: ] |
| 9. | Hatanaka Y, Yamashita Y, Takahashi M, Koga Y, Saito R, Nakashima K, Urata J, Miyao M. Unresectable hepatocellular carcinoma: analysis of prognostic factors in transcatheter management. Radiology. 1995;195:747-752. [Cited in This Article: ] |
| 10. | Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574-1581. [Cited in This Article: ] |
| 11. | Ikeda M, Okada S, Yamamoto S, Sato T, Ueno H, Okusaka T, Kuriyama H, Takayasu K, Furukawa H, Iwata R. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol. 2002;32:455-460. [Cited in This Article: ] |
| 12. | Wigmore SJ, Redhead DN, Thomson BN, Parks RW, Garden OJ. Predicting survival in patients with liver cancer considered for transarterial chemoembolization. Eur J Surg Oncol. 2004;30:41-45. [Cited in This Article: ] |
| 13. | Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY, Park YO, Kim WT, Byun JH. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352-361. [Cited in This Article: ] |
| 14. | Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50-57. [Cited in This Article: ] |
| 15. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [Cited in This Article: ] |
| 16. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [Cited in This Article: ] |
| 17. | Greene FL, Balch CM, Fleming ID, Fritz A, Haller DG, Morrow M, Page DL. AJCC Cancer Staging Handbook. New York: Springer Verlag 2002; . [Cited in This Article: ] |
| 18. | Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386-391. [Cited in This Article: ] |
| 19. | Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100:1995-2004. [Cited in This Article: ] |
| 20. | Carr BI, Buch SC, Kondragunta V, Pancoska P, Branch RA. Tumor and liver determinants of prognosis in unresectable hepatocellular carcinoma: a case cohort study. J Gastroenterol Hepatol. 2008;23:1259-1266. [Cited in This Article: ] |
| 21. | Bonnetain F, Paoletti X, Collette S, Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Barbare JC, Bedenne L. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res. 2008;17:831-843. [Cited in This Article: ] |
| 22. | O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325-331. [Cited in This Article: ] |
| 23. | Savastano S, Miotto D, Casarrubea G, Teso S, Chiesura-Corona M, Feltrin GP. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with Child’s grade A or B cirrhosis: a multivariate analysis of prognostic factors. J Clin Gastroenterol. 1999;28:334-340. [Cited in This Article: ] |
| 24. | Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, Benjamin MS, Sherman M, Gallinger S, Burnett B, Feld R, Chen E, Greig PD, Grant DR, Knox JJ. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol). 2006;18:684-692. [Cited in This Article: ] |
| 25. | Ahmad SA, Bilimoria MM, Wang X, Izzo F, Delrio P, Marra P, Baker TP, Porter GA, Ellis LM, Vauthey JN. Hepatitis B or C virus serology as a prognostic factor in patients with hepatocellular carcinoma. J Gastrointest Surg. 2001;5:468-476. [Cited in This Article: ] |
| 26. | Zhang SM, Zeng ZC, Tang ZY, Sun J, Cheng JM, Liu R, Wang P, Zhang BH. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int. 2008;2:237-243. [Cited in This Article: ] |
| 27. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [Cited in This Article: ] |
| 28. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [Cited in This Article: ] |
| 29. | Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123-128. [Cited in This Article: ] |