Published online Sep 7, 2009. doi: 10.3748/wjg.15.4150
Revised: July 21, 2009
Accepted: July 28, 2009
Published online: September 7, 2009
AIM: To explore the possibility of using the Non-invasive Micro-test Technique (NMT) to investigate the role of Transient Receptor Potential Canonical 1 (TRPC1) in regulating Ca2+ influxes in HL-7702 cells, a normal human liver cell line.
METHODS: Net Ca2+ fluxes were measured with NMT, a technology that can obtain dynamic information of specific/selective ionic/molecular activities on material surfaces, non-invasively. The expression levels of TRPC1 were increased by liposomal transfection, whose effectiveness was evaluated by Western-blotting and single cell reverse transcription-polymerase chain reaction.
RESULTS: Ca2+ influxes could be elicited by adding 1 mmol/L CaCl2 to the test solution of HL-7702 cells. They were enhanced by addition of 20 μmol/L noradrenalin and inhibited by 100 μmol/L LaCl3 (a non-selective Ca2+ channel blocker); 5 μmol/L nifedipine did not induce any change. Overexpression of TRPC1 caused increased Ca2+ influx. Five micromoles per liter nifedipine did not inhibit this elevation, whereas 100 μmol/L LaCl3 did.
CONCLUSION: In HL-7702 cells, there is a type of TRPC1-dependent Ca2+ channel, which could be detected via NMT and inhibited by La3+.
- Citation: Zhang ZY, Wang WJ, Pan LJ, Xu Y, Zhang ZM. Measuring Ca2+ influxes of TRPC1-dependent Ca2+ channels in HL-7702 cells with Non-invasive Micro-test Technique. World J Gastroenterol 2009; 15(33): 4150-4155
- URL: https://www.wjgnet.com/1007-9327/full/v15/i33/4150.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4150
Changes in the concentration of Ca2+ in the cytoplasmic space play a central role in intracellular signaling pathways in liver cells, including glucose, fatty acid, amino acid and xenobiotic metabolism, bile acid secretion, protein synthesis and secretion, the movement of lysosomes and other vesicles, the cell cycle and cell proliferation, and apoptosis and necrosis[1-4]. In earlier reports, it has been shown that ligand-gated, store-operated, receptor-activated, and stretch-activated Ca2+-permeable channels are expressed in hepatocytes and in liver cell lines. No voltage-operated Ca2+ channels (VOCCs) have been detected[5-8]. There is increasing evidence that members of the canonical subgroup of Transient Receptor Potential (TRP) proteins constitute tetramers of both receptor-activated and store-operated Ca2+ channels (SOCs)[9-11], and Transient Receptor Potential Canonical 1 (TRPC1) is considered as one of the most likely candidates in forming Ca2+ channels in mammalian cells[12-15].
The Non-invasive Micro-test Technique (NMT) was developed in the late 20th century, and is a new technology for obtaining dynamic information on specific ionic/molecular activities on material surfaces, non-invasively. This technique incorporates different temporal and spatial resolution domains from other traditional methods, and its 3-dimensional measurement capability enables us to observe the physiological characteristics of biological phenomena that would be difficult or even impossible with other techniques[16]. To date, Ca2+, H+, K+, Cl-, NO-, Mg2+, Cd2+, Al3+, and O2 have be detected as sensors for ionic/molecular species.
In the present study, we used NMT to study the Ca2+ influxes elicited by extracellular elevations of Ca2+ concentration, and the inhibitory effects of several Ca2+ channel blockers, to investigate the role of TRPC1 in regulating Ca2+ fluxes in HL-7702 cells.
Nifedipine, noradrenalin, protease inhibitor Cocktail, Fast Red TR, Naphthol AS-MX phosphate, and Calcium Ionophore I [Cocktail A were bought from the Sigma-Aldrich Company (Catalog Number: 21048)]. Lipofectamine 2000 was purchased from Invitrogen. The TRPC1 polyclonal antibody was acquired from the Abnova Company. Peroxidase-conjugated secondary antibody was obtained from the Beijing Zhongshan Golden Bridge Co.. All the other reagents were of reagent grade.
The human liver cell line, HL-7702, bought from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, was maintained in RPMI-1640 containing 10% FBS, 1% Penicillin, and Streptomycin. Plasmid pBS-T-TRPC1 was constructed and verified as previously described[17]. HL-7702 cells were grown to 75%-80% confluence in 35 mm dishes in advance and transfection was carried out with 1 μg/mL of the recombinant plasmid and Lipofectamine 2000 according to the manufacturer’s protocol.
Measurements of net influxes of Ca2+ were performed using NMT (BIO-001A, Younger USA Sci. & Tech. Co., Amherst, MA, USA; Applicable Electronics Inc., Forestdale, MA, USA; and ScienceWares Inc., East Falmouth, MA, USA). The electrode was controlled to move with an excursion of 10 μm at a programmable frequency in the range of 0.3-0.5 Hz; this minimized mixing of the bathing saline.
To construct the microelectrodes, borosilicate micropipettes (2-4 μm aperture, XYPG120-2, Xuyue (Beijing) Science and Technology Co., Ltd., Beijing, China) were silanized with tributylchlorosilane and the tips filled with Calcium Ionophore I - Cocktail A. An Ag/AgCl wire electrode holder (XYEH01-1) was inserted in the back of the electrode to make electrical contact with the electrolyte solution. Only electrodes with Nernstian slopes > 25 mV were used. Ca2+ fluxes were calculated by Fick’ law of diffusion: J0 = -[D × (dC/dX)] where J0 represents the net Ca2+ flux (in μmol/cm per second), D is the self-diffusion coefficient for Ca2+ (in cm2/s), dC is the difference value of Ca2+ concentrations between the two positions, and dX is the 10 μm excursion over which the electrode moved in our experiments. Data and image acquisition, preliminary processing, control of the three-dimensional electrode positioner, and stepper-motor-controlled fine focus of the microscope stage were performed with ASET software.
Single cell RT-PCR was performed to determine whether the cells measured by NMT were successfully transfected, using a previously described method, with some modifications[18]. Directly after the Ca2+ influx assay, the contents of tested cell were aspirated into a microelectrode. The tip of the electrode was then broken in a PCR tube and stored at -80°C until use. Reverse transcription was carried out using a kit from TIANGEN (Beijing, China) according to the manufacturer’s instructions. The first PCR was performed using specific primers (Forward: GCAATGATACCTTCCATTCGTTC; Reverse: CGATGCACTAGGCAGCAGATC) and the following conditions: pre-denaturation at 94°C for 3 min; followed by 30 cycles of denaturation at 94°C for 30 s and annealing at 60°C for 30 s, then synthesis at 72°C for 60 s; the last step was extension at 72°C for 5 min. After the first PCR, 1 μL of the reaction products was used as the template for a secondary PCR with the same conditions as above and 25 cycles. The predicted size of the TRPC1 amplicons were 455 bp and reaction products were confirmed and analyzed by agarose gel electrophoresis.
Total proteins were obtained from cultured cells by using lysis buffer (35 mmol/L Tris at pH 7.4, 0.4 mmol/L EDTA, 10 mmol/L MgCl2, 0.1% protease inhibitor Cocktail). For western blotting analysis, 20 μg proteins were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto to polyvinylidene difluoride membranes for 2 h at 20 V. The membranes were then blocked for 2 h with blocking solution (5% bovine serum albumin) and probed with anti-TRPC1 antibodies. The primary antibodies were incubated for 1 h at room temperature and, after washing, the membranes were incubated with peroxidase-conjugated secondary antibodies for 1 h. Finally, the proteins on the membranes were dyed by staining solution containing Fast Red TR and Naphthol AS-MX phosphate. Immunoblots were then scanned to obtain images.
Data were expressed as mean ± SD of n cells from at least six cell culture dishes. The statistical significance of diversities between means was determined using the DUNNET t-test. A value of P < 0.05 was considered significant.
Before the experiment, 35 mm dishes with pre-dispersed normal HL-7702 cells were perfused with test solution containing (in mmol/L): 2.3 NaHCO3, 27 Na2SO4, 9.7 KCl, 61.1 MgCl2, and 366.7 NaCl. The Ca2+ influxes were then measured by NMT. The background noise was recorded for three min before 1 mmol/L CaCl2 was added to elicit an inward Ca2+ current. As shown in Figure 1A, the Ca2+ selective microelectrode moved between two positions close to the tested cells constantly to acquire experimental data. Net Ca2+ fluxes are depicted in Figure 1B, a giant wave trough emerged shortly after CaCl2 was added.
To further identify the property of the Ca2+ influxes of HL-7702 cells, three drugs were selected as tools in the following experiments. After background noise was recorded for three min, a Ca2+ channel agonist, noradrenalin (20 μmol/L), or two types of inhibitors, nifedipine (5 μmol/L) or LaCl3 (100 μmol/L) were added into the test solution together with 1 mmol/L CaCl2. The effects of the three drugs on Ca2+ influxes are shown in Figure 1C-E. Net Ca2+ fluxes were significantly influenced by noradrenalin and La3+; however, nifedipine did not induce any change. A bar graph of the maximum net Ca2+ fluxes in the four groups (three drug-treated groups and control group) is depicted in Figure 1F; these experiments were repeated six times (n = 6).
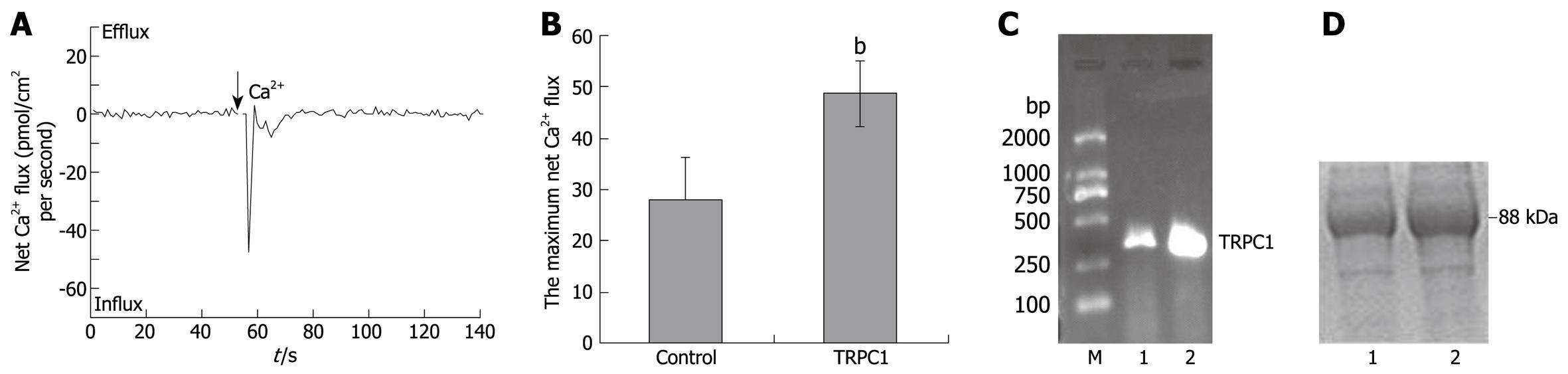
When TRPC1-transfected HL-7702 cells were measured by NMT, the maximum net Ca2+ influxes increased to 48.9 ± 6.4 pmol/cm2 per second (n = 6) and a deeper wave trough was observed (Figure 2A), The bar graph shown in Figure 2B shows that the statistical difference between the TRPC1-transfected group and the control group was significant (P < 0.01). Single cell RT-PCR and western blotting were performed after NMT experiments. The results of agarose gel electrophoresis and SDS-PAGE are shown in Figure 2C and D, respectively. They demonstrated that TRPC1-expressions in tested cells were elevated after transfection.
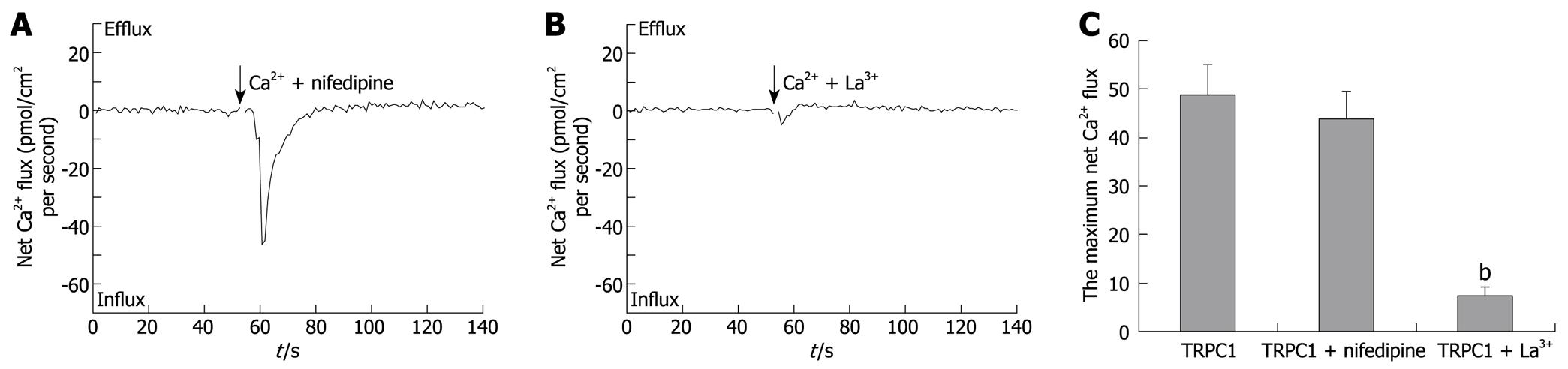
TRPC1-transfection induced increases in Ca2+ influxes. To investigate the effects of nifedipine and La3+ on these increased Ca2+ influxes, the two drugs were added into the test solution together with 1 mmol/L CaCl2. As shown in Figure 3A-C, the maximum net Ca2+ fluxes maintained an average of 44.0 ± 5.7 pmol/cm2 per second (n = 6) when 5 μmol/L nifedipine was applied, whereas 100 μmol/L LaCl3 made reduced the maximum value to 7.6 ± 1.9 pmol/cm2 per second (n = 6). A significant statistical difference only existed between the TRPC1 + La3+-treated group and the TRPC1-transfected group (P < 0.01). Single cell RT-PCR and western blotting were performed after NMT experiments to confirm the increased TRPC1 expression (data not shown).
Initially, we detected Ca2+ influxes in HL-7702 cells using NMT when 1 mmol/L CaCl2 was added to the test solution and the extracellular Ca2+ concentration markedly changed. Ca2+ influx could be influenced by two drugs, noradrenalin, and LaCl3; however nifedipine did not induce any change.
As a known neurotransmitter, noradrenalin can act through α1-adrenoceptors to activate phospholipase C. This generates inositol 1,4,5-trisphosphate (IP3) within the cell, which in turn mediates the rise of Ca2+ concentration by release from intracellular stores[19,20] and opening of receptor operated Ca2+ entry at the plasma membrane[21-23]. Thus, the increase in Ca2+ influx induced by noradrenalin together with 1 mmol/L external CaCl2 could be explained if receptor operated Ca2+ channels played a central role in these experiments. On the other hand, Ca2+ influxes were prominently inhibited by La3+. Analogs often effect their action through competitive inhibition with their common receptors or channels. La3+ has a similar size of ionic radius to that of Ca2+, which enables La3+ to compete with Ca2+, which makes La3+ a non-selective Ca2+ channel blocker. In addition, a study showed that La3+ could inhibit both influx and efflux of Ca2+ in lacrimal cells[24], which was consistent with the present study. Most importantly, nifedipine, as an antagonist of L-type Ca2+ channels, did not induce any change in Ca2+ influxes, which would indicate that no VOCCs existed or that transmembrane Ca2+ influxes elicited by external Ca2+ did not pass through VOCCs in HL-7702 cells.
TRPC1 is one of seven members of the TRPC sub-family of non-selective cation channels and is expressed in a wide variety of cell types and tissues[25-27]. It is likely that TRPC1 plays a significant part in intracellular Ca2+ homeostasis. In salivary gland cells, the current through the endogenous SOCs was the same as the membrane current which was activated by the depletion of intracellular Ca2+ stores in cells in which TRPC1 was ectopically expressed and the endogenous SOCs were decreased by transfection with antisense TRPC1[28]. In kidney epithelial cells, a new receptor-operated channel formed by heteromeric assembly of TRPP2 and TRPC1 subunits was discovered[29]. In T cells, intracellular Ca2+ elevation induced by Δ9-tetrahydrocannabinol was attributable entirely to extracellular Ca2+ influxes, which were not dependent on store depletion, but mediated through TRPC1 channels[30]. The results of the second part of our study do not conflict with these reports. In HL-7702 cells, overexpression of TRPC1 causes an increase of Ca2+ influxes induced by adding external Ca2+, and the non-selective Ca2+ channel blocker, La3+, can attenuate this elevation. In summary, there is a TRPC1-dependent Ca2+-type channel(s), either receptor-activated or store-operated present in HL-7702 cells, which can be inhibited by La3+.
Taken together, NMT is a powerful tool for ion channel research, which has been effectively applied in various systems[31-33]. We used NMT to explore the properties of Ca2+ channels, and found that there was a TRPC1-dependent Ca2+-type channel(s), which could be detected via NMT and inhibited by La3+, in HL-7702 cells.
Ca2+ plays an important role in intracellular signaling pathways and Transient Receptor Potential Canonical 1 (TRPC1) is considered as one of the most likely candidates in forming Ca2+ channels in mammalian cells. As a technology to obtain dynamic information of specific ionic/molecular activities on material surfaces non-invasively, Non-invasive Micro-test Technique (NMT) is being increasingly applied to study characters of ion channels.
TRPC1 has been verified as a molecular candidate or a regulator of Ca2+ channels in several mammalian cells. However, the role of TRPC1 in normal human liver cells has not been elucidated. In this study, the authors demonstrate that a TRPC1-dependent Ca2+-type channel(s) exists in HL-7702 cells, using NMT, a non-invasive technique.
This is the first study to investigate the role of TRPC1 in regulating Ca2+ channels in normal human liver cells. Furthermore, measuring Ca2+ influxes was performed non-invasively, which cannot be accomplished with other traditional techniques.
Cytoplasmic Ca2+ overloading might cause damage to liver cells. By understanding the role of TRPC1 in mediating extracellular Ca2+ influxes, this study might represent a future strategy for preventing or treating diseases induced by dysfunctions of Ca2+ channels in the clinic, such as hepatic ischemia-reperfusion injury.
The canonical transient receptor potential (TRPC) channel subfamily consists of seven mammalian cation channels and is expressed in almost every tissue, including the liver. The TRPC1 channel is permeable to Ca2+ and is the most likely candidate for receptor-operated Ca2+ channels. In addition, TRPC1 also plays a dominant role in mediating store-operated Ca2+ channels in many types of cells.
The authors recorded Ca2+ influxes elicited by adding external Ca2+ into a test solution in several different conditions. It revealed that there is a TRPC1-dependent Ca2+-type channel(s), which can be detected via NMT, in normal human liver cells. This is an interesting study.
| 1. | Nieuwenhuijs VB, De Bruijn MT, Padbury RT, Barritt GJ. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig Dis Sci. 2006;51:1087-1102. |
| 2. | Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther. 2005;313:1305-1313. |
| 3. | O'Brien EM, Gomes DA, Sehgal S, Nathanson MH. Hormonal regulation of nuclear permeability. J Biol Chem. 2007;282:4210-4217. |
| 4. | Enfissi A, Prigent S, Colosetti P, Capiod T. The blocking of capacitative calcium entry by 2-aminoethyl diphenylborate (2-APB) and carboxyamidotriazole (CAI) inhibits proliferation in Hep G2 and Huh-7 human hepatoma cells. Cell Calcium. 2004;36:459-467. |
| 5. | Graf J, Häussinger D. Ion transport in hepatocytes: mechanisms and correlations to cell volume, hormone actions and metabolism. J Hepatol. 1996;24 Suppl 1:53-77. |
| 6. | Sawanobori T, Takanashi H, Hiraoka M, Iida Y, Kamisaka K, Maezawa H. Electrophysiological properties of isolated rat liver cells. J Cell Physiol. 1989;139:580-585. |
| 7. | Auld A, Chen J, Brereton HM, Wang YJ, Gregory RB, Barritt GJ. Store-operated Ca(2+) inflow in Reuber hepatoma cells is inhibited by voltage-operated Ca(2+) channel antagonists and, in contrast to freshly isolated hepatocytes, does not require a pertussis toxin-sensitive trimeric GTP-binding protein. Biochim Biophys Acta. 2000;1497:11-26. |
| 8. | Brereton HM, Harland ML, Froscio M, Petronijevic T, Barritt GJ. Novel variants of voltage-operated calcium channel alpha 1-subunit transcripts in a rat liver-derived cell line: deletion in the IVS4 voltage sensing region. Cell Calcium. 1997;22:39-52. |
| 9. | Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233-252. |
| 10. | Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25-36. |
| 11. | Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757-810. |
| 12. | Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479-495. |
| 13. | Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195:287-296. |
| 14. | Brereton HM, Chen J, Rychkov G, Harland ML, Barritt GJ. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim Biophys Acta. 2001;1540:107-126. |
| 15. | Chen J, Barritt GJ. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca(2+)-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem J. 2003;373:327-336. |
| 16. | Ding YN, Xu Y. Non-invasive micro-test technology and its applications in biology and medicine. Physics. 2007;36:548-558. |
| 17. | Zhang ZY, Zhang ZM, Pan LJ, Shui CX, Wang YY. Construction of human TRPC1 eukaryotic expression vector and its expression in HL-7702 cells. Zhonghua Shiyan Waike Zazhi. 2009;26:976-978. |
| 18. | Lambolez B, Audinat E, Bochet P, Crépel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247-258. |
| 19. | Burgess GM, Godfrey PP, McKinney JS, Berridge MJ, Irvine RF, Putney JW Jr. The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984;309:63-66. |
| 20. | Joseph SK, Thomas AP, Williams RJ, Irvine RF, Williamson JR. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984;259:3077-3081. |
| 22. | Irvine RF. Inositol phosphates and Ca2+ entry: toward a proliferation or a simplification? FASEB J. 1992;6:3085-3091. |
| 23. | Putney JW Jr. Excitement about calcium signaling in inexcitable cells. Science. 1993;262:676-678. |
| 24. | Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JW Jr. Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990;258:C1006-C1015. |
| 25. | Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25-32. |
| 26. | Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53-60. |
| 27. | Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619-647. |
| 28. | Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. J Biol Chem. 2003;278:11337-11343. |
| 29. | Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472-479. |
| 30. | Rao GK, Kaminski NE. Induction of intracellular calcium elevation by Delta9-tetrahydrocannabinol in T cells involves TRPC1 channels. J Leukoc Biol. 2006;79:202-213. |
| 31. | Knox RJ, Jonas EA, Kao LS, Smith PJ, Connor JA, Kaczmarek LK. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J Physiol. 1996;494:627-639. |