Published online Oct 7, 2009. doi: 10.3748/wjg.15.4653
Revised: September 12, 2009
Accepted: September 19, 2009
Published online: October 7, 2009
Anemia of diverse etiology is a common complication of chronic liver diseases. The causes of anemia include acute or chronic gastrointestinal hemorrhage, and hypersplenism secondary to portal hypertension. Severe hepatocellular disease predisposes to hemorrhage because of impaired blood coagulation caused by deficiency of blood coagulation factors synthesized by hepatocytes, and/or thrombocytopenia. Aplastic anemia, which is characterized by pancytopenia and hypocellular bone marrow, may follow the development of hepatitis. Its presentation includes progressive anemia and hemorrhagic manifestations. Hematological complications of combination therapy for chronic viral hepatitis include clinically significant anemia, secondary to treatment with ribavirin and/or interferon. Ribavirin-induced hemolysis can be reversed by reducing the dose of the drug or discontinuing it altogether. Interferons may contribute to anemia by inducing bone marrow suppression. Alcohol ingestion is implicated in the pathogenesis of chronic liver disease and may contribute to associated anemia. In patients with chronic liver disease, anemia may be exacerbated by deficiency of folic acid and/or vitamin B12 that can occur secondary to inadequate dietary intake or malabsorption.
- Citation: Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol 2009; 15(37): 4653-4658
- URL: https://www.wjgnet.com/1007-9327/full/v15/i37/4653.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4653
| Treatment of variceal bleeding |
| Pharmacological therapy |
| Somatostatin: initial bolus (24-48 h) and a perfusion (5 d) |
| Octreotide: initial bolus (24-48 h) and a perfusion (5 d) |
| Terlipressin: important side effects |
| Primary prophylaxis |
| β-blockers non-selective: propranolol, nadolol |
| Isosorbide 5-mononitrate |
| Secondary prophylaxis |
| β-blockers non-selective: propranolol, nadolol |
| Isosorbide 5-mononitrate |
| Endoscopy therapy: band ligation, sclerotherapy |
| Cause of low hematocrit | Possible contributing factors |
| Hemorrhage and/or iron deficiency | Alcoholic gastritis |
| Portal hypertension | |
| Peptic ulceration | |
| Hemolysis | Chronic liver disease and/or cirrhosis |
| Zieve syndrome | |
| Spur cell anemia of severe liver disease | |
| Reduced erythropoiesis | Anemia of chronic disease |
| Nutritional (e.g. folic acid deficiency) | |
| Sideroblastic anemia | |
| Alcohol toxicity | |
| Hypersplenism | Portal hypertension |
| Hemodilution | Fluid retention of chronic liver disease |
| Aggressive intravenous fluid therapy |
Chronic liver diseases frequently are associated with hematological abnormalities. Anemia of diverse etiology occurs in about 75% of patients with chronic liver disease[1].
A major cause of anemia associated with chronic liver disease is hemorrhage, especially into the gastrointestinal tract. Patients with severe hepatocellular disease develop defects of blood coagulation as a consequence of endothelial dysfunction, thrombocytopenia, deficiencies of coagulation factors and various associated disorders[2].
In severe hepatocellular disease, decreased synthesis of liver-produced plasma proteins leads to reduced serum levels of several blood clotting factors. Hemorrhage may occur as a complication of chronic liver disease because of a lack of one or more liver-produced blood clotting factors, thrombocytopenia, and/or defective platelet function. Hemorrhage in such patients may also occur from esophageal or gastric varices secondary to portal hypertension. The biosynthetic pathways of blood coagulation factors II, VII, IX and X are within the hepatocyte and are dependent on vitamin K[3]. Low serum levels of these factors are associated with prolongation of the prothrombin time (PT). When attributable to hepatocellular disease, they are not improved by administration of vitamin K; correction of the associated impaired blood coagulation necessitates infusion of preparations of the deficient factors.
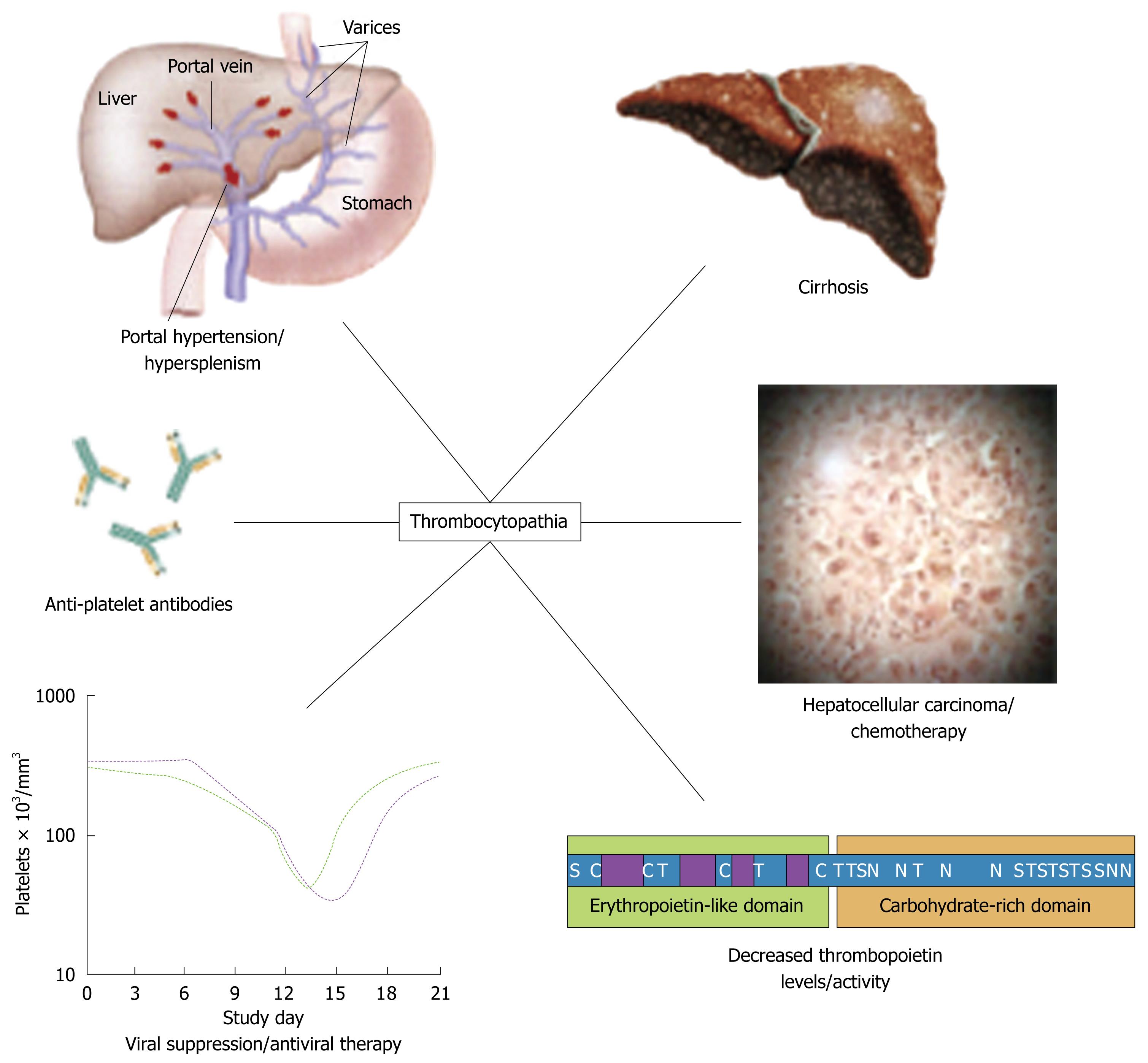
Splenomegaly, which is usually caused by portal hypertension in patients with chronic liver disease, may lead to secondary hemolysis, an increase in plasma volume, macrocytosis and megaloblastic anemia. Alcohol, a common etiologic factor of chronic liver disease, is toxic to the bone marrow. Alcoholics often develop secondary malnutrition, a manifestation of which may be anemia caused by folic acid deficiency. In some patients, bone marrow failure and aplastic anemia develop after an episode of hepatitis. Finally, anemia is a recognized complication of treatment of chronic hepatitis C with a combination of interferon and ribavirin: anemia in this context is predominantly caused by ribavirin-induced hemolysis[4].
The frequent association of anemia with chronic liver disease and/or hepatocellular failure provides a rationale for examining the role of the liver in the formation and destruction of erythrocytes. Indeed, the liver itself may be implicated in a variety of different mechanisms that contribute to the development of anemia in patients with chronic liver disease. This paper provides an overview of anemia that may complicate chronic liver diseases and the mechanisms responsible.
The frequent association of anemia with chronic liver disease and/or hepatocellular failure provides a rationale for examining the role of the liver in the formation and destruction of red blood cells. Indeed, a variety of different mechanisms may be implicated in the development of anemia in patients with liver disease.
Acute gastrointestinal hemorrhage is a common and potentially serious complication of portal hypertension[5-8]. It is usually caused by rupture of an esophageal varix. Hemorrhage caused by this mechanism is the second most common cause of mortality in patients with cirrhosis. In such patients, a ruptured esophageal varix is the cause of approximately 70% of episodes of upper gastrointestinal hemorrhage[6]. Acute hemorrhage may induce severe hypovolemia and subsequently secondary iron deficiency anemia. The initial aim of treatment is correction of hypovolemia and restoration of stable hemodynamic function; minimal values for mean arterial pressure and for hemoglobin of 80 mmHg and 8 g/100 mL, respectively, should be maintained. Initially, gelatin-based colloids or solutions of human albumin may be infused to correct hypovolemia. However, infusions of packed erythrocytes in plasma are ideal in this context since such infusions have the potential of correcting, not only hypovolemia, but also secondary anemia. First-line management involves institution of both medical and endoscopic treatments (Table 1)[6]. Medical therapy includes administration of vasoactive drugs, such as somatostatin, octreotide or terlipressin. Optimal endoscopic treatment involves ligation of esophageal varices and obturation of gastric varices with tissue adhesives.
In some patients with cirrhosis, chronic hemorrhage into the gastrointestinal tract occurs. Esophageal and gastric varices and/or portal hypertensive gastropathy may be associated with slow chronic loss of blood into the gut and development of chronic iron deficiency anemia. The most important approach to management is prevention of variceal hemorrhage[5,7,8]. The annual incidence of initial variceal hemorrhage in patients with cirrhosis is estimated to be about 4%, but for the group with medium-sized or large varices, the incidence is about 15%[6]. β-blockers or isosorbide 5-mononitrate may reduce the rate of transformation of small varices into large varices and decrease the incidence of variceal hemorrhage in patients with small varices[5]. In patients who survive a first episode of variceal hemorrhage, the risk of recurrent hemorrhage is > 60%. Accordingly, all patients surviving variceal hemorrhage should receive active treatment aimed at preventing recurrence. Non-selective β-blockers or isosorbide 5-mononitrate and endoscopic therapy, including ligation of and/or sclerotherapy of varices, are the first-line treatments for preventing recurrence of variceal hemorrhage[5,8]; a combination of both these approaches constitutes optimal management. Additional treatment with oral iron supplementation is indicated for iron deficiency anemia caused by chronic blood loss. In some cases of advanced chronic liver disease, intravenous iron formulations may be administrated to increase plasma levels and tissue deposits of iron.
Hypersplenism secondary to portal hypertension is another mechanism of anemia in patients with chronic liver disease. Hypersplenism is associated with splenomegaly. In addition to chronic liver disease, thrombosis of the splenic vein may also be a cause of an increase in pressure within the portal venous system, which can lead to secondary hypersplenism. The main characteristics of hypersplenism are those attributable to pancytopenia. Hemolytic anemia occurs because of intrasplenic destruction of erythrocytes. Destruction of megakaryocytes and leukocyte precursors results in thrombocytopenia and leukopenia[9]. Symptoms and signs of hypersplenism are influenced by the primary underlying disease; they include abdominal pain and/or discomfort, and, in advanced cases, gastrointestinal hemorrhage secondary to portal hypertension. There may be hyperplasia of the progenitor cells in the bone marrow. It is important to determine the cause of hypersplenism. The main therapeutic approach for this syndrome is management directed at the underlying primary disease, usually chronic liver disease. When chronic liver disease is advanced, additional therapeutic options may need to be adopted. After assessing the severity of impaired hepatocellular function in a patient with advanced chronic liver disease, splenectomy may be considered if the splenic vein is thrombosed. An alternative approach is partial or total embolization of the splenic artery, which, in some recent studies, has been associated with good results, in particular, lower morbidity and mortality rates than those associated with surgery. Partial embolization preserves the immunological function of the spleen and is the preferred option for patients with cirrhosis[10].
The liver plays a central role in blood coagulation. Acute and chronic hepatocellular diseases are usually associated with defective blood coagulation due to a variety of different causes. These include: decreased hepatic synthesis of factors II, VII, IX and X; the presence of inhibitors of these factors; decreased clearance of activated coagulation factors; thrombocytopenia; impaired platelet function; hyperfibrinolysis; and disseminated intravascular coagulation[11,12]. Coagulation defects complicating liver disease predispose to an increased bleeding tendency, which increases both morbidity and mortality [11-13].
Defective blood coagulation associated with hepatocellular disease may be monitored using global screening tests, such as the PT and the activated partial thromboplastin time. In mild hepatocellular disease, PT usually is within the normal range or only modestly prolonged. In more advanced hepatocellular disease, prolongation of PT tends to reflect the severity of hepatocellular failure. Vitamin K routinely is administered parenterally (usually only once) to patients with liver disease and a prolonged PT, to exclude vitamin K deficiency as a cause of the prolonged PT[11].
Thrombocytopenia (platelet count < 150 000/L) is common in patients with chronic liver disease; it has been reported in as many as 76% of patients with cirrhosis[4,12]. The pathogenesis of the thrombocytopenia is complex; it includes splenic pooling, and increased destruction and impaired production of platelets (Figure 1). Impaired production of platelets is caused, at least in part, by low levels of thrombopoietin. Prolonged bleeding time, and impaired aggregation, reduced adhesiveness and abnormal ultrastructure of platelets reflect abnormal platelet function; these abnormalities have been attributed to an intrinsic platelet defect. Specific treatments to attempt to reverse the effects of this defect are not usually given, but platelet transfusions or platelet-stimulating agents have been administered in some cases.
An important coagulation defect associated with chronic liver disease is low levels of factor VIIa. In recent years, the hemostatic agent recombinant factor VIIa has become available as a potentially new therapeutic agent for use in the management of coagulopathy in patients with cirrhosis. This agent may enhance initial control of acute variceal bleeding[14]. However, such therapy is associated with significant side effects, such as vascular injury and thrombosis.
Hyperfibrinolysis is another cause of impaired hemostasis in patients with liver disease. In a non-randomized trial[15], antifibrinolytic amino acids were administered to patients with acute or chronic liver disease, who had upper gastrointestinal bleeding and acquired defects of blood coagulation. However, administration of such amino acids does not have an established place in therapy.
Thrombotic events, although rare in patients with cirrhosis, may occur. They tend to involve particularly the portal and/or mesenteric veins.
A rational approach to managing disorders of blood coagulation in patients with liver disease is important because of the high risk of associated secondary hemorrhage.
Aplastic anemia associated with liver disease is characterized by development of pancytopenia and hypocellular bone marrow in relation to the occurrence of hepatitis[16]. The main feature of this syndrome is injury to or loss of pluripotent hematopoietic stem cells, in the absence of infiltrative disease of the bone marrow[16-19].
Hepatitis-associated aplastic anemia (HAA) has been defined as a variant of aplastic anemia, which occurs concurrently with or within 6 mo of an increase in the serum level of alanine aminotransferase to at least five times the upper limit of the reference range. Severe marrow aplasia may be induced by hepatitis viruses, such as hepatitis B virus and hepatitis C virus (HCV), and also by other viruses, such as human immunodeficiency virus, Epstein-Barr virus, transfusion-transmitted virus and echovirus[16,20]. Parvovirus B19 commonly infects pro-erythroblasts and may induce transient red-cell aplasia, particularly in patients with chronic hemolytic anemia. It has been postulated that viruses and/or antigens, through the mediation of γ interferon or the cytokine cascade, induce lymphocyte activation and ultimately apoptotic death of hematopoietic cells in the bone marrow[17].
Clinical presentation includes symptoms and signs related to pancytopenia, such as pallor, fatigue, hemorrhagic manifestations, progressive anemia, and bacterial infections. The diagnosis of HAA is suggested by a complete blood count, which reveals pancytopenia (including anemia) together with absolute reticulocytopenia[16]. A bone marrow biopsy typically reveals hypocellularity that affects red and white cell precursors and megakaryocytes; residual hematopoietic cells appear morphologically normal[19].
The two major options for treating severe HAA are hematopoietic cell transplantation and immunosuppressive therapy. According to recent reviews, response rates to these approaches are 75%-88% and 75%-80%, respectively[16,18]. Blood and platelet infusions are often necessary before instituting specific treatment; before administration blood products should be irradiated to avoid sensitization.
Currently, optimal treatment for chronic infection with HCV infection is a combination of therapy with pegylated interferon and ribavirin. Of hematological abnormalities that may be associated with such combination therapy, the most common is anemia[21]. Significant anemia (hemoglobin < 10 g/dL) has been observed in 9%-13% of patients receiving interferon and ribavirin; moderate anemia (hemoglobin < 11 g/dL) occurs in about 30% of patients undergoing such treatment[20-22]. There are several mechanisms by which anemia may occur during combination therapy for HCV infection, and ribavirin and/or interferons may contribute to anemia. In this context, hemoglobin concentrations decrease mainly as a result of ribavirin-induced hemolysis[20].
Anemia caused to ribavirin leads to modifications of the dose in up to 25% of patients, and this type of anemia may be problematic in patients with HCV infection, especially those who also have renal or cardiovascular disorders. Adherence to ribavirin therapy is one factor that is critically important in the treatment of HCV infection. Although ribavirin-associated anemia can be reversed by reducing the dose of ribavirin or by discontinuing the drug altogether, this approach compromises outcomes by significantly decreasing rates of sustained virological response. A recent study reviewed the predictors of anemia in patients undergoing treatment for HCV infection[21]. Patients with impaired renal function may be at an increased risk of ribavirin-related anemia and, accordingly, should be monitored carefully. Furthermore, a decrease in hemoglobin concentration of ≥ 1.5 g/dL by week 2 of treatment has been found to be an excellent early predictor of subsequent substantial decreases in hemoglobin. This predictor might be applied to identify candidates for early intervention for management of anemia to facilitate maintenance of the dose of ribavirin. One of the specific approaches to manage ribavirin-associated anemia is administration of recombinant human erythropoietin[21]. After 16 wk of ribavirin therapy, patients who had also been given erythropoietin alfa had significantly higher mean hemoglobin levels than patients in a control group. In patients with chronic hepatitis C, viramidine, a prodrug of ribavirin that is selectively taken up by the liver, has the potential of maintaining the antiviral efficacy of ribavirin, while decreasing the risk of hemolytic anemia[4].
Interferons may also contribute to anemia. Their main relevant action is induction of bone marrow suppression. This effect of interferon results in suppression of compensatory reticulocytosis associated with ribavirin-induced hemolytic anemia. Thus, the bone-marrow-suppressive effect of interferon may contribute to anemia, which complicates therapy with combination of interferon and ribavirin[4].
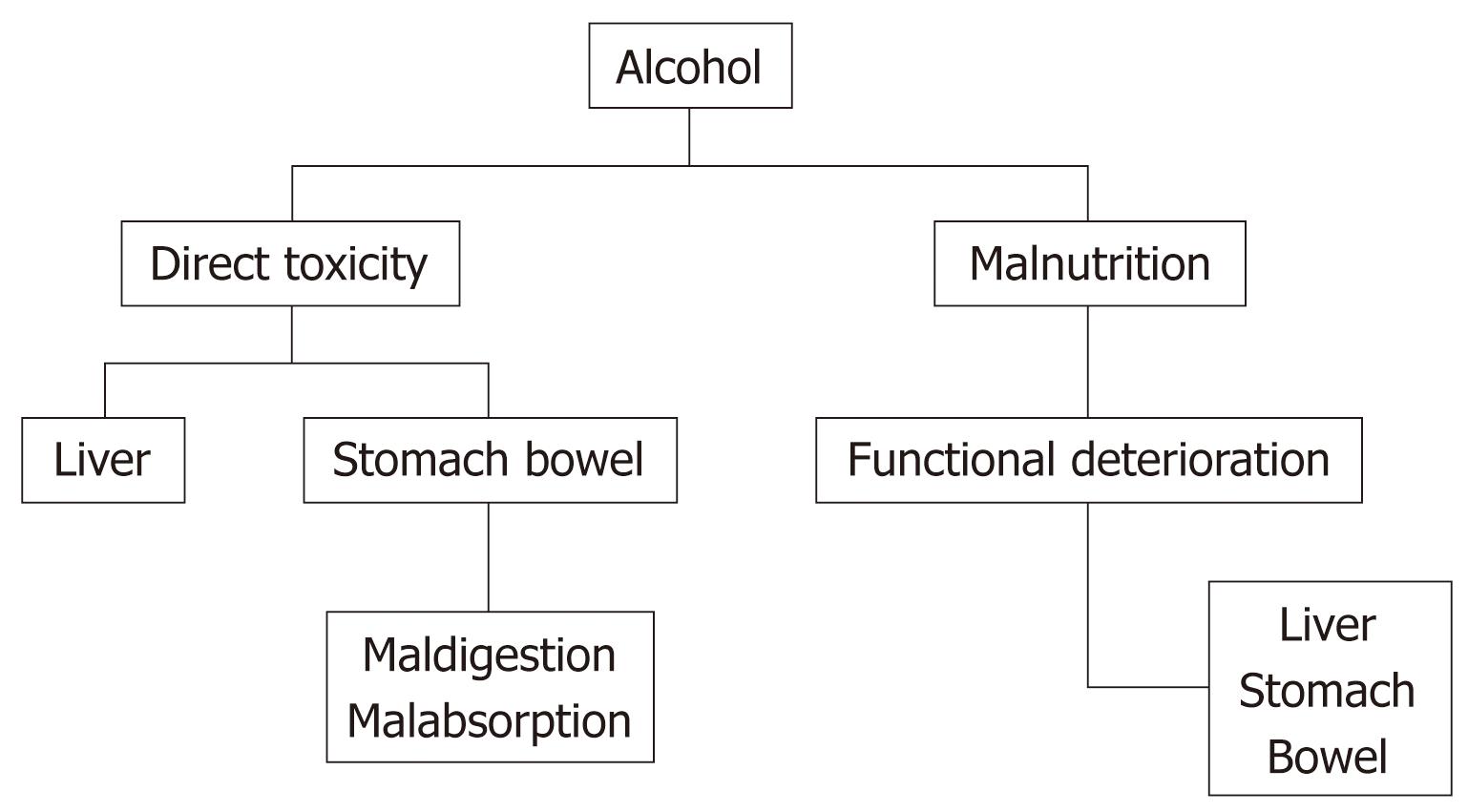
Alcohol is implicated in the pathogenesis of chronic liver disease; it may contribute to anemia secondary to its direct effects on the liver and also to other diverse mechanisms (Figure 2)[23].
Markers of iron overload tend to be higher among those who consume more than two alcoholic drinks per day than among non-drinkers, after adjusting for potential confounding factors[24]. Consumption of alcohol appears to be associated with an approximately 40% reduction in the risk of development of iron deficiency anemia.
Folic acid and vitamin B12 deficiencies develop frequently in patients with cirrhosis. These deficiencies may be related to inadequate food intake or intestinal malabsorption. They are suspected when examination of a blood film reveals hypersegmented cells and oval macrocytes, in addition to round macrocytes characteristic of chronic liver disease. When anemia is caused by these deficiencies, the mean corpuscular volume is increased and bone marrow shows megaloblastic erythropoiesis.
Anemia due to folic acid deficiency may result, not only from a lack of folic acid in the diet, but also the weak antifolate action of ethanol. Folic acid deficiency is the most common cause of a low hematocrit in hospitalized patients who are alcoholics[25,26]. Parenterally administered vitamin B12 not only corrects anemia caused by vitamin B12 deficiency, but may also induce improvement in the peripheral neuropathy that are associated with this deficiency[23]. Supplements of vitamins A, B and C may be administered empirically to patients with advanced alcoholic disease.
Anemia in an alcoholic may also arise as a consequence of the direct toxic effects of alcohol on erythrocyte precursors in the bone marrow. Management of alcohol-induced suppression of erythropoiesis includes abstinence from alcohol and a nutritious diet with appropriate supplements.
Other factors that may contribute to anemia and a low hematocrit in alcoholic patients are given in Table 2.
Liver diseases are frequently associated with hematological abnormalities. Anemia of diverse etiology occurs in many of these patients. Bleeding is one of the most severe causes of anemia, with a high mortality, and defective blood coagulation contributes to the anemia. Other mechanisms of anemia include aplastic anemia secondary to previous hepatitis, or side effects of treatment of hepatitis with interferon and ribavirin. In patients with alcoholic liver disease, different effects of alcohol may contribute to anemia, such as malabsorption, malnutrition or direct toxic effect. The pathogenesis of the anemia in each case is different and it is important to begin the correct therapy.
Peer reviewer: Carla W Brady, MD, MHS, Duke University Medical Center, Division of Gastroenterology, DUMC Box 3913, Durham, NC 27705, United States
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | McHutchison JG, Manns MP, Longo DL. Definition and management of anemia in patients infected with hepatitis C virus. Liver Int. 2006;26:389-398. [Cited in This Article: ] |
| 2. | Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, Tripodi A, Sanyal AJ. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039-1046. [Cited in This Article: ] |
| 3. | Pereira SP, Langley PG, Williams R. The management of abnormalities of hemostasis in acute liver failure. Semin Liver Dis. 1996;16:403-414. [Cited in This Article: ] |
| 4. | Van Vlierbergh H, Delanghe JR, De Vos M, Leroux-Roel G. Factors influencing ribavirin-induced hemolysis. J Hepatol. 2001;34:911-916. [Cited in This Article: ] |
| 5. | Garcia-Pagan JC, De Gottardi A, Bosch J. Review article: the modern management of portal hypertension--primary and secondary prophylaxis of variceal bleeding in cirrhotic patients. Aliment Pharmacol Ther. 2008;28:178-186. [Cited in This Article: ] |
| 6. | Abraldes JG, Bosch J. The treatment of acute variceal bleeding. J Clin Gastroenterol. 2007;41 Suppl 3:S312-S317. [Cited in This Article: ] |
| 7. | Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. J Clin Gastroenterol. 2007;41 Suppl 3:S318-S322. [Cited in This Article: ] |
| 8. | Albillos A. Preventing first variceal hemorrhage in cirrhosis. J Clin Gastroenterol. 2007;41 Suppl 3:S305-S311. [Cited in This Article: ] |
| 9. | Laffi G, Marra F, Tarquini R, Abbate R. Coagulation defects in cirrhosis--old dogmas not yet ready for burial. J Thromb Haemost. 2006;4:2068-2069. [Cited in This Article: ] |
| 10. | Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Liu JD, Chang CC, Chen YY. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;13:619-622. [Cited in This Article: ] |
| 11. | Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83-96. [Cited in This Article: ] |
| 12. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [Cited in This Article: ] |
| 13. | Reverter JC. Abnormal hemostasis tests and bleeding in chronic liver disease: are they related? Yes. J Thromb Haemost. 2006;4:717-720. [Cited in This Article: ] |
| 14. | Levy JH, Fingerhut A, Brott T, Langbakke IH, Erhardtsen E, Porte RJ. Recombinant factor VIIa in patients with coagulopathy secondary to anticoagulant therapy, cirrhosis, or severe traumatic injury: review of safety profile. Transfusion. 2006;46:919-933. [Cited in This Article: ] |
| 15. | Marti-Carvajal AJ, Pérez-Requejo JL. Antifibrinolytic amino acids for acquired coagulation disorders in patients with liver disease. Cochrane Database Syst Rev. 2007;46:CD006007. [Cited in This Article: ] |
| 16. | Gonzalez-Casas R, Garcia-Buey L, Jones EA, Gisbert JP, Moreno-Otero R. Systematic review: hepatitis-associated aplastic anaemia--a syndrome associated with abnormal immunological function. Aliment Pharmacol Ther. 2009;30:436-443. [Cited in This Article: ] |
| 17. | Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519. [Cited in This Article: ] |
| 18. | Davies JK, Guinan EC. An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol. 2007;136:549-564. [Cited in This Article: ] |
| 19. | Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162-168. [Cited in This Article: ] |
| 20. | Cariani E, Pelizzari AM, Rodella A, Gargiulo F, Imberti L, Manca N, Rossi G. Immune-mediated hepatitis-associated aplastic anemia caused by the emergence of a mutant hepatitis B virus undetectable by standard assays. J Hepatol. 2007;46:743-747. [Cited in This Article: ] |
| 21. | Ong JP, Younossi ZM. Managing the hematologic side effects of antiviral therapy for chronic hepatitis C: anemia, neutropenia, and thrombocytopenia. Cleve Clin J Med. 2004;71 Suppl 3:S17-S21. [Cited in This Article: ] |
| 22. | Reau N, Hadziyannis SJ, Messinger D, Fried MW, Jensen DM. Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon alfa-2a (40KD) plus ribavirin. Am J Gastroenterol. 2008;103:1981-1988. [Cited in This Article: ] |
| 23. | Moreno Otero R, Cortés JR. [Nutrition and chronic alcohol abuse]. Nutr Hosp. 2008;23 Suppl 2:3-7. [Cited in This Article: ] |
| 24. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293-1301. [Cited in This Article: ] |
| 25. | Lindenbaum J, Roman MJ. Nutritional anemia in alcoholism. Am J Clin Nutr. 1980;33:2727-2735. [Cited in This Article: ] |
| 26. | Lewis G, Wise MP, Poynton C, Godkin A. A case of persistent anemia and alcohol abuse. Nat Clin Pract Gastroenterol Hepatol. 2007;4:521-526. [Cited in This Article: ] |