Published online Jan 21, 2009. doi: 10.3748/wjg.15.321
Revised: December 2, 2008
Accepted: December 9, 2008
Published online: January 21, 2009
AIM: To investigate the therapeutic effects of four strains of probiotics (E. feacalis, L. acidophilus, C. butyricum and B. adolescentis) on dextran sulphate sodium (DSS)-induced experimental colitis in Balb/c mice.
METHODS: Eighty Balb/c mice were randomly divided into 8 groups. Weight-loss, fecal character, fecal occult blood and hematochezia were recorded daily. Disease activity index (DAI) scores were also evaluated everyday. Length of colon was measured and histological scores were evaluated on the 13th day. Myeloperoxidase (MPO) activity was detected. Interleukin-1 (IL-1) and IL-4 expression was detected by ELISA and RT-PCR.
RESULTS: The four strains of probiotics relieved the inflammatory condition of DSS-induced experimental colitis in mice. Weight loss was slowed down in all probiotics-treated mice. Even weight gain was observed by the end of probiotics treatment. The DAI and histological scores of probiotics-treated mice were lower than those of mice in the control group (1.9 ± 0.2 vs 8.6 ± 0.4, P < 0.05 for E. faecalis). The length of colon of probiotics-treated mice was longer than that of mice in the control group (10.3 ± 0.34 vs 8.65 ± 0.77, P < 0.05 for E. faecalis). The four strains of probiotics decreased the MP activity and the IL-1 expression, but increased the IL-4 expression. E. faecalis had a better effect on DSS-induced experimental colitis in mice than the other three strains.
CONCLUSION: The four strains of probiotics have beneficial effects on experimental colitis in mice. E. faecalis has a better effect on DSS-induced experimental colitis in mice than the other three strains. Supplement of probiotics provides a new therapy for UC.
- Citation: Chen LL, Wang XH, Cui Y, Lian GH, Zhang J, Ouyang CH, Lu FG. Therapeutic effects of four strains of probiotics on experimental colitis in mice. World J Gastroenterol 2009; 15(3): 321-327
- URL: https://www.wjgnet.com/1007-9327/full/v15/i3/321.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.321
Ulcerative colitis (UC) is a non-specific chronic inflammation of intestinal tract. The primary therapies with salazosulfamide, glucocorticoids and immunodepressant probiotics are limited by their side-effects, poor compliance of patients and high relapse rates[1–3].
It is well known that consumption of certain bacteria improves intestinal health[4–6]. Animal and clinical studies also indicate that gastrointestinal bacteria play an important role in the development of UC[78]. Supplements of probiotics provide a new therapy for UC. Bibiloni et al[9] found that VSL#3 (including 4 strains of Lactobacilli, 3 strains of Bifidobacteria and 1 strain of Streptococcus) displays a beneficial effect on UC. Kruis et al[10] showed that Escherichia coli and Nissle 1917 have a similar effect and safety on 5-aminosalicylic acid[10]. It was reported that administration of Lactobacillus casei obviously decreases histological damage, while administration of Escherichia coli O83 and Nissle 1917 is beneficial for immunological regulation[11]. Peran et al[12] showed that probiotics can relieve UC symptoms through different mechanisms of action[12].
Because of the specific damage site of UC and the different colonizations of each bacterium, different probiotics have different effects on UC[13–15]. In order to find out effective strains, we compared the effects of four strains of probiotics on experimental colitis in mice.
L. acidophilus, C. butyricum, B. adolescentis and E. feacalis were isolated from intestinal tract of healthy adults and identified in our facility.
Salicylazosulfapyridine (SASP) was purchased from Fuda Pharmaceutical Co. Ltd (China). Dextran sulphate sodium was produced by MW 5000, Sigma-Aldrich Co (USA). ELISA kit was bought from Boster Biological Technology, Ltd (China), RT-PCR correlate agents were purchased from Promega Biotech Co., Ltd (USA) and Myeloperoxidase (MPO) diagnostic kit was bought from Jiancheng biotech Co (China).
Six-eight-week-old Balb/c mice (half males and half females, weighing 20.0 ± 2.0 g) provided by Hunan Agricultural University (China) were randomly divided into 8 groups, housed in clean filter-top cages under standard conditions (50% ± 10% humidity) in a 12-h dark/12-h light cycle, and fed with standard mouse chow. All mice were fed under standard conditions for 5 d. All mice were divided as follows: (1) Normal group: Normal diet without special treatment; (2) Model group: drinking 5% DSS for modeling; (3) NS group: 5% DSS for modeling + NS (100 &mgr;L/10 g) by gavage (4) SASP group: 5% DSS for modeling + SASP (50 mg/mL) by gavage; (5) L. acidophilus group: 5% DSS for modeling + 109 U/mL L. acidophilus by gavage; (6) C. butyricum group: 5% DSS for modeling +109 U/mL C. butyricum by gavage; (7) B. adolescentis group: 5% DSS for modeling +109 U/mL B. adolescentis by gavage; (8) E. faecalis group: 5% DSS for modeling +109 U/mL E. faecalis by gavage; The four strains of probiotics were grown overnight in culture media and suspended in normal saline (NS) to a concentration of 109 U/mL. SASP was dissolved in NS to a concentration of 50 mg/mL. The mice were fed with 200 &mgr;L of this daily-prepared suspension via an intragastric tube. On the 13th day, all mice were sacrificed.
To reflect the general conditions of mice, DAI scores were determined by an investigator blind to the protocol by scoring the extent of body weight loss, fecal character, fecal occult blood or hematochezia as previously described[16]. On the 13th day, blood was collected and all mice were sacrificed. The colon, from the colo-cecal junction to the anus was excised with its length measured, rinsed with 5 mL of 0.01 mol/L PBS (pH 7.4) to remove the fecal remnants, cut open longitudinally at the mesenteric attachment. One cm of the distal colon was removed and fixed for 48 h in PBS-buffered 10% formalin. The tissue was then processed for paraffin embedding and cut into 5-&mgr;m thick sections. The sections were stained with hematoxylin and eosin (HE). Other part of the colon was preserved in liquid nitrogen.
MPO activity was measured as an indicator of neutrophil accumulation in colonic mucosa as preciously described[17].
All primers were designed by software Primer5 (Table 1). The anneal temperature was 58-53°C for 45 min at 30 amplification (Table 1).
| Primer | Product (bp) | ||
| IL-1β | Sense: | 5'-AGCCCATCCTCTGTGACTCATG-3' | 422 |
| Antisense: | 5'-GCTGATGTACCAGTTGGGGAAC-3' | ||
| IL-4 | Sense: | 5'-ACTTCAGTGGCTGGATTTAT-3' | 424 |
| Antisense: | 5' ATTCCCTGAAAGGCTTGGTC-3' | ||
| β-actin | Sense: | 5' ATGGATGACGATATCGCT-3' | 569 |
| Antisense: | 5'-ATGAGGTAGTCTGTCAGGT-3' |
The concentrations of IL-1 and IL-4 were measured in homogenized colons with an ELISA kit according to its manufacturers protocol and expressed as per milligram of protein.
Statistical analyses were performed using SPSS for Windows, version 13.0. All results were expressed as mean ± SD. Data sets were analyzed by one-way analysis of variance (ANOVA) and Fishers’ protected LSD post hoc-test. Those with a significant difference were further analyzed by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
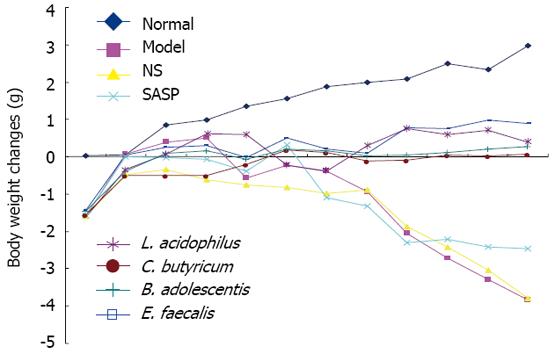
No difference was observed in body weight of mice among the 4 groups on day 0 (ANOVA). Body weight increased gradually in the normal group. Mice in the model group had a weight gain during the first 4 d due to lack of gavage stimulation, and a significant weight loss from day 5. Because of the double stimulation of DSS and gavages, mice in the NS group had an obviously weight loss. Mice in the SASP group maintained their body weight at its base level during the first 7 d, but had a significant weight loss from day 8 due to severe experimental colitis. The four strains of probiotics, especially E. feacalis, could inhibit the weight loss (Figure 1).
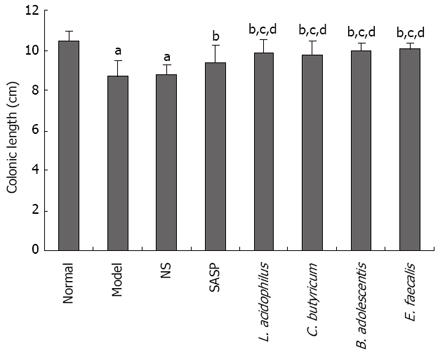
The length of colon of mice in the model and NS groups was significantly decreased compared with the SASP group. C. butyricum, L. acidophilus, B. adolescentis, E. faecalis could effectively prevent the shortening of colon (Figure 2).
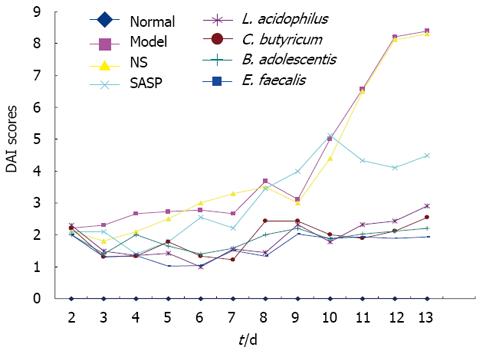
Weight loss, fecal character, fecal occult blood and hematochezia were evaluated individually as previously described[16]. The highest DAI score was observed in model and NS groups. SASP had a good effect on early experimental colitis, but a poor long-term effect on severe experimental colitis. The DAI scores of the four groups were low (Figure 3).
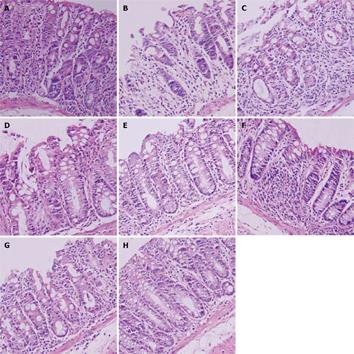
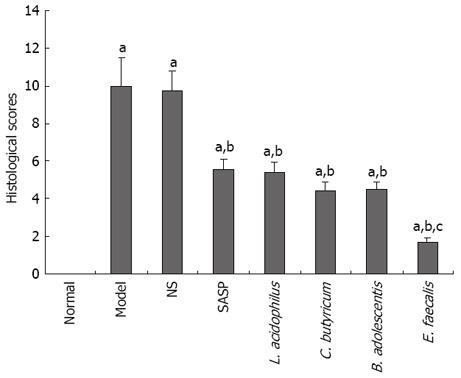
Histological changes in mice of the 4 groups were evaluated individually as previously described[18] (Figure 4). The highest score was found in mice of the model and NS groups, and a lower score was observed in mice of the SASP and probiotics treatment groups compared to mice of the model group, especially the mice in E. faecalis treatment group (1.67 ± 0.27 vs 9.99 ± 1.48, P < 0.05) (Figure 5).
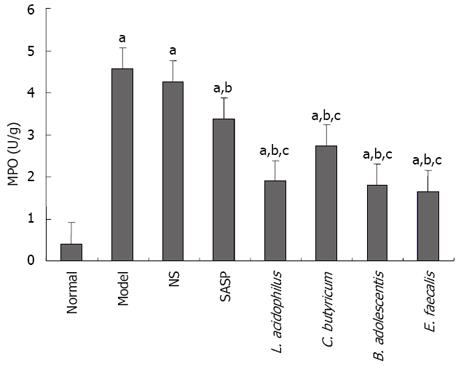
The level of MPO activity was low in normal group, high in model group, and lower in SASP and probiotics treatment groups than in model group, especially in E. faecalis treatment group (Figure 6).
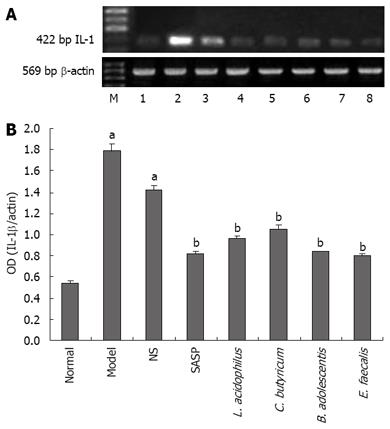
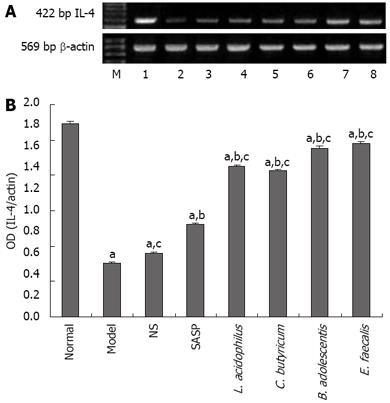
The level of IL-1β mRNA was the lowest in normal group, the highest in model group, and lower in SASP and probiotics treatment groups than in model group (0.82 ± 0.03 vs 1.78 ± 0.07, P < 0.05) (Figure 7A-B). On the contrary, the level of IL-4 mRNA was the highest in normal group, the lowest in model group, higher in SASP and probiotics treatment groups than in model group (0.98 ± 0.01 vs 0.30 ± 0.01, P < 0.05) (Figure 8A-B)
The level of IL-1β and IL-4 was similar with that of mRNA. The level of IL-1β was the lowest in L. acidophilus and E. faecalis treatment groups (105.25 ± 7.79 vs 166.93 ± 13.69, P < 0.05), and the highest in L. acidophilus, B. adolescentis, and E. faecalis treatment groups (184.85 ±11.51 vs 119.33 ± 10.86, P < 0.05) (Table 2).
| Groups | Number | IL-1β (pg/mg.protein) | IL-4 (pg/mg.protein) |
| Norml | 10 | 84.64 ± 7.02 | 205.81 ± 14.83 |
| Model | 10 | 166.93 ± 13.69a | 119.33 ± 10.86a |
| NS | 10 | 147.36 ± 12.61a | 124.37 ± 9.85a |
| SASP | 10 | 125.1 ± 12.04ac | 147.02 ± 12.02ac |
| DSS + L. acidophilus | 10 | 109.43 ± 11.98ace | 177.81 ± 10.29ace |
| DSS + C. butyricum | 10 | 121.25 ± 12.42ace | 150.76 ± 9.98ac |
| DSS + B. adolescentis | 10 | 120.27 ± 10.90ac | 173.69 ± 11.98ace |
| DSS + E. faecalis | 10 | 105.25 ± 7.79ace | 184.85 ± 11.51ce |
Studies showed that intestinal bacteria play an important role in the development of UC[519], and supplement of probiotic is beneficial for UC[1314]. Because the damage site of UC resides mostly at the colon or rectum and different bacteria have different secretion functions or metabolism in intestinal tract, complement of local bacteria is more beneficial for UC. There are 300-500 different species of bacteria in the intestinal tract[5], and their location remains unclear at present. Since probiotics isolated from intestinal tract have an excellent permanent planting ability, obviously effective probiotics should be selected in a comparative study about their effects on experimental colitis.
Several studies on Escherichia coli Nissle, Lactobacillus casei, Bifidobacterium lactis, Lactobacillus acidophilus, etc, showed that these probiotics can be used in treatment of inflammatory bowel disease (IBD)[111220]. In the present study, the effects of the four strains of probiotics on experimental colitis were compared.
The results of our study indicate that the four strains of probiotics had therapeutic effects on experimental colitis, confirming that probiotics can be used in treatment of colitis. Weight loss was slowed down and even weight gain was observed by the end of our experiment. The DAI and histological scores were low in probiotics treatment groups. These results agree with the reported findings[1321]. The MPO activity decreased significantly in all probiotics treatment groups, especially in E. faecalis treatment group, suggesting that the four stains of probiotics can relieve the symptoms of experimental colitis by decreasing the infiltration of neutrophils.
Balish et al[22] revealed that IBD occurs in germ-free IL-10-/- mice when they are colonized with a pure culture of E. faecalis, indicating E. faecalis is a conditioned pathogen. However, our study showed that E. faecalis could relieve the symptoms of experimental colitis and decrease the infiltration of neutrophils. E. faecalis had a better effect on experimental colitis than the other three strains of probiotics. Ruiz et al[23] reported that the expression of pro-inflammatory cells is transient 1 wk after E. faecalis treatment in intestinal epithelial cells from wild-type mice, suggesting that lack of protective TGF-β/Smad signaling and failing to inhibit TLR2-mediated pro-inflammatory gene expression in the intestinal epithelium might be a partial mechanism of IBD developed in IL-10-/- mice.
Because E. faecalis, L. acidophilus and some other probiotics have different effects on experimental colitis in wild-type mice rather than in immunodeficient mice, there should be more immunological mechanisms against experimental colitis except for the signal pathway or innate immunological mechanism. Some inflammatory cytokines, especially IL-1 and IL-4, are closely related with the development and progress of experimental colitis and IBD. IL-1 is secreted by mononuclear macrophages and high doses of IL-1, especially IL-1β, can result in UC[24]. IL-4 is synthesized by lamina propria intestinal lymphocytes after in vitro polyclonal activation. Compared with peripheral lymphocytes, intestinal epithelial and lamina propria lymphocytes spontaneously secrete IL-4[25], which can inhibit secretion of IL-1β by monocytes in a dose-dependent manner[26], suggesting that IL-4 has a protective function on regulatiing immunological reaction in intestinal tract. Our study showed that the expression of IL-1β was lower in probiotics treatment groups than in model group, and similar to that in SASP treatment group. The expression of IL-4 in probiotics treatment groups was increased. The expression of IL-4 was higher in E. feacalis treatment group than in treatment SASP group. The general condition, DAI scores, histological scores, and MPO activity were maintained at a parallel level, supporting the effects of probiotics at cytokine level. Changes in IL-1 and IL-4 level represent the inflammation degree of experimental colitis. Our experimental results also indicate that there should be some other inflammatory cytokines involved in the difference of adaptive immunological mechanisms in experimental colitis of wide-type and immunodeficient mice.
In summary, L. acidophilus, C. butyricum, B. adolescentis, E. faecalis are effective against DSS-induced acute experimental colitis. Reduced infiltration of neutrophils, decreased expression of IL-1 and increased expression of IL-4 might be a partial immunological mechanism of probiotics on experimental colitis in mice.
Ulcerative colitis (UC) is a non-specific chronic inflammation of intestinal tract and the primary therapies with probiotics are limited by their side-effects, poor compliance of patients and high relapse rates. Supplement of probiotics provides a new therapy for UC.
Bacteria play an important role in pathogenesis of UC. Supplement of probiotics provide a new therapy for UC. Because of the specific damage site of UC and the different colonization sites of bacteria, different probiotics display different effects on UC. A more effective strain of probiotics should be selected for UC.
They compared the effects of four strains of probiotics isolated from healthy human feces in order to find one or two more effective strains. E. faecalis had a better effect than the other three strains. Their study showed that E. faecalis had different effects on experimental colitis in wild-type mice. The experimental results indicate that there should be some other inflammatory cytokines involved in experimental colitis of wide-type and immunodeficient mice except for IL-1 and IL-4.
The results of their study indicate that there should be some other inflammatory cytokines involved in experimental colitis of wide-type and immunodeficient mice except for IL-1 and IL-4.
The effects of four strains of probiotics on DSS-induced colonic inflammation in mice were clarified. The authors showed that the four strains of probiotics derived from healthy human feces could relieve colonic inflammation as sulfasalazine. Of the four strains, E. faecalis was most beneficial for DSS-induced colitis. Methods employed and results obtained are reasonable.
| 1. | Regueiro M, Curtis J, Plevy S. Infliximab for hospitalized patients with severe ulcerative colitis. J Clin Gastroenterol. 2006;40:476-481. [Cited in This Article: ] |
| 2. | Jakobovits SL, Jewell DP, Travis SP. Infliximab for the treatment of ulcerative colitis: outcomes in Oxford from 2000 to 2006. Aliment Pharmacol Ther. 2007;25:1055-1060. [Cited in This Article: ] |
| 3. | Lewis JD, Gelfand JM, Troxel AB, Forde KA, Newcomb C, Kim H, Margolis DJ, Strom BL. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1428-1435; quiz 1436. [Cited in This Article: ] |
| 4. | Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, Campieri M, Morgenstern S, Rachmilewitz D. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399-406. [Cited in This Article: ] |
| 5. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [Cited in This Article: ] |
| 6. | Dieleman LA, Goerres MS, Arends A, Sprengers D, Torrice C, Hoentjen F, Grenther WB, Sartor RB. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52:370-376. [Cited in This Article: ] |
| 7. | Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:260-263. [Cited in This Article: ] |
| 8. | Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286-299. [Cited in This Article: ] |
| 9. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. [Cited in This Article: ] |
| 10. | Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [Cited in This Article: ] |
| 11. | Kokesova A, Frolova L, Kverka M, Sokol D, Rossmann P, Bartova J, Tlaskalova-Hogenova H. Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia Microbiol (Praha). 2006;51:478-484. [Cited in This Article: ] |
| 12. | Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836-844. [Cited in This Article: ] |
| 13. | Chapman TM, Plosker GL, Figgitt DP. Spotlight on VSL#3 probiotic mixture in chronic inflammatory bowel diseases. BioDrugs. 2007;21:61-63. [Cited in This Article: ] |
| 14. | Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371-1387. [Cited in This Article: ] |
| 15. | Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1012-1018. [Cited in This Article: ] |
| 16. | Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin Exp Immunol. 1999;117:462-468. [Cited in This Article: ] |
| 17. | Fabia R, Ar'Rajab A, Johansson ML, Willen R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155-162. [Cited in This Article: ] |
| 18. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [Cited in This Article: ] |
| 19. | Torres MI, Rios A. Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol. 2008;14:1972-1980. [Cited in This Article: ] |
| 20. | Hudcovic T, Stepankova R, Kozakova H, Hrncir T, Tlaskalova-Hogenova H. Effects of monocolonization with Escherichia coli strains O6K13 and Nissle 1917 on the development of experimentally induced acute and chronic intestinal inflammation in germ-free immunocompetent and immunodeficient mice. Folia Microbiol (Praha). 2007;52:618-626. [Cited in This Article: ] |
| 21. | Kamada N, Maeda K, Inoue N, Hisamatsu T, Okamoto S, Hong KS, Yamada T, Watanabe N, Tsuchimoto K, Ogata H. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun. 2008;76:214-220. [Cited in This Article: ] |
| 22. | Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253-2257. [Cited in This Article: ] |
| 23. | Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. IL-10 gene-deficient mice lack TGF-beta/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J Immunol. 2005;174:2990-2999. [Cited in This Article: ] |
| 24. | Ashwood P, Harvey R, Verjee T, Wolstencroft R, Thompson RP, Powell JJ. Functional interactions between mucosal IL-1, IL-ra and TGF-beta 1 in ulcerative colitis. Inflamm Res. 2004;53:53-59. [Cited in This Article: ] |
| 25. | Carol M, Lambrechts A, Van Gossum A, Libin M, Goldman M, Mascart-Lemone F. Spontaneous secretion of interferon gamma and interleukin 4 by human intraepithelial and lamina propria gut lymphocytes. Gut. 1998;42:643-649. [Cited in This Article: ] |
| 26. | Kucharzik T, Lugering N, Weigelt H, Adolf M, Domschke W, Stoll R. Immunoregulatory properties of IL-13 in patients with inflammatory bowel disease; comparison with IL-4 and IL-10. Clin Exp Immunol. 1996;104:483-490. [Cited in This Article: ] |