Published online Feb 7, 2009. doi: 10.3748/wjg.15.599
Revised: December 3, 2008
Accepted: December 10, 2008
Published online: February 7, 2009
AIM: To determine if disruption of the cagA gene of Helicobacter pylori (H pylori) has an effect on the expression of other proteins at proteome level.
METHODS: Construction of a cagA knock out mutant Hp27_ΔcagA (cagA-) via homologous recombination with the wild-type strain Hp27 (cagA+) as a recipient was performed. The method of sonication-urea-CHAPS-DTT was employed to extract bacterial proteins from both strains. Soluble proteins were analyzed by two-dimensional electrophoresis (2-DE). Images of 2-DE gels were digitalized and analyzed. Only spots that had a statistical significance in differential expression were selected and analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). Biological information was used to search protein database and identify the biological function of proteins.
RESULTS: The proteome expressions between wild-type strain and isogenic mutant with the cagA gene knocked-out were compared. Five protein spots with high abundance in bacteria proteins of wild-type strains, down-regulated or absently expressed in bacteria proteins of mutants, were identified and analyzed. From a quantitative point of view, the identified proteins are related to the cagA gene and important antioxidant proteins of H pylori, including alkyl hydroperoxide reductase (Ahp), superoxide dismutase (SOD) and modulator of drug activity (Mda66), respectively, suggesting that cagA is important to maintain the normal activity of antioxidative stress and ensure H pylori persistent colonization in the host.
CONCLUSION: cagA gene is relevant to the expressions of antioxidant proteins of H pylori, which may be a novel mechanism involved in H pylori cagA pathogenesis.
-
Citation: Huang ZG, Duan GC, Fan QT, Zhang WD, Song CH, Huang XY, Zhang RG. Mutation of cytotoxin-associated gene A affects expressions of antioxidant proteins of
Helicobacter pylori . World J Gastroenterol 2009; 15(5): 599-606 - URL: https://www.wjgnet.com/1007-9327/full/v15/i5/599.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.599
Helicobacter pylori (H pylori), a spiral-shaped bacterium that colonizes the human gastric mucosa, is estimated to inhabit at least half of the world’s human population[1]. Infection with H pylori is associated with development of peptic ulcer, gastric carcinoma and gastric mucosa-associated lymphoid tissue lymphomas[2–4]. However, little is known about the molecular mechanisms of pathogenesis induced by H pylori and the fundamental causes of diversity in infection outcomes. Cytotoxin-associated gene A protein (CagA) is a H pylori immuno-dominant antigen with its gene residing in the cag pathogenicity island, which is a 40-kilobase insertion containing genes involved in virulence[5]. Recent studies indicate that CagA is delivered from H pylori into the cytoplasm of H pylori-attached gastric epithelial cells via the type-IV secretion system[6]. Upon membrane localization, translocated CagA interacts with a number of host proteins involving cell signaling in a tyrosine phosphorylation-dependent and -independent manner[78]. Epidemiological studies have shown that cagA- positive H pylori strains are associated with higher grades of gastric mucosal inflammation as well as severe atrophic gastritis and gastric carcinoma[910]. CagA is considered a marker of increased pathogenic potential and may play an important role in the pathogenesis induced by H pylori.
China is one of the nations with the highest H pylori infection incidence[11]. More than 90% isolated strains possess cagA gene[12]. However, the biological activity of cagA gene still remains unclear. In the present study, we analyzed two related H pylori strains, Hp27 and Hp27_ΔcagA, through a proteomic approach. Wild type strain Hp27 is a cagA gene possessor, while strain Hp27_ΔcagA is an isogenic cagA knock out mutant. Proteins with altered expressions can be separated by two-dimensional electrophoresis (2-DE) and conclusively identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the peptide digests. This study was to determine if disruption of the cagA gene has an effect on the expression of other proteins of H pylori at proteome level.
Hp27 (cagA+) isolated from a patient with chronic atrophy gastritis was grown on Brucella agar plates containing 10% sheep blood supplemented with 10 mg/L vancomycin, 2500 U/L polymyxin B, 2 mg/L amphotericin and 5 mg/L trimethoprim, in an anaerobic jar consisting of 50 mL/L O2, 100 mL/L CO2, and 850 mL/L N2 at 37°C for 3 d. Kanamycin (25 mg/L) was added for mutated strains selection.
Isogenic Hp27_ΔcagA mutant was obtained from strain Hp27 as follows. In brief, we produced a Hp27_ΔcagA isogenic mutant harboring a total cagA deletion as previously described[13]. H pylori were grown on blood agar plates under microaerophilic conditions for 3 d and genomic DNA was extracted with a genomic DNA purification kit (Takara). An upstream (U fragment) and a downstream region (D fragment) of the cagA gene were amplified over the genomic DNA as homologous arms using the P1/P2 and P3/P4 primer pairs, respectively. A kanamycin-resistant gene was amplified as a screening marker from pEGFP-N2 vector (Clontech) using the P5/P6 primer pair. The primer sequences are as follows (restriction sites are underlined): P1-Xho I: 5'-GCGCTCGAGACTTTCTTGTAGCTGTC-3'; P2-Hind III: 5'-GGCAAGCTTTGTTTCTCCTTTACT-3'; P3-Pst I: 5'-GGCTGCAGAGGATTGAGGAATAC-3'; P4-Xba I: 5'-CGTCTAGATTTTAGCGATCAAACAAC-3'; P5-Hind III: 5'-GCAAGCTTATGATTGAACAAGATGGATTG-3'; P6-Pst I: 5'-GGCTGCAGTCAGAAGAACTCGTCAAGAAG-3'.
After digestion of PCR products with Xho I/Hind III (U fragment) and Pst I/Xba I (D fragment), the kanamycin resistance gene digested with Hind III/Pst I was introduced between the U and D fragments. The resulting chimera was cloned into the Xho I/Xba I-digested pBluescript SK II (-) vector (Stratagene) to obtain the construct targeting vector: pBSKΔcagA_kan. Hp27 served as a recipient strain was electrotransformated with pBSKΔcagA_kan. Bacteria were grown on blood agar plates for 48 h and then streaked on fresh selective plates supplemented with 25 &mgr;g/mL of kanamycin. A real cagA isogenic mutant (Hp27_ΔcagA) was selected from kanamycin-resistant colonies and the corresponding insertional DNA region was checked by PCR to confirm the exact recombination.
A wild-type strain of Hp27 and an isogenic mutant of Hp27_ΔcagA were harvested from Brucella agar plates and then washed 3 times with ice-cold PBS. Total protein was extracted with an appropriate volume (300 &mgr;L) of lysis buffer containing 8 mol/L urea, 65 mmol/L DTT, 2% CHAPS, 2 mmol/L PMSF, 0.5% IPG buffer, and protease inhibitor mixture. The extraction mixture was sonicated with parameters of 120 W, 5 min, pulse: 1S, 2S. The protein mixture was centrifuged at 12 000 r/min for 40 min. After transferred to a clean tube, the supernatant was stored at -70°C as aliquots. The protein concentration was determined by Bradford dye-binding assay with bovine serum albumin as the standard.
Two-dimensional electrophoresis was carried out as follows. Precast IPG strips (pH 3-10 linear, 18 cm, Amersham Pharmacia Biotechnology Inc.) were used in the first dimension. A total amount of 1000 &mgr;g protein was diluted to a total volume of 350 &mgr;L with the buffer containing 8 mol/L urea, 20 g/L CHAPS, 5 g/L IPG buffer 3-10, 20 mol/L DTT and a trace of bromophenol blue. After loaded on IPG strips, IEF was carried out on IPGphor (Bio-Rad,USA) according to the following protocol: rehydration for 16 h at 50 V, 1 h at 200 V, 1 h at 500 V, 1 h at 1000 V , 5 h at 10 000 V and 60 000 V h at 10 000 V (total 112.5 kVh). The current was limited to 50 &mgr;A per gel. After IEF separation, the strips were immediately equilibrated for 2 × 15 min with an equilibration solution containing 50 mmol/L Tris-HCl (pH 6.8), 6 mol/L urea, 300 g/L glycerol and 20 g/L SDS. Then, 20 mmol/L DTT was included in the first equilibration solution, and 20 g/L iodoacetamide was added in the second equilibration step to alkylate thiols. Electrophoresis in the second dimension was carried out on 15% SDS-PAGE gels (18 cm × 20 cm × 0.1 cm). The strips were held in place with 5 g/L agarose dissolved in a SDS/Tris running buffer and electrophoresis was carried out at a constant power (2.5 W/gel for 40 min and 15 W/gel for 5 h) using a Protean II xi cell gel SDS-PAGE system (Bio-Rad, USA).
After electrophoresis, gels were stained with Coomassie brilliant blue, equilibrated in a solution containing 500 mL/L methanol, 50 mL/L acetic acid and 25 g/L Coomassie brilliant blue R-250 for at least 2 h, and rinsed in 300 mL/L ethanol containing 70 mL/L acetic acid. Digitalized images were obtained by ImageScanner GS-800 (Bio-Rad, USA) scanning of the gels, and analyzed qualitatively and quantitatively by the PDQuest gel image analysis software 7.1 (Bio-Rad, USA). To determine variation, three gels were prepared for each sample. The computer analysis allowed automatic detection and quantification of protein spots as well as matching. The normalized volume of protein spots was used to analyze the differential level of protein expression. Only those spots that had a statistical significance in differential expression were selected for further investigation.
Differential protein spots were cut out from the gel. After being washed with 300 &mgr;L milliQ water for 15 min, each protein spot was decolorized with the successive action of 50 &mgr;L of 15 mmol/L potassium ferricyanide and 50 mmol/L sodium thiosulphate for 5-10 min. Faded gel pieces were dried in a vacuum centrifuge tube for 5 min. Cysteine reduction and alkylation were performed and incubated with 10 mmol/L DTT, 100 mmol/L NH4HCO3 at 56°C for 1 h in the dark. Gel pieces were dried again and incubated with 50 mmol/L fresh iodoacetamide in 100 mmol/L NH4HCO3 at room temperature for 30 min and rehydrated in digestion buffer containing 20 &mgr;L of 12.5 &mgr;g/mL modified trypsin and 20 mmol/L NH4HCO3 for 30 min in ice. Excess liquid was removed and the gel pieces were digested continuously at 30°C overnight (> 16 h). The resulting peptide mixture was extracted from the digested solution by centrifugation and resuspended in 10 &mgr;L of 50% CH3CN and 0.1% trifluoroacetic acid (TFA) for 10 min at 30°C on a shaking platform. Peptide mass maps were generated by Applied Biosystems Voyager System 6192 MALDI-TOF-mass spectrometry (ABI, USA). Peptide masses were analyzed using the MS-Fit search program (http://prospector.ucsf.edu/ucsfhtml4.0u/msfit.htm). The searching parameters were set up as follows: acquisition mass ranges 900-3500 Da, the mass tolerance was ± 0.5 Da; the number of missed cleavage sites was allowed up to 1; the minimum number of matched peptide was four; species was set as bacteria; and the searching range was within the experimental pI value ± 0.5 pH unit and experimental Mr ± 20%.
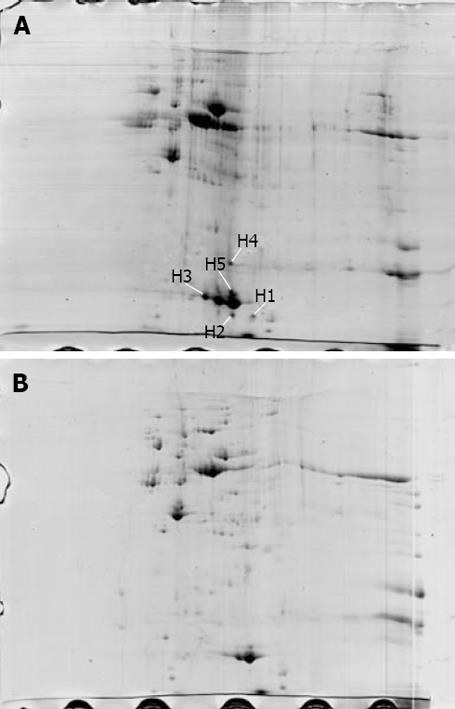
The proteome maps of H pylori strains Hp27 and Hp27_ΔcagA were produced and compared (Figure 1). After spot detection, background subtraction, volume normalization, differentially expressed proteins were detected in wide-type strain versus isogenic mutant. The arrows indicate five protein spots whose expression levels were significantly differently represented in strain Hp27 as compared to strain Hp27_ΔcagA.
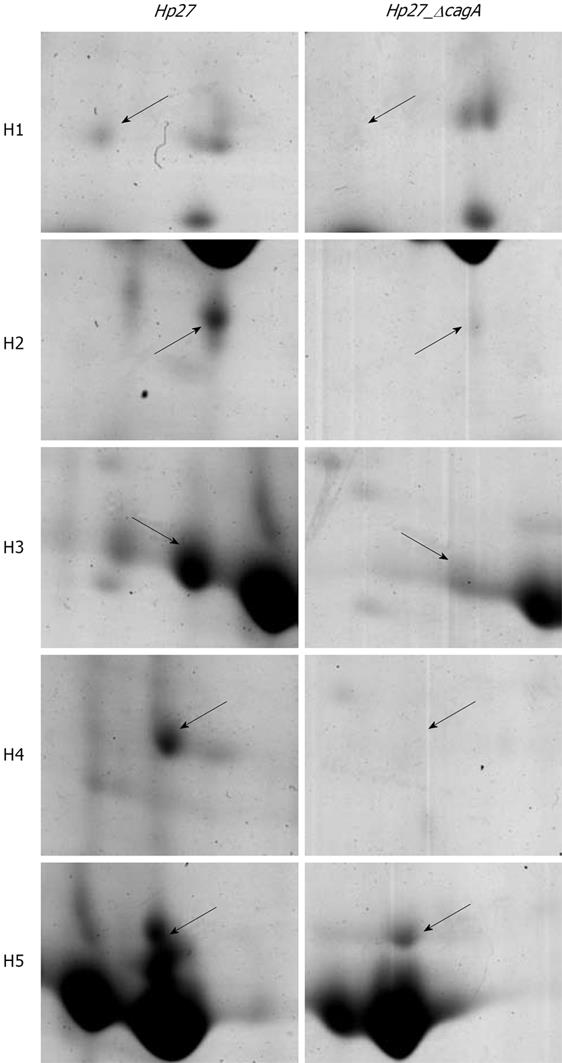
Expression levels of the two proteins were obtained by calculating the relative spot volume of each protein versus the total amount of protein in the gel. Segments of 2-DE gel map for five proteins derived from strains Hp27 and Hp27_ΔcagA are shown in Figure 2.
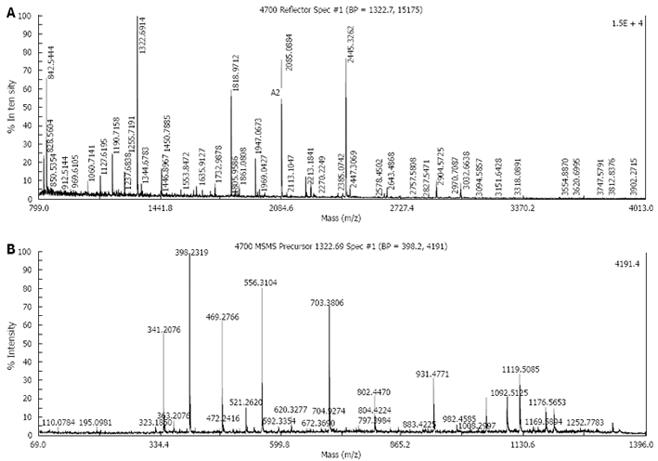
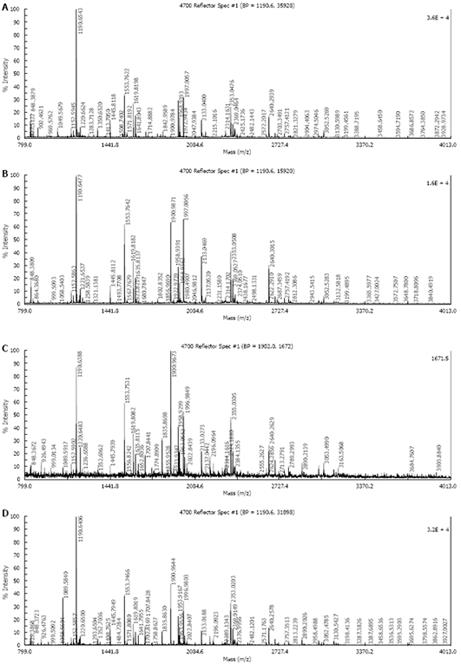
Five protein spots differentially expressed in strains Hp27 and Hp27_ΔcagA were excised from 2-DE gels and identified by peptide mass fingerprinting (Figures 3 and 4).
Mascot searches using the peptide mass fingerprinting data indicated that the differentially expressed proteins were alkyl hydroperoxide reductase (Ahp), superoxide dismutase (Sod) and modulator of drug activity (Mda66) (Table 1). These three proteins are all important antioxidant proteins of H pylori. From a quantitative point of view, these proteins are novel, and have not been reported previously in relation to cagA gene.
| Spot | Accession No. | MW | PI | Description | MOWS E score | Sequence coverage (%) |
| H1 | gi|2313748 | 21 591 | 6.59 | Modulator of drug activity (Mda66) | 62 | 46 |
| H2 | gi|2314747 | 22 221 | 5.88 | Alkyl hydroperoxide reductase (AhpC) | 111 | 67 |
| H3 | gi|2314747 | 22 221 | 5.88 | Alkyl hydroperoxide reductase (AhpC) | 140 | 76 |
| H4 | gi|2313490 | 24 602 | 5.77 | Superoxide dismutase (SOD) | 86 | 62 |
| H5 | gi|2313490 | 24 602 | 5.77 | Superoxide dismutase (SOD) | 132 | 69 |
A comparative proteome analysis was carried out between the two H pylori strains: Hp27, a wild-type strain and Hp27_ΔcagA, a cagA- isogenic mutant. Five differential protein spots, which are abundant in bacteria proteins of wild-type strains and are down-regulated or absently-expressed in bacteria proteins of mutants, were selected to perform in-gel trypsin digestion and MALDI-TOF-MS-based PMF analysis. The three identified proteins are Ahp, Sod and Mda66. Of the 5 position identifications, spots H2 and H3 were identified as the same protein Ahp, and spots H4 and H5 were also identified as the same protein Sod, indicating that these gene products are present as isoforms with post-translational modification[14].
Alkyl hydroperoxide reductase (AhpC), a thioredoxin (Trx)-dependent AhpC, is a member of the 2-Cys peroxiredoxins family (2-Cys Prxs). A group of thiol-specific antioxidant enzymes, which catalyze the reduction of hydrogen peroxide and organic hydroperoxides, are ubiquitous proteins that protect organisms from damage by reactive oxygen species[15]. H pylori are oxygen-sensitive microaerophilic bacteria, and contain many antioxidant proteins, among which AhpC is most abundant. The function of AhpC is to protect H pylori from a hyperoxidative environment by reducing toxic organic hydroperoxides[16].Wang et al[17] reported that mutant cells defective in AhpC are more sensitive to oxidative stress conditions, accumulate more free (toxic) iron, and suffer more DNA fragmentation compared to wild type cells. Olczak et al[18] tested the ability of strains with mutation in ahpC (encoding alkyl hydroperoxide reductase) to colonize the stomachs of mice, and showed that the mutant is clearly more sensitive than the parent strain to both oxygen and cumene hydroperoxide and unable to colonize mouse stomachs, whereas 78% of the mice inoculated with the parent strain become H pylori positive. Recently, Chuang et al[19] revealed that AhpC of H pylori acts as a peroxide reductase in reducing organic hydroperoxides and as a molecular chaperone in preventing protein misfolding under oxidative stress. Besides, AhpC could also influence the activity of other proteins. Catalase in ahpC mutant partially inactivated (approximately 50%) in comparison with the parent strain, indicating that organic hydroperoxides (the substrate of AhpC), which accumulate in ahpC mutant cells, are responsible for the inactivation of catalase[20]. In this study, the expression of AhpC was down-regulated in the cagA gene knocked-out mutant (Hp27_ΔcagA), suggesting that AhpC, one of the most important anti-oxidative stress proteins of H pylori, is related with the cagA gene.
Superoxide dismutase (SOD), a nearly ubiquitous enzyme in organisms exposed to toxic environments, is able to catalyze the conversion of superoxide radicals to hydrogen peroxide and molecular oxygen. Single SOD in H pylori, encoded by the sodB gene, has been suspected to be a virulence factor for this pathogenic microaerophile[21]. Seyler et al[22], who first isolated mutants with interruptions in the sodB gene, found that the sodB mutants are devoid of SOD activity, and more sensitive to O2 and H2O2 for both growth and viability. Since oxidative stress is correlated with DNA damage, they studied the frequency of spontaneous mutation to rifampin resistance. The frequency of mutagenesis of the sodB mutant strain is about 15-fold greater than that of the wild-type strain. Wang et al[17] also reported that mutant cells defective in SOD are more sensitive to oxidative stress conditions and suffer more DNA fragmentation compared to wild type cells, and that a significant proportion of cells of sodB mutant strains develop into stress-induced coccoid form or lysed as well as that they also contain a significantly higher amount of 8-oxo-guanine associated with their DNA, compared to wild type cells. Seyler et al[22] observed that only 1 out of 23 mice inoculated with a SOD-deficient mutant of a mouse-adapted strain became H pylori positive, while 15 out of 17 mice inoculated with the wild-type strain harbored the organism, in a mouse colonization model, indicating that SOD is a virulence factor affecting the ability of H pylori to colonize the mouse stomach and is important for the growth and survival of H pylori under oxidative stress conditions.
Mda66, identified in the ahpC napA double mutant by two-dimensional gel electrophoresis combined with N-terminal protein sequencing, is another possible antioxidant protein[23]. Single bacterial homologue is a MdaB protein of Escherichia coli (E. coli), first identified as a modulator of drug activity (named mda66) because the gene is mapped at 66 min on the E. coli chromosome[24]. Wang et al[25] demonstrated that, like its homologue in E. coli[26], Mda66 protein is a NADPH quinone reductase and able to reduce quinone to quinol. Quinone metabolism within cells has a direct effect on the cell’s ability to deal with oxidative stress[27]. Therefore, the reduced status of H pylori Mda66 protein plays an important role in the management of oxidative stress. Wang et al[25] reported that the wild-type strain could tolerate 10% oxygen, but the growth of mdaB mutant was significantly inhibited by 10% oxygen. The mda66 mutant was also more sensitive to H2O2, organic hydroperoxides, and paraquat, an agent generated by superoxide. Although the wild-type strain survived more than 10 h after air exposure, exposure of the mutant strain to air for 8 h resulted in no recovery of viable cells. It was reported that oxidative stress sensitivity of mda66 mutant can reduced the ability of mda66 mutant to colonize mouse stomachs[25]. H pylori were recovered from 10 of 11 mouse stomachs inoculated with the wild-type strain, with about 5000-45 000 CFU/g of stomach, while only 3 of 12 mice inoculated with the mdaB mutant strain did not harbor any H pylori, and contained less than 2000 CFU/g of stomach[25]. Therefore, the physiological function of H pylori Mda66 protein is similar to that of NADPH quinone reductase that plays an important role in the management of oxidative stress and contributes to successful colonization of the host.
Oxidative stress resistance is one of the key properties enabling pathogenic bacteria to escape the effects of reactive oxygen produced in the host. Therefore, proteins (enzymes) involved in oxidative stress resistance are the important factors for bacterial colonization and pathogenesis[28]. Microaero philic organisms, such as H pylori, are particularly vulnerable to the detrimental effects of oxygen and oxidative stress. Nevertheless, enzymes including AhpC, catalase (KatA), SOD, thioperoxidase (Tpx), etc., can maintain persistent infection by using a variety of protective enzymatic systems that eliminate or minimize toxic oxygen-derived products.
In this study, three important antioxidant proteins of H pylori including AhpC, SOD and Mda66 were identified from the 2-DE maps showing differentially expressed proteins between the wild-type strain (Hp27) and isogenic cagA knock out mutant (Hp27_ΔcagA), indicating that the cagA gene is relevant to the expressions of antioxidant proteins of H pylori. Disruption of the target gene was found to have a certain effect on the expression of other genes that its encoded protein was shown to play a direct or indirect role in the regulation of protein biosynthesis, suggesting that cagA gene quantitatively influences ahpC, sodB and mda66 transcription or their subsequent translation and correct folding.
Regulatory genes are usually located in the genome upstream of genes. With reference to the genomic sequence of H pylor 26695, cagA is only located in the upstream of mda66, and it is difficult to explain how CagA regulates other antioxidant proteins. Since there are no DNA-binding motifs or motifs suggestive of a two-component regulatory system in CagA, the protein may act as a signal transducer by means of other proteins[829]. It was recently reported that a ferric uptake regulator (Fur) and a post-transcriptional regulator CsrA play a key role in the regulation of antioxidative stress enzymes[21]. We presumed that cagA might be related to Fur and/or CsrA, through which cagA influences the expression of antioxidant protein. However, the correlation between cagA and Fur and/or CsrA needs to be further studied.
In this study, cagA was found to be correlated with the three stress-resistant enzymes, suggesting that cagA gene may be of importance for H pylori to maintain the normal activity of antioxidative stress and to keep long-term persistence in the host, which is a novel mechanism involved in cagA pathogenesis. Based on our results and the reported linkage between cagA and motility[30], our conclusion is that cagA has virulence effects and may play a specific role in H pylori pathogenesis by influencing the expression of other proteins (enzymes).
Helicobacter pylori (H pylori) is a causative agent of gastritis, peptic ulcer and gastric cancer. CagA, a major virulence factor of H pylori, is considered a marker of increased pathogenic potential and may play an important role in the pathogenesis of cagA gene. However, little is known about the molecular mechanism and multiple biological functions of cagA.
CagA is a H pylori immunodominant antigen whose gene resides in the cag pathogenicity island, a 40-kilobase insertion containing genes involved in virulence. A previous study showed that cagA is related with the motility of H pylori. However, its exact mechanism still remains unclear. In this study, a proteomic approach was employed to determine if disruption of the cagA gene has some effects on the expression of other proteins of H pylori, through which various functions of the cagA gene can be recognized.
A proteomic approach combined with MALDI-TOF-MS was employed to investigate changes in expression of the wild-type strain of Hp27 (cagA+) and the cagA gene knock out isogenic mutant. Three antioxidant proteins were identified, including alkyl hydroperoxide reductase (Ahp), superoxide dismutase (SOD) and modulator of drug activity (Mda66), respectively. It is implied that cagA is essential to maintain the normal activity of antioxidative stress and ensure H pylori persistent colonization in the host, which may be a novel mechanism involved in cagA pathogenesis.
These results provide a novel mechanism involved in H pylori cagA pathogenesis. The cagA gene may be of importance for H pylori to maintain the normal activity of antioxidative stress and keep long-term persistence in the host.
CagA: Cytotoxin-associated gene A protein encoded by cytotoxin-associated gene pathogenicity island, a major virulence factor of H pylori. Two-dimensional electrophoresis: A two-step method to separate soluble proteins. Soluble proteins were first separated by isoelectric focusing (IEF) electrophoresis, and then according to the difference in molecular weight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antioxidant protein: Proteins (enzymes) involved in oxidative stress resistance, which are important factors for bacterial colonization and pathogenesis.
The results of this study are interesting. The findings are novel. However, how the absence of antioxidant proteins should be further studied.
| 1. | Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720-741. [Cited in This Article: ] |
| 2. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [Cited in This Article: ] |
| 3. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [Cited in This Article: ] |
| 4. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [Cited in This Article: ] |
| 5. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [Cited in This Article: ] |
| 6. | Al-Ghoul L, Wessler S, Hundertmark T, Krüger S, Fischer W, Wunder C, Haas R, Roessner A, Naumann M. Analysis of the type IV secretion system-dependent cell motility of Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun. 2004;322:860-866. [Cited in This Article: ] |
| 7. | Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971-980. [Cited in This Article: ] |
| 8. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263-1268. [Cited in This Article: ] |
| 9. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [Cited in This Article: ] |
| 10. | Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138-1144. [Cited in This Article: ] |
| 11. | Zhang L, Jiang J, Pan KF, Liu WD, Ma JL, Zhou T, Perez Perez GI, Blaser MJ, Chang YS, You WC. Infection of H.pylori with cagA+ strain in a high-risk area of gastric cancer. Shijie Huaren Xiaohua Zazhi. 1998;6:40-41. [Cited in This Article: ] |
| 12. | Zhang L, Yan XJ, Zhang LX, Han FC, Zhang NX, Hou Y, Liu YG. Seroepidemiological study of Hp and CagA+Hp infection. Shijie Huaren Xiaohua Zazhi. 2000;8:389-392. [Cited in This Article: ] |
| 13. | Huang ZG, Duan GC, Fan QT, Huang XY. Construction and identification of Chinese mutant Helicobacter pylori strain with absent expression of cagA gene. Shijie Huaren Xiaohua Zazhi. 2006;14:3190-3194. [Cited in This Article: ] |
| 14. | Steel LF, Shumpert D, Trotter M, Seeholzer SH, Evans AA, London WT, Dwek R, Block TM. A strategy for the comparative analysis of serum proteomes for the discovery of biomarkers for hepatocellular carcinoma. Proteomics. 2003;3:601-609. [Cited in This Article: ] |
| 15. | Wang G, Olczak AA, Walton JP, Maier RJ. Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect Immun. 2005;73:378-384. [Cited in This Article: ] |
| 16. | Papinutto E, Windle HJ, Cendron L, Battistutta R, Kelleher D, Zanotti G. Crystal structure of alkyl hydroperoxide-reductase (AhpC) from Helicobacter pylori. Biochim Biophys Acta. 2005;1753:240-246. [Cited in This Article: ] |
| 17. | Wang G, Conover RC, Olczak AA, Alamuri P, Johnson MK, Maier RJ. Oxidative stress defense mechanisms to counter iron-promoted DNA damage in Helicobacter pylori. Free Radic Res. 2005;39:1183-1191. [Cited in This Article: ] |
| 18. | Olczak AA, Seyler RW Jr, Olson JW, Maier RJ. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect Immun. 2003;71:580-583. [Cited in This Article: ] |
| 19. | Chuang MH, Wu MS, Lo WL, Lin JT, Wong CH, Chiou SH. The antioxidant protein alkylhydroperoxide reductase of Helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc Natl Acad Sci USA. 2006;103:2552-2557. [Cited in This Article: ] |
| 20. | Wang G, Conover RC, Benoit S, Olczak AA, Olson JW, Johnson MK, Maier RJ. Role of a bacterial organic hydroperoxide detoxification system in preventing catalase inactivation. J Biol Chem. 2004;279:51908-51914. [Cited in This Article: ] |
| 21. | Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847-860. [Cited in This Article: ] |
| 22. | Seyler RW Jr, Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001;69:4034-4040. [Cited in This Article: ] |
| 23. | Li R, Bianchet MA, Talalay P, Amzel LM. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc Natl Acad Sci USA. 1995;92:8846-8850. [Cited in This Article: ] |
| 24. | Chatterjee PK, Sternberg NL. A general genetic approach in Escherichia coli for determining the mechanism(s) of action of tumoricidal agents: application to DMP 840, a tumoricidal agent. Proc Natl Acad Sci USA. 1995;92:8950-8954. [Cited in This Article: ] |
| 25. | Wang G, Maier RJ. An NADPH quinone reductase of Helicobacter pylori plays an important role in oxidative stress resistance and host colonization. Infect Immun. 2004;72:1391-1396. [Cited in This Article: ] |
| 26. | Hayashi M, Ohzeki H, Shimada H, Unemoto T. NADPH-specific quinone reductase is induced by 2-methylene-4-butyrolactone in Escherichia coli. Biochim Biophys Acta. 1996;1273:165-170. [Cited in This Article: ] |
| 27. | Søballe B, Poole RK. Microbial ubiquinones: multiple roles in respiration, gene regulation and oxidative stress management. Microbiology. 1999;145:1817-1830. [Cited in This Article: ] |
| 28. | Ramarao N, Gray-Owen SD, Meyer TF. Helicobacter pylori induces but survives the extracellular release of oxygen radicals from professional phagocytes using its catalase activity. Mol Microbiol. 2000;38:103-113. [Cited in This Article: ] |
| 29. | Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745-755. [Cited in This Article: ] |
| 30. | Figura N, Trabalzini L, Mini R, Bernardini G, Scaloni A, Talamo F, Lusini P, Ferro E, Martelli P, Santucci A. Inactivation of Helicobacter pylori cagA gene affects motility. Helicobacter. 2004;9:185-193. [Cited in This Article: ] |