Published online Nov 21, 2009. doi: 10.3748/wjg.15.5455
Revised: October 12, 2009
Accepted: October 19, 2009
Published online: November 21, 2009
AIM: To investigate factors predicting failure of percutaneous endoscopic gastrostomy (PEG) to eliminate gastroesophageal reflux (GER).
METHODS: Twenty-nine consecutive mechanically ventilated patients were investigated. Patients were evaluated for GER by pH-metry pre-PEG and on the 7th post-PEG day. Endoscopic and histologic evidence of reflux esophagitis was also carried out. A beneficial response to PEG was considered when pH-metry on the 7th post-PEG day showed that GER was below 4%.
RESULTS: Seventeen patients responded (RESP group) and 12 did not respond (N-RESP) to PEG. The mean age, sex, weight and APACHE II score were similar in both groups. GER (%) values were similar in both groups at baseline, but were significantly reduced in the RESP group compared with the N-RESP group on the 7th post-PEG day [2.5 (0.6-3.8) vs 8.1 (7.4-9.2, P < 0.001)]. Reflux esophagitis and the gastroesophageal flap valve (GEFV) grading differed significantly between the two groups (P = 0.031 and P = 0.020, respectively). Histology revealed no significant differences between the two groups.
CONCLUSION: Endoscopic grading of GEFV and the presence of severe reflux esophagitis are predisposing factors for failure of PEG to reduce GER in mechanically ventilated patients.
- Citation: Douzinas EE, Andrianakis I, Livaditi O, Bakos D, Flevari K, Goutas N, Vlachodimitropoulos D, Tasoulis MK, Betrosian AP. Reasons of PEG failure to eliminate gastroesophageal reflux in mechanically ventilated patients. World J Gastroenterol 2009; 15(43): 5455-5460
- URL: https://www.wjgnet.com/1007-9327/full/v15/i43/5455.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5455
Gastroesophageal reflux (GER) is a common problem in critically ill patients and constitutes a major mechanism of esophageal mucosal injury or erosive esophagitis in mechanically ventilated patients[1,2]. Nasogastric tube (NGT) plays a significant role in the development of GER in these patients[3]. GER is considered to be a major risk for ventilator-associated pneumonia (VAP), which is associated with prolongation of Intensive Care Unit (ICU) stay and increased mortality[4,5]. The judicious use of NGT, the semi-recumbent position and the application of strategies to improve gastric emptying are the proposed measures for preventing GER and thus minimizing VAP[5-7].
The relationship between percutaneous endoscopic gastrostomy (PEG) and subsequent development of GER is complex and not well understood. The reported effects of PEG on GER are contradictory. Some authors reported the development or worsening of GER after PEG[8,9], while others claimed the opposite[10,11]. Johnson et al[11] reported that PEG tube placement decreases GER by increasing the lower esophageal sphincter pressure (LES), and showed that the anterior apposition of the stomach by PEG has a similar effect to gastropexy and increases LES pressure. The latter was argued by Coben et al[12], who found that gastrostomy tubes had no effect on basal LES pressure. Few studies, however, have evaluated the effect of PEG on GER in mechanically ventilated patients. In a prospective randomized trial, we have shown that PEG when combined with the semi-recumbent position and control of gastric residue, can decrease GER in the majority of these patients[13]. In this new prospective study we aimed to explore the factors that interfere with failure of PEG to reduce GER in critically ill, mechanically ventilated patients.
Patients eligible for this study were all mechanically ventilated, were tolerating nasogastric feeding via NGT and had prolonged ICU stay. Enteral feeding was performed at a continuous infusion rate of 80 to 100 mL/h. Exclusion criteria were unstable hemodynamic state, the administration of morphine, atropine, theophylline, barbiturates or cisapride, the presence of bloated intestine, and a past history of gastroesophageal reflux disease or hiatus hernia. The patients were divided into 2 groups based on whether GER decreased to less than 4% (responders, RESP group) or remained unchanged or worsened (non-responders, N-RESP group) after PEG placement. The institutional review board approved the study and informed consent was obtained from each patient’s next of kin.
Antacids, H2 receptor blockers or proton pump inhibitors were withdrawn 7 d before the study; sucralfate (2 g twice daily via NGT) was provided thereafter for gastric mucosa protection. Patients were neither sedated nor paralyzed. Enteral feeding was stopped 6 h before and during the period of pH-metry, which was performed on a 24 h basis. Gastrostomy was performed by the pull through (Ponsky) technique the day after[14]. Monitoring of pH was repeated on the 7th post-PEG day in order to assess the effect of PEG on eliminating or reducing GER%.
On the second post-PEG day, patients were administered continuous drip PEG-feeding with a polymeric diet at 60 mL/h and an energy content of 1000 kcal/L (Fresenius, Bad Homburg, Germany). Patients were placed in a semi-recumbent position (30°) and the volume of the gastric nutrient residue was determined to obviate gastric stasis. This was realized by aspirating the gastric content with a syringe at 8-h intervals to verify if the nutrient volume exceeded 200 mL. If the volume did exceed 200 mL, feeding was withheld and if volume persisted, the nutrient was drained. Feeding was re-administered when the volume was decreased.
The presence or absence of hiatus hernia, reflux esophagitis and the grading of gastroesophageal flap valve (GEFV) were examined and scored according to the criteria below by two endoscopists. Biopsy specimens were obtained from the lower 3 cm of the esophagus for histological examination.
Reflux esophagitis: Reflux esophagitis was graded according to the Los Angeles classification[15].
GEFV: This was assessed by the grading system of Hill et al[16], as follows: Grade I: Normal ridge of tissue closely approximated the shaft of the retroflexed scope. Grade II: The ridge is slightly less well defined and opens with respiration. Grade III: The ridge is barely present and the hiatus is patulous. Grade IV: There is no muscular ridge and the hiatus is wide open at all times. GEFV grades I and II were regarded as representing normal GEFV, while grades III and IV were regarded as representing abnormal GEFV.
Esophageal pH-metry: The presence of gastroesophageal reflux was demonstrated by using 24-h double sensor catheter system pH monitoring. Baseline and 7th d post-PEG pH-metry was carried out using the portable recorder Digitrapper Mk III (Synectics Medical AB, Stockholm, Sweden). Briefly, after ordinary NGT removal, the sensor probe was introduced via the nose into the stomach until acid pH was recorded with the distal sensor as previously described[13]. The probe was then slowly withdrawn until the distal sensor channel detected a sudden pH change from acid (< 4) to above 5. The electrode was then withdrawn another 5 cm. In this way, the distal and proximal sensors were located at 5 and 20 cm above the lower esophageal sphincter, respectively. The correct positioning of the electrode was verified at the end of pH-metry and before its withdrawal by a chest x-ray. The recording device measured pH values every 4 s and stored the mean of 20 values every 80 s. Gastroesophageal reflux was expressed as the percentage of time that the esophageal pH was less than 4 in the given 24-h period [GER (%)], with a value of > 4% considered abnormal[17]. Additionally, the number of reflux episodes per hour and the number of reflux episodes lasting longer than 5 min in 24 h were measured and recorded. The pH-metry from the lower esophageal sensor consistently recorded GER 15%-20% higher than that from the upper sensor. However, since the importance of reflux detection in the upper part of the esophagus is greater in relation to aspiration, only data from the upper sensor are presented.
Biopsies were assessed by two experienced histopathologists and scored for surface ulceration (present or absent), basal cell hyperplasia (score 0-3), acute inflammatory infiltration of the epithelium (score 0-3) and the lamina propria (score 0-3) and presence of long papillae (score 0-3) to produce an overall histological score (ranging from 0 to 12). The interobserver variability was < 5%. In case of disagreement, reevaluation was performed jointly by the two observers so that a consensus could be reached. Complete surface ulceration was scored as 12 and no other epithelial changes were assessed. Overall histological score of 0-2 corresponded to grade zero, 3-4 corresponded to grade I, 5-8 to grade II and 9-12 to grade III histological esophagitis.
Data are reported as median and inter-quartile range (Q1-Q3). Comparisons among groups (responders, non-responders) were made using the Mann-Whitney test. Comparisons (number of reflux episodes) within groups were performed by the Wilcoxon’s rank sum test. The Pearson’s χ2 test or Fisher’s exact test when appropriate, were used to examine the association between qualitative variables. The Spearman rank correlation coefficient (rs) was calculated to describe the relationships between variables. A P-value less than 0.05 was considered significant.
A cohort of 29 consecutive mechanically ventilated patients undergoing PEG was prospectively evaluated. Demographic data are listed in Table 1. Patients were divided into the RESP group (n = 17) and the N-RESP group (n = 12). The mean age, sex, weight and APACHE II score were similar in both groups. The reasons for PEG tube placement were as follows: cerebrovascular attack 48.2% (n = 14), neuromuscular disorder 13.7% (n = 4), COPD-pneumonia 20.6% (n = 6), and trauma 17.2% (n = 5). The median number of days of nasogastric tube placement before PEG was 29.5 in the RESP group vs 45 in the N-RESP group (NS, Table 1), which denotes a similar pre-PEG timing in the two groups.
| RESP group (n = 17) | N-RESP group (n = 12) | P value | |
| Age, years | 55 (32-65) | 65 (49.5-68) | NS |
| Sex (M/F) | (12/5) | (5/7) | NS |
| Weight, kg | 75 (70-80) | 77 (68.5-80) | NS |
| APACHE II | 17 (15-20) | 15.5 (13.5-22.5) | NS |
| Indication for PEG placement | |||
| Cerebrovascular attack | 9 | 5 | |
| Neuromuscular disorder | 4 | 2 | |
| COPD-pneumonia | 2 | 2 | |
| Head trauma | 2 | 3 | |
| Days on NGT | 29.5 (25-38) | 45 (25-53) | NS |
| Weaning, days | 10 (6.5-14) | 13 (12-20) | NS |
| Outcome (survived/died) | (14/3) | (7/5) | NS |
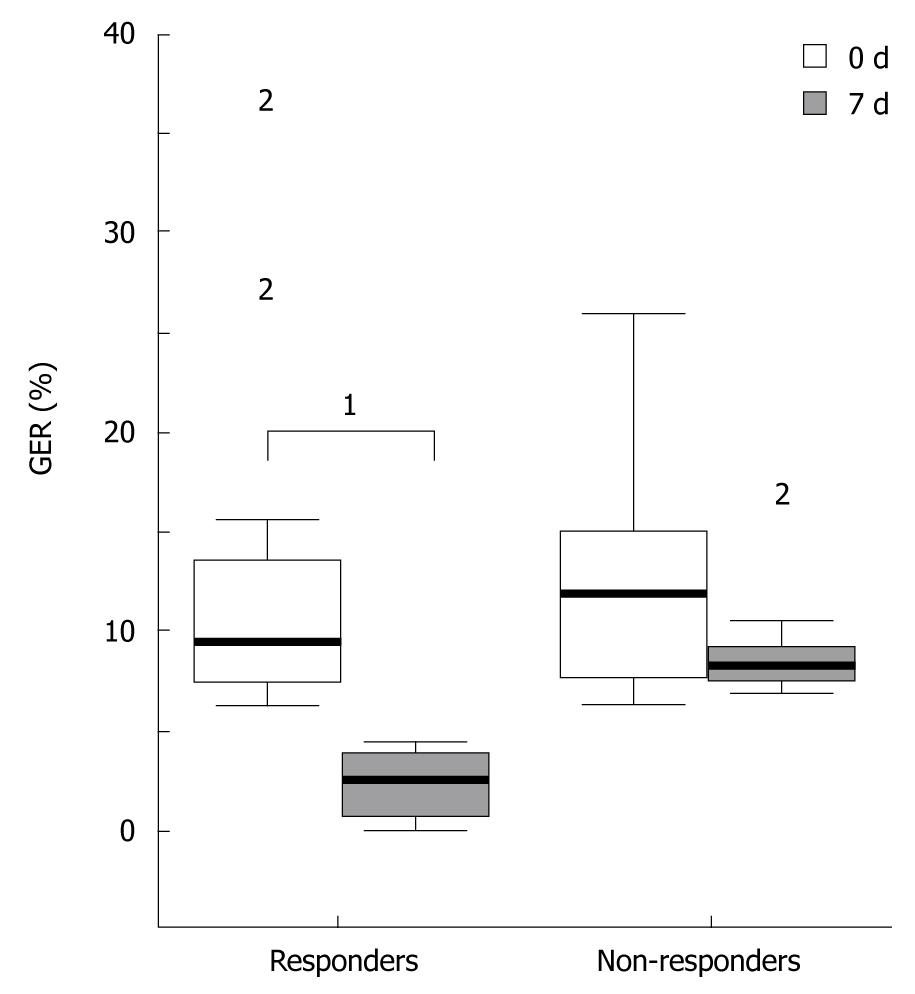
All variables from esophageal pH-metry are listed in Table 2. GER (%) values were similar in both groups at baseline, but were significantly reduced in the RESP group compared with the N-RESP group at the 7th post-PEG day (P < 0.001, Figure 1). The median (range) number of reflux episodes > 5 min and the number of reflux episodes/h decreased significantly at the 7th post-PEG day in the RESP group (P < 0.001) compared to baseline, but not in the N-RESP group (NS). Both variables were significantly higher at baseline in the RESP group compared with in the N-RESP group [7 (4-9) vs 3.5 (2-6), P = 0.045 and 8 (3-10) vs 2.9 (2-4.4), P = 0.020, respectively].
| RESP group (n = 17) | N-RESP group (n = 12) | P value | |
| Gastroesophageal reflux GER (%) | |||
| Baseline | 9.4 (7.4-13.5) | 11.7 (7.6-15) | NS |
| 7th post-PEG day | 2.5 (0.6-3.8) | 8.1 (7.4-9.2) | < 0.001b |
| No. of reflux episodes/h | |||
| Baseline | 8 (3-10) | 2.9 (2-4.4) | 0.020a |
| 7th post-PEG day | 1.3 (0.5-2) | 2.9 (2.3-3.4) | 0.002a |
| No. of reflux episodes > 5 min | |||
| Baseline | 7 (4-9) | 3.5 (2-6) | 0.045a |
| 7th post-PEG day | 1 (0-2) | 3 (1.5-5) | 0.003a |
Reflux esophagitis assessed at PEG tube placement was observed in 100% of the study population (Table 3). There was a significant difference between the two groups: grade A:B:C:D = 11:6:0:0 in the RESP group (n = 17) vs 4:4:4:0 in the N-RESP group (n = 12), P = 0.031, respectively. The presence of reflux esophagitis correlated with GEFV grading (rs = 0.465, P = 0.011). Hiatus hernia was not observed in any of the patients studied.
The GEFV appearance differed significantly between the two groups (P = 0.020, Table 3). Ten patients in the RESP group and 2 in the N-RESP group were evaluated as normal GEFV, while 7 and 10 patients, respectively, were evaluated as abnormal GEFV.
The histologic results are summarized in Table 3. The histology of esophageal biopsies was normal in 10.3% (2/17 and 1/12 in the RESP and N-RESP groups, respectively). There was no significant difference between the two groups: grade 0:1:2:3 = 2:8:6:1 in the RESP group (n = 17) vs 1:3:5:3 in the N-RESP (n = 12), respectively, P = NS. Histologic grading showed a good correlation with endoscopic grading (rs = 0.705, P = 0.000).
Weaning from mechanical ventilation was achieved in 13/17 (76.4%) of responders within 10 (6.5-14) d, whereas 7/12 (58%) of non-responders required 13 (12-20) d (NS). Three patients in the RESP group (17.6%) and five in the N-RESP group (41.6%) died while in the ICU (NS). The deaths were unrelated to the PEG tube procedure.
The results of this study confirm previous findings that PEG tube placement can eliminate GER in mechanically ventilated patients[13]. Our findings also provided evidence that the presence of severe reflux esophagitis and the gastro-esophageal flap valve grade are factors that predict failure of PEG to control GER in these patients.
The mechanisms that cause GER in mechanically ventilated patients differ from conscious patients[1]. Transient lower esophageal sphincter relaxation (TLESR) is believed to be the major mechanism of acid reflux in awake patients, with a defective basal LES pressure caused by hiatus hernia and straining associated with increased abdominal pressure being contributing factors[18,19]. In contrast, the absent or very low basal LES pressure induced by mechanical ventilation, opiates, endotoxemia-related sepsis and nasogastric tube are the major causes of reflux in critically ill patients[1,2,7,20]. The latter is considered to be a key factor for both the development and the degree of GER[21]. In several studies, 48%-60% of ICU patients were found to have erosive esophageal lesions induced by GER, 3 or 5 d after NGT placement[2,22]. Furthermore, the degree of GER correlates with the duration of NGT. In an earlier study, we showed a positive correlation between the duration of nasogastric intubation and the degree of GER[13]. These findings were also validated in the current study, which showed 100% incidence of GER in patients with long-standing NGT.
Few data exist in the literature regarding the influence of PEG tube placement on GER in mechanically ventilated adult patients. We have reported the effectiveness of PEG in decreasing GER (%) by more than 60%, compared to baseline, at the 7th post-PEG day in 16 critically ill patients[13]. Our data are in agreement with previous findings and show a significant reduction in GER% at the 7th post-PEG day in 17/29 (58.6%) of the studied population. Similarly, both the number of reflux episodes/h and those lasting > 5 min were significantly reduced in the RESP group. However, this was not the case for the N-RESP group, although these variables were significantly increased at baseline in the former group of patients (Table 2). Overall, PEG tube placement improved (or at least did not deteriorate) both GER (%) and the two indices tested for gastroesophageal reflux. This is in agreement with previous findings which demonstrated that GER is not a contraindication to PEG[23].
Reflux esophagitis occurs in approximately 25%-30% of critically ill patients undergoing endoscopy and GER is considered a major cause[1,7]. However, not all patients with gastroesophageal reflux have esophagitis. In this study, reflux esophagitis assessed by endoscopy, was present in 100% of the study population and showed a good correlation with GER (rs = 0.465, P = 0.011). The increased incidence of reflux esophagitis and GER (%) in this study could be attributed to prolonged NGT placement [mean 29.5 (25-38) and 45 (25-53) d for the RESP and N-RESP groups, respectively] and to the removal of proton pump inhibitors 7 d prior to the study. It is well known that these agents bind with and inactivate the gastric parietal cell proton pump (H+/K+-ATPase) which, in turn, inhibits gastric acid production and raises gastric pH[24].
Grading of GEFV differed significantly between the two groups (P = 0.020). Approximately 83% of subjects with abnormal GEFV (grades III and IV) were in the N-RESP group vs 41% of the RESP group, suggesting that endoscopic grading of GEFV pre-PEG provides useful information for predicting failure of PEG to reduce GER.
Heikenen et al[25], have demonstrated a poor validity between the severity of histologic evidence of esophagitis and significant GER. Our data are in keeping with this concept and showed no significant correlation between pre-PEG histological evaluation and the development of GER post-PEG. That is, evaluation of esophageal histology pre-PEG does not reliably predict the development of reflux after PEG placement.
Our study has some strengths and limitations. The strengths include the prospective design and the follow-up pH-metry on the 7th post-PEG day. In contrast, the study is limited due to the small sample size, so data should be extrapolated with caution.
In conclusion, the results of this study indicate that the endoscopic grading of GEFV and the presence of severe reflux esophagitis graded LA classification C or D are predisposing factors of failure of PEG to reduce GER in mechanically ventilated patients. We also provided evidence that gastroesophageal reflux is not a contraindication for PEG tube placement. The latter seems to eliminate or at least not exacerbate GER in these patients.
Gastroesophageal reflux (GER) is a common problem in mechanically ventilated patients and contributes to the development of esophageal mucosal injury and even erosive esophagitis.
One of the main factors that have been implicated in the pathogenesis of GER in these patients is the placement and duration of a nasogastric tube (NGT). NGT may lead to GER through relaxation of the lower esophageal sphincter (LES) pressure. The authors have previously shown that percutaneous endoscopic gastrostomy (PEG) can eliminate GER by disengaging patients from the presence of NGT. In this study, the aforementioned findings are further confirmed and the authors identified factors, including the presence of severe reflux esophagitis and gastroesophageal flap valve grade that predict failure of PEG to eliminate GER.
The presence of NGT and mechanical ventilation are factors predisposing to the development of GER. However, few data exist regarding the effect of PEG tube placement in the prevention of GER in mechanically ventilated adult patients. In the present study the authors identified factors which could predict PEG failure to reduce GER.
By identifying the factors that predict failure of PEG to decrease GER, this study may represent a reference in deciding which patients are likely to benefit from PEG tube placement and thus protect them from the development of esophagitis and even ventilator-associated pneumonia (VAP).
Percutaneous endoscopic gastrostomy: Percutaneous endoscopic gastrostomy (PEG) is a procedure in which a feeding tube is passed through the abdominal wall directly into the stomach under endoscopic guidance, enabling nutrients, medication and fluids to be delivered directly into the stomach; Gastroesophageal reflux: GER is defined as the movement of stomach contents (solids or liquids) into the esophagus, and is caused mainly by dysfunction of the lower esophageal sphincter.
This is an interesting prospective study that provides valuable information regarding factors that predict failure of PEG to eliminate GER. The results are interesting and suggest that severe reflux esophagitis and the gastro-esophageal flap valve grade are the main contributing factors.
Authors are grateful to L Haziroglou, BSc for his editorial assistance and language review.
Peer reviewer: Jamie S Barkin, MD, Professor of Medicine, Chief, Sinai Medical Center Division of Gastroenterology, Mt. Sinai Medical Center, University of Miami, School of Medicine, 4300 Alton Road, Miami Beach, FL 33140, United States
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Nind G, Chen WH, Protheroe R, Iwakiri K, Fraser R, Young R, Chapman M, Nguyen N, Sifrim D, Rigda R. Mechanisms of gastroesophageal reflux in critically ill mechanically ventilated patients. Gastroenterology. 2005;128:600-606. [Cited in This Article: ] |
| 2. | Wilmer A, Tack J, Frans E, Dits H, Vanderschueren S, Gevers A, Bobbaers H. Duodenogastroesophageal reflux and esophageal mucosal injury in mechanically ventilated patients. Gastroenterology. 1999;116:1293-1299. [Cited in This Article: ] |
| 3. | Ferrer M, Bauer TT, Torres A, Hernandez C, Piera C. Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med. 1999;130:991-994. [Cited in This Article: ] |
| 4. | Torres A, El-Ebiary M, Soler N, Monton C, Fabregas N, Hernandez C. Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator-associated pneumonia. Eur Respir J. 1996;9:1729-1735. [Cited in This Article: ] |
| 5. | Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858. [Cited in This Article: ] |
| 6. | Ibanez J, Penafiel A, Raurich JM, Marse P, Jorda R, Mata F. Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. JPEN J Parenter Enteral Nutr. 1992;16:419-422. [Cited in This Article: ] |
| 7. | Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222-1241. [Cited in This Article: ] |
| 8. | Isch JA, Rescorla FJ, Scherer LR 3rd, West KW, Grosfeld JL. The development of gastroesophageal reflux after percutaneous endoscopic gastrostomy. J Pediatr Surg. 1997;32:321-322; discussion 322-323. [Cited in This Article: ] |
| 9. | Balan KK, Vinjamuri S, Maltby P, Bennett J, Woods S, Playfer JR, Critchley M. Gastroesophageal reflux in patients fed by percutaneous endoscopic gastrostomy (PEG): detection by a simple scintigraphic method. Am J Gastroenterol. 1998;93:946-949. [Cited in This Article: ] |
| 10. | Razeghi S, Lang T, Behrens R. Influence of percutaneous endoscopic gastrostomy on gastroesophageal reflux: a prospective study in 68 children. J Pediatr Gastroenterol Nutr. 2002;35:27-30. [Cited in This Article: ] |
| 11. | Johnson DA, Hacker JF 3rd, Benjamin SB, Ciarleglio CA, Chobanian SJ, Van Ness MM, Cattau EL Jr. Percutaneous endoscopic gastrostomy effects on gastroesophageal reflux and the lower esophageal sphincter. Am J Gastroenterol. 1987;82:622-624. [Cited in This Article: ] |
| 12. | Coben RM, Weintraub A, DiMarino AJ Jr, Cohen S. Gastroesophageal reflux during gastrostomy feeding. Gastroenterology. 1994;106:13-18. [Cited in This Article: ] |
| 13. | Douzinas EE, Tsapalos A, Dimitrakopoulos A, Diamanti-Kandarakis E, Rapidis AD, Roussos C. Effect of percutaneous endoscopic gastrostomy on gastro-esophageal reflux in mechanically-ventilated patients. World J Gastroenterol. 2006;12:114-118. [Cited in This Article: ] |
| 14. | Ponsky JL, Gauderer MW, Stellato TA. Percutaneous endoscopic gastrostomy. Review of 150 cases. Arch Surg. 1983;118:913-914. [Cited in This Article: ] |
| 15. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [Cited in This Article: ] |
| 16. | Hill LD, Kozarek RA, Kraemer SJ, Aye RW, Mercer CD, Low DE, Pope CE 2nd. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44:541-547. [Cited in This Article: ] |
| 17. | Kim GH, Kang DH, Song GA, Kim TO, Heo J, Cho M, Kim JS, Lee BJ, Wang SG. Gastroesophageal flap valve is associated with gastroesophageal and gastropharyngeal reflux. J Gastroenterol. 2006;41:654-661. [Cited in This Article: ] |
| 18. | Richter JE. Gastrooesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2007;21:609-631. [Cited in This Article: ] |
| 19. | van Herwaarden MA, Samsom M, Smout AJ. Excess gastroesophageal reflux in patients with hiatus hernia is caused by mechanisms other than transient LES relaxations. Gastroenterology. 2000;119:1439-1446. [Cited in This Article: ] |
| 20. | Park H, Clark E, Cullen JJ, Koland JG, Kim MS, Conklin JL. Expression of inducible nitric oxide synthase in the lower esophageal sphincter of the endotoxemic opossum. J Gastroenterol. 2002;37:1000-1004. [Cited in This Article: ] |
| 21. | Nagler R, Spiro HM. Persistent gastroesophageal reflux induced during prolonged gastric intubation. N Engl J Med. 1963;269:495-500. [Cited in This Article: ] |
| 22. | Gastinne H, Canard JM, Pillegand B, Voultoury JC, Catanzano A, Claude R, Gay R. [Oesophagitis during mechanical ventilation]. Nouv Presse Med. 1982;11:3029-3032. [Cited in This Article: ] |
| 23. | Samuel M, Holmes K. Quantitative and qualitative analysis of gastroesophageal reflux after percutaneous endoscopic gastrostomy. J Pediatr Surg. 2002;37:256-261. [Cited in This Article: ] |
| 24. | Savarino V, Di Mario F, Scarpignato C. Proton pump inhibitors in GORD An overview of their pharmacology, efficacy and safety. Pharmacol Res. 2009;59:135-153. [Cited in This Article: ] |