Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2396
Revised: November 5, 2009
Accepted: November 12, 2009
Published online: May 21, 2010
AIM: To investigate whether narrow band imaging (NBI) is a useful tool for the in vivo detection of angiogenesis in inflammatory bowel disease (IBD) patients.
METHODS: Conventional and NBI colonoscopy was performed in 14 patients with colonic inflammation (8 ulcerative colitis and 6 Crohn’s disease). Biopsy samples were taken and CD31 expression was assayed immunohistochemically; microvascular density was assessed by vessel count.
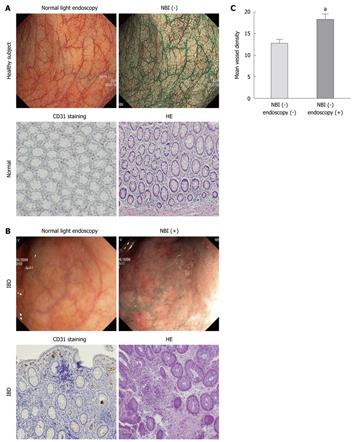
RESULTS: In areas that were endoscopically normal but positive on NBI, there was a significant (P < 0.05) increase in angiogenesis (12 ± 1 vessels/field vs 18 ± 2 vessels/field) compared with areas negative on NBI. In addition, in areas that were inflamed on white light endoscopy and positive on NBI, there was a significant (P < 0.01) increase in vessel density (24 ± 7 vessels/field) compared with NBI-negative areas.
CONCLUSION: NBI may allow in vivo imaging of intestinal angiogenesis in IBD patients.
- Citation: Danese S, Fiorino G, Angelucci E, Vetrano S, Pagano N, Rando G, Spinelli A, Malesci A, Repici A. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: A pilot study. World J Gastroenterol 2010; 16(19): 2396-2400
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2396
Angiogenesis plays a crucial role in neoplastic and non-neoplastic chronic inflammatory disorders[1-4], including the inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC)[4,5]. Potent angiogenic activity has been demonstrated in histological specimens from the mucosa of both UC and CD patients, as assessed by CD31 staining[6]. Several reports have shown that blockade of angiogenesis in preclinical models of IBD is a promising new therapeutic approach[7,8]. Narrow band imaging (NBI) is a new endoscopic technology that highlights mucosal surface structures and microcapillaries. Optical filters achieve sequential green and blue illumination, narrowing the bandwidth of spectral transmittance, and thus obtaining tissue illumination at selected narrow wavelength bands[9,10] to achieve the greatest contrast between vascular structures and the surrounding mucosa.
The diagnostic accuracy of NBI in detecting colorectal neoplasia in patients with or without concomitant UC is superior to conventional colonoscopy and equivalent to that of chromoendoscopy[11,12]. A recent meta-analysis concluded that NBI is accurate, with high diagnostic precision for in vivo diagnosis of neoplasia across a range of organs (colon, esophagus, duodenal ampulla and lung)[13].
Very recently, NBI has been proposed as a tool to assess the grade of inflammation in patients with inactive or mildly active UC[14]. In the preliminary study described herein, we investigated whether NBI colonoscopy could be a useful tool to detect in vivo angiogenesis in IBD patients with colonic inflammation.
This was an open study involving patients with a diagnosis of IBD referred to our Gastrointestinal Endoscopy Unit for follow-up colonoscopy. A total of 14 patients were included (8 UC and 6 colonic CD). The extent of the disease was determined by previous colonoscopy. At the time of enrollment in the study, 3 (3/8) UC patients presented with inactive disease (Mayo score = 0) while 5 (5/8) had active disease (2 patients Mayo score = 1, 2 patients Mayo score = 2 and 1 patient Mayo score = 1); 3 (3/6) patients with CD presented with inactive disease and 3 (3/6) had active disease. For CD patients endoscopic activity was assessed by Crohn’s Disease Endoscopic Index of Severity (CDEIS). After obtaining informed consent from all patients, white light colonoscopy and NBI (Olympus Medical System, Tokyo, Japan) examinations were performed.
For the white light colonoscopy, the vascular pattern was defined as normal if it did not show any irregularities, or as distorted if the pattern was tortuous. When the vascular pattern intensity was visualized with NBI, we were able to distinguish 2 different mucosal patterns: a stronger (blacker) capillary vascular pattern (NBI+), and a milder or regular capillary vascular pattern (NBI-). For this reason, in our study the vascular pattern could be classified into 4 categories: normal (with white light colonoscopy) and NBI-; distorted (with white light colonoscopy) and NBI-; normal (with white light colonoscopy) and NBI+; distorted (with white light colonoscopy) and NBI+.
For each patient, after determining the vascular pattern by NBI, biopsy specimens were obtained from 5 areas that were normal with conventional endoscopy and NBI-, 5 areas that were normal with conventional endoscopy but NBI+, and 5 areas that were endoscopically inflamed and NBI+. CD31 staining was performed by immunohistochemistry, and microvascular density was assessed by vessel count in colonic biopsies. The pathologist was blinded to the subjects.
The parametric data are expressed as the mean ± SD and non parametric data as percent. Fischer’s exact probability test and the χ2 test were used to evaluate statistical differences. A P-value less than 0.05 was considered statistically significant.
For each of the 14 patients enrolled in the study, the mucosal vascular pattern was assessed by both conventional and NBI colonoscopy.
In the uninflamed mucosa of patients with IBD, classified as normal with white light colonoscopy and NBI-, the NBI pattern was similar to that in the mucosa from healthy control individuals. At the same time, the vascularization pattern and the microvessel density, as revealed by CD31 staining, were similar in the specimens from the uninflamed, NBI- mucosa from patients with IBD and from healthy controls. No differences were found between UC and CD patients.
Compared to areas that were endoscopically normal under white light colonoscopy and NBI-, endoscopically normal but NBI+ areas displayed a significant (P < 0.05) increase in angiogenesis (12 ± 1 vessels/field vs 18 ± 2 vessels/field) (Figure 1A and B). The importance of our findings lies in the evidence that in patients with normal white light colonoscopy, areas positive on NBI showed an increased leukocyte infiltrate and a significantly increased microvessel density (P < 0.05) as assessed by histological analysis (Figure 1A-C). As revealed by staining for CD31, the mean microvessel diameter in IBD was 0.1 mm, a size histologically compatible with the diameter of a dot observed on the NBI image. No differences were found between UC and CD patients (not shown).
Lastly, in areas from IBD patients that were inflamed under white light endoscopy and were NBI+, a significant (P < 0.01) increase in vessel density (24 ± 7 vessels/field) was found compared with endoscopically normal, NBI- areas (Figure 2A and B), a finding compatible with a high degree of microscopical inflammation and immune-driven angiogenesis. No differences were found according to Mayo score in vessel density (not shown). No differences were found between UC and CD patients (not shown).
Angiogenesis is an integral component of non-neoplastic chronic inflammatory disorders, such as IBD[15,16]. Microscopic imaging is the approach that has thus far proven most valid for quantification of vasculature in normal and pathological tissue[17-19]. NBI has been successfully used to visualize angiogenesis and thereby to detect cancerous areas in the colon. Because of the improved mucosal contrast provided, NBI may improve the detection of colon polyps compared with standard white light colonoscopy. NBI has also been widely used to detect dysplasia in patients with long-standing UC, achieving good results in terms of diagnostic accuracy and detection of polyps, although if did not show any statistically significant differences compared with standard white light colonoscopy[19,20].
In a recently published study, NBI was proposed as a tool to assess the grade of inflammation in patients with inactive or mildly active UC[12]. In our study, we found that NBI could also be used to visualize areas of abnormal microvascular changes, not observed at white light colonoscopy. A statistically significant correlation exists between NBI pattern positivity (both in inflamed and normal areas at conventional endoscopic examination) and microvessel density, as confirmed by CD31 staining in histological specimens from the same areas. In patients who were normal under standard colonoscopy, there was increased leukocyte infiltration and microvessel density in NBI+ areas, as assessed by histological analysis.
Blockade of angiogenesis could be beneficial in patients with chronic inflammation and some drugs that have demonstrated efficacy for the treatment of IBD, such as tumor necrosis factor-α inhibitors, have potent antiangiogenic activity. Our preliminary findings suggest that NBI could be a novel tool for the in vivo assessment of mucosal angiogenesis. However since our study is still a preliminary study because of the limited number of patients, a larger study should be performed to define the exact role of NBI in IBD patients, and the correlation of mucosal angiogenesis with endoscopic activity.
Endoscopic imaging and monitoring of angiogenesis has the potential to be a valuable biomarker in monitoring the grade of intestinal inflammation in vivo, monitoring response to and optimization of available treatments, and finally in evaluating the response to new agents for the treatment of IBD in randomized clinical trials.
Angiogenesis plays a crucial role in neoplastic and non-neoplastic chronic inflammatory disorders, including inflammatory bowel diseases (IBD).
Narrow-band imaging (NBI) is a new endoscopic technology that highlights mucosal surface structures and microcapillaries. A recent meta-analysis concluded that NBI is accurate, with high diagnostic precision for in vivo diagnosis of neoplasia across a range of organs (colon, esophagus, duodenal ampulla and lung).
Very recently, NBI has been proposed as a tool to assess the grade of inflammation in patients with inactive or mildly active ulcerative colitis. In this preliminary study, the authors investigated whether NBI colonoscopy could be a useful tool to detect in vivo angiogenesis in IBD patients with colonic inflammation.
Several reports have shown that blockade of angiogenesis in preclinical models of IBD is a promising new therapeutic approach. Visualize angiogenesis in vivo may represent the first step for such a therapeutic approach.
Angiogenesis: the process of new capillary formation from pre-existing vasculature in adult tissues; Narrow-band imaging: a new endoscopic technology that highlights mucosal surface structures and microcapillaries.
The authors report that NBI may be a novel modality for imaging of intestinal angiogenesis in IBD. The results provide sufficient evidence to draw scientific conclusions. The statistical data reflect the results and are adequate for a clinical study. The discussion is well organized, and valuable conclusions are provided.
Peer reviewers: Atsushi Nakajima, MD, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan; Javier San Martín, MD, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay
S- Editor Wang JL L- Editor Cant MR E- Editor Lin YP
| 1. | Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931-10934. [Cited in This Article: ] |
| 2. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [Cited in This Article: ] |
| 3. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [Cited in This Article: ] |
| 4. | Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-660. [Cited in This Article: ] |
| 5. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [Cited in This Article: ] |
| 6. | Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060-2073. [Cited in This Article: ] |
| 7. | Danese S, Scaldaferri F, Vetrano S, Stefanelli T, Graziani C, Repici A, Ricci R, Straface G, Sgambato A, Malesci A. Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease. Gut. 2007;56:1248-1256. [Cited in This Article: ] |
| 8. | Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585-595.e5. [Cited in This Article: ] |
| 9. | Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76-81. [Cited in This Article: ] |
| 10. | Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kwon R, Mamula P, Rodriguez B, Shah RJ. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581-589. [Cited in This Article: ] |
| 11. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [Cited in This Article: ] |
| 12. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [Cited in This Article: ] |
| 13. | East JE, Tan EK, Bergman JJ, Saunders BP, Tekkis PP. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther. 2008;28:854-867. [Cited in This Article: ] |
| 14. | Kudo T, Matsumoto T, Esaki M, Yao T, Iida M. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis. 2009;24:495-501. [Cited in This Article: ] |
| 15. | Szekanecz Z, Koch AE. Vascular endothelium and immune responses: implications for inflammation and angiogenesis. Rheum Dis Clin North Am. 2004;30:97-114. [Cited in This Article: ] |
| 16. | Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457-465. [Cited in This Article: ] |
| 17. | Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-180. [Cited in This Article: ] |
| 18. | Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249-1264. [Cited in This Article: ] |
| 19. | Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227-1230. [Cited in This Article: ] |
| 20. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. [Cited in This Article: ] |