Published online Dec 28, 2008. doi: 10.3748/wjg.14.7361
Revised: September 4, 2008
Accepted: September 11, 2008
Published online: December 28, 2008
AIM: To assess the safety of bismuth used in Helicobacter pylori (H pylori) eradication therapy regimens.
METHODS: We conducted a systematic review and meta-analysis. MEDLINE and EMBASE were searched (up to October 2007) to identify randomised controlled trials comparing bismuth with placebo or no treatment, or bismuth salts in combination with antibiotics as part of eradication therapy with the same dose and duration of antibiotics alone or, in combination, with acid suppression. Total numbers of adverse events were recorded. Data were pooled and expressed as relative risks with 95% confidence intervals (CI).
RESULTS: We identified 35 randomised controlled trials containing 4763 patients. There were no serious adverse events occurring with bismuth therapy. There was no statistically significant difference detected in total adverse events with bismuth [relative risk (RR) = 1.01; 95% CI: 0.87-1.16], specific individual adverse events, with the exception of dark stools (RR = 5.06; 95% CI: 1.59-16.12), or adverse events leading to withdrawal of therapy (RR = 0.86; 95% CI: 0.54-1.37).
CONCLUSION: Bismuth for the treatment of H pylori is safe and well-tolerated. The only adverse event occurring significantly more commonly was dark stools.
-
Citation: Ford AC, Malfertheiner P, Giguère M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for
Helicobacter pylori eradication: Systematic review and meta-analysis. World J Gastroenterol 2008; 14(48): 7361-7370 - URL: https://www.wjgnet.com/1007-9327/full/v14/i48/7361.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7361
| Study | Country | No. of centres | Bismuth compound used1 | Duration of bismuth therapy (days) | Total dose (mg/d) used | Method of collection of adverse event data | Generation of randomization schedule provided | Method of concealment of allocation provided | Double-blind |
| Bujanda 2001[15] | Spain and Portugal | Multi-centre | RBC | 7 | 800 | Unclear | Yes | No | Yes |
| Burette 1992[16] | Belgium | 1 | CBS | 10 | 480 | Unclear | No | No | No |
| Buzas 2001[17] | Hungary | 1 | RBC | 7 | 800 | Unclear | No | No | No |
| Carpintero 1997[18] | Spain | 1 | CBS | 42 | 480 | Unclear | Yes | No | No |
| Carvalho 1998[19] | Brazil | 1 | BSN | 14 | 1200 | Unclear | No | No | No |
| Catalano 2000[20] | Italy | 1 | RBC | 10 | 800 | Questionnaire | Yes | No | Yes |
| Chuang 2001[22] | Taiwan | 1 | RBC | 7 | 800 | Unclear | No | No | Yes |
| Dal Bo 1998[23] | Italy | 1 | TDB | 14 | 480 | Unclear | No | No | No |
| Danese 2001[24] | Italy | 1 | RBC | 7 | 800 | Validated questionnaire | No | No | No |
| Eberhardt 1990[25] | Germany | 4 | BSS | 28 | 1800 | Unclear | No | No | No |
| Fakheri 2004[26] | Iran | 1 | CBS | 14 | 480 | Unclear | Yes | No | No |
| Forne 1995[27] | Spain | 1 | CBS | 7 | 480 | Diary cards | No | Yes | No |
| Gasbarrini 2000[28] | Italy | 1 | RBC | 7 | 800 | Validated questionnaire | No | No | No |
| Georgopoulos 1999[29] | Greece | 3 | RBC | 7 | 800 | Unclear | No | No | No |
| Gisbert 2000[30] | Spain | 1 | RBC | 7 | 800 | Unclear | Yes | No | No |
| Graham 1998[31] | USA | 111 | RBC | 28 | 800 | Unclear | No | No | Yes |
| Hung 2002[32] | Hong Kong | 3 | RBC | 7 | 800 | Diary | Yes | Yes | No |
| Lanza 1989[33] | USA | 1 | BSS | 21 | 2100 | Unclear | No | No | Yes |
| Lanza 1998[34] | USA | 47 | RBC | 28 | 800 | Diary cards | No | No | Yes |
| Liu 1999[35] | China | 1 | TDB | 7 | 480 | Diary | No | No | No |
| Mao 2000[36] | Vietnam | 1 | RBC | 10 | 400 | Diary | No | No | No |
| Marshall 1988[7] | Australia | 1 | CBS | 56 | 480 | Unclear | No | No | Yes |
| Masci 1995[37] | Italy | 1 | CBS | 28 to 56 | 480 | Unclear | Yes | No | Yes |
| Nafeeza 1992[38] | Malaysia | 1 | CBS | 28 | 480 | Unclear | No | No | Yes |
| Pare 1999[39] | Multi-national | Multi-centre | RBC | 28 | 800 | Unclear | Yes | No | Yes |
| Perri 2002[40] | Italy | 1 | RBC | 7 | 800 | Questionnaire | No | No | No |
| Peterson 1996[41] | USA | 38 | RBC | 28 | 800 | Unclear | No | No | Yes |
| Rokkas 1988[42] | UK | 1 | CBS | 56 | 480 | Unclear | No | No | Yes |
| Spadaccini 1998[43] | Italy | 1 | RBC | 7 | 800 | Face-to-face interview | No | No | No |
| Spiliadis 1998[44] | Greece | 3 | CBS | 14 | 1200 | Unclear | No | No | No |
| Spinzi 2000[45] | Italy | 6 | RBC | 7 | 800 | Face-to-face interview | No | No | No |
| Sung 1998[46] | Hong Kong | 1 | RBC | 7 | 800 | Telephone interview | No | No | No |
| Whitehead 2000[47] | UK | 1 | CBS and BSN | 28 | Unclear | Unclear | Yes | Yes | Yes |
| Wong 2001[48] | Hong Kong | 1 | RBC | 7 | 800 | Diary | Yes | Yes | No |
| Xiao 2001[21] | China | Multi-centre | CBS | 7 | 480 | Diary | No | No | No |
| Adverse event | Number of trials | Total number of patients | Number of patients in bismuth arms | Number of patients in comparison arms | Number of adverse events in bismuth arms (%) | Number of adverse events in comparison arms (%) | Relative risk of adverse events with bismuth versus comparison regimen (95% CI) |
| Any | 25 | 3180 | 1585 | 1595 | 431 (27.2) | 419 (26.3) | 1.01 (0.87-1.16) |
| Abdominal pain | 13 | 2439 | 1221 | 1218 | 63 (5.2) | 61 (5.0) | 1.06 (0.64-1.74) |
| Dark stools | 4 | 467 | 233 | 234 | 39 (16.7) | 5 (2.1) | 5.06 (1.59-16.12) |
| Diarrhoea | 22 | 3406 | 1761 | 1645 | 124 (7.0) | 113 (6.9) | 1.01 (0.72-1.42) |
| Dizziness | 8 | 1630 | 867 | 763 | 54 (6.2) | 49 (6.4) | 1.18 (0.81-1.72) |
| Headache | 14 | 2433 | 1276 | 1157 | 41 (3.2) | 28 (2.4) | 1.31 (0.81-2.11) |
| Metallic taste | 14 | 2475 | 1260 | 1215 | 124 (9.8) | 116 (9.6) | 1.02 (0.81-1.28) |
| Nausea and/or vomiting | 20 | 3417 | 1767 | 1650 | 111 (6.3) | 86 (5.2) | 1.16 (0.89-1.52) |
| Leading to withdrawal of therapy | 28 | 3951 | 2033 | 1918 | 33 (1.6) | 38 (2.0) | 0.86 (0.54-1.37) |
Bismuth salts have been used for centuries in medicine. From a gastroenterology perspective these drugs have been used to treat peptic ulcer disease, dyspepsia, parasitic infections, microscopic colitis, and infectious diarrhoea[1]. The discovery of Helicobacter pylori (H pylori) in 1983 by Warren and Marshall revolutionised the management of peptic ulcer disease[2], and led to a renewed interest in bismuth compounds, largely because bismuth was found to inhibit the growth of H pylori and was effective in eradicating the organism (when combined with antibiotics or in combination with antibiotics and acid suppression therapy[3,4]).
The first randomised controlled trial (RCT) of bismuth in H pylori-positive individuals suggested that bismuth was superior to erythromycin monotherapy in eradicating the infection[5]. A further RCT of 6 wk of colloidal bismuth subcitrate versus cimetidine, in H pylori-positive duodenal ulcer patients, demonstrated that bismuth successfully eradicated the bacterium in up to 50% of patients[6]. Subsequently, an RCT of both colloidal bismuth subcitrate and cimetidine, alone or in combination with tinidazole, confirmed that colloidal bismuth subcitrate and tinidazole cleared the infection in almost 75% of patients[7]. With the addition of a second antibiotic, tetracycline or amoxicillin, eradication rates in later RCTs exceeded 80%[8-10]. However, there were some problems associated with bismuth-based triple therapy, which included the number of tablets patients were required to take, the duration of therapy, and side effects such as altered taste, nausea, and diarrhoea.
There are a variety of bismuth salts currently available on the market. All are inorganic, poorly soluble and therefore less than 1% is typically absorbed systemically[11]. Blood concentrations of bismuth do rise when these compounds are ingested however, and there is therefore the potential for toxicity, though levels less than 50 μg/mL are unlikely to be associated with any meaningful toxicity in man[11]. In the 1970s, high doses of bismuth salts were used for long periods and were associated with neurotoxicity. In France, there were almost 1000 cases of bismuth-associated encephalopathy of which 72 were fatal[1]. The doses of bismuth used in H pylori eradication are administered for a much shorter duration, typically 1 to 2 wk. In a recent bioavailability study, where bismuth salts were given in combination with omeprazole for 6 d[12], plasma levels of bismuth remained well below 50 μg/mL, but a review of their safety profile would provide additional evidence that such low doses of bismuth, given for a short period of time, do not expose patients to undue risks. We have therefore conducted a systematic review and meta-analysis of available published literature to assess the magnitude of the risk of adverse events experienced when bismuth salts are used, either alone or in combination with one or more antibiotics, to eradicate H pylori.
Primary outcomes: The primary aim of this systematic review and meta-analysis was to assess the total number of adverse events occurring following treatment for H pylori with bismuth compounds, either alone, or in combination with antibiotics and/or acid suppression therapy, compared to treatment with antibiotics alone, acid suppression therapy alone, a combination of the two, or no treatment/placebo.
Secondary outcomes: The secondary aims were to evaluate the number of specific individual adverse events occurring and the number of withdrawals of therapy due to adverse events, and to assess the effect of long-term (defined as 1 mo or more) therapy on number of adverse events (both total number and by specific category) and withdrawals due to adverse events.
Types of studies: In order to best estimate adverse events that were directly attributable to the use of bismuth, studies were only eligible for inclusion in this systematic review if they were RCTs that compared bismuth monotherapy with either acid suppression therapy alone, placebo, or no treatment, or compared bismuth compounds in combination with either antibiotics, or antibiotics and acid suppression therapy as part of a recognised efficacious eradication regimen with an identical dose and duration of antibiotics either alone or in combination with acid suppression therapy. We defined an efficacious bismuth-containing eradication regimen as any one of: bismuth triple therapy (bismuth in combination with two antibiotics); bismuth quadruple therapy (as for triple therapy, but with the addition of acid suppression therapy); or ranitidine bismuth citrate dual (with one antibiotic) or triple (with two antibiotics) therapy.
Types of participants: Patients were required to be H pylori-positive adults (over the age of 16 years) taking any bismuth compound for more than 1 d with a comparison group of H pylori-positive patients who were not taking bismuth.
Types of assessment: Bismuth toxicity had to be assessed and recorded using one or more of the following methods: medical databases; face-to-face interviews; telephone interviews; symptom diaries; or questionnaire in order for studies to be eligible for inclusion. The questionnaire used was not required to be previously validated but, if there were sufficient studies using questionnaires, we aimed to assess the impact of this in a sensitivity analysis.
Types of outcome measures: The proportion of patients that reported any adverse event and the proportion experiencing specific individual adverse events were assessed wherever trial reporting allowed this.
Search strategy: Two authors performed searches of the medical literature to identify articles from MEDLINE (from 1966 up to October 2007), EMBASE (from 1988 up to October 2007), and the Cochrane Library and Current Contents electronic databases. RCTs using bismuth salts were identified using the medical subject heading term “bismuth”. These studies were combined using the set operator and with papers that used a variety of free text terms including “Denol”, “Pepto-Bismol”, “bismuth”, “subsalicylate”, “tripotassium dicitrato bismuthate”, “subnitrate”, “subgallate”, “ranitidine bismuth citrate”, “pylorid”, “quadruple therapy”, “pylera”, and “bismuth subcitrate potassium”. There were no language restrictions, and papers published in abstract form only were also eligible for inclusion in the review. The abstracts of all papers identified by the initial search were evaluated for appropriateness to the study question, and all potentially relevant studies were retrieved and examined in greater detail to determine whether or not they met all eligibility criteria. The bibliographies of identified studies were then used to perform a recursive search of the literature to identify other potentially eligible studies. In addition, Digestive Disease Week, United European Gastroenterology Week, and European H pylori Study Group conference abstract books between 2000 and 2007 were hand-searched.
Selection of studies: Two reviewers screened all titles and abstracts of trials that were identified by the search strategy as being potentially eligible for inclusion in the systematic review to confirm or refute eligibility. This was performed using pre-designed eligibility forms. A third reviewer adjudicated where any disagreements arose, and a consensus view was taken.
Assessment of study quality: The quality of studies was assessed according to the following pre-defined criteria: method of assessment of occurrence of adverse events (interview, diary, and questionnaire), generation of randomisation schedule, method of allocation of concealment, and blinding of assessor as to patient allocation to therapy.
Data concerning total number of adverse events and number of specific individual adverse events were extracted on to specially developed forms by two reviewers and all data extraction was checked by a third reviewer. These verified data were then entered onto a Microsoft Excel spreadsheet (XP professional edition; Microsoft Corp, Redmond, WA, USA), and again this was double-checked by a third reviewer. Trial characteristics including setting (population-based, primary care, secondary care), country of origin, number of centres involved, duration of bismuth therapy and dosage schedule, type of bismuth compound, mean age of included patients, and proportion of male patients were recorded to allow exploration of potential reasons for any heterogeneity detected between trial results.
Data were extracted as dichotomous outcomes and pooled using a random effects model[13], where sufficient data were available. The impact of bismuth therapy on the incidence of total and specific individual adverse effects versus comparison regimen was expressed as a combined relative risk (RR) with a 95% confidence interval (CI). The number needed to harm with bismuth therapy to cause one adverse event, and a 95% CI, were calculated as the reciprocal of the risk difference from the meta-analysis, and where this was statistically significant the results were reported.
Due to differences in methodology, patient populations, and outcome measures between eligible trials, the results of individual studies can be very diverse and therefore when they are included in the same meta-analysis this may affect the accuracy of the overall result. This inconsistency within a single meta-analysis can be quantified with a statistical test of heterogeneity, to assess whether the variation across trials is due to true heterogeneity, or chance. This quantity is termed I2, and its value ranges from 0 to 100 percent, with 0 percent representing no observed heterogeneity, and larger values indicating increasing heterogeneity. A value below 25 percent is arbitrarily chosen to represent low levels of heterogeneity[14]. Where the degree of statistical heterogeneity is greater than this, clinical reasons within individual trials that may account for some of this inconsistency can be explored. Wherever statistically significant heterogeneity existed between trial results in this systematic review, possible explanations were investigated informally using sensitivity analyses. These are exploratory only, and may explain some of the observed variability, but the results should be interpreted with caution.
All statistical analyses were performed using Stats Direct version 2.2.4 (Stats Direct Ltd, Sale, Cheshire, UK), which was used to generate Forest plots of pooled relative risks for total adverse event rates and specific individual adverse event rates by category, as well as funnel plots to assess for evidence of publication bias.
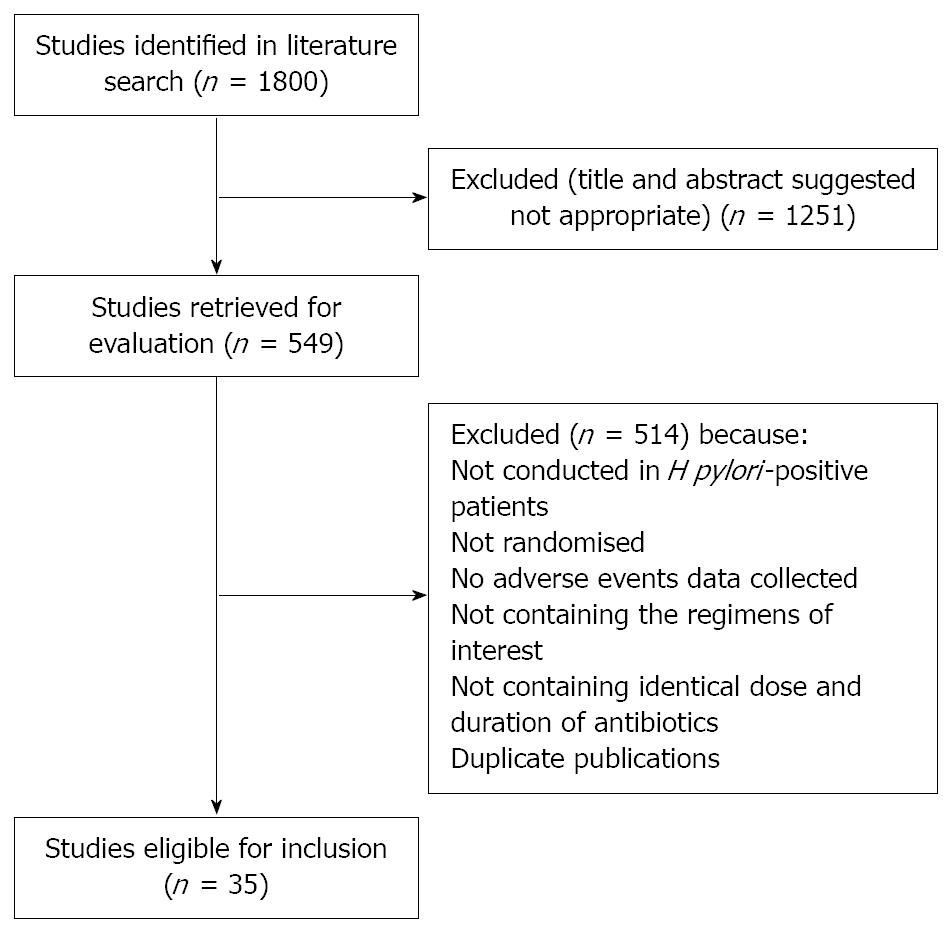
The search strategy identified 1800 studies, of which 549 were possibly eligible. After reviewing the abstracts of these it became clear that 209 were RCTs of bismuth, and these were retrieved for further assessment. Of these, 35 were eligible for inclusion in the meta-analysis[7,15-48], reporting on 4763 H pylori-positive patients, 2435 of whom received bismuth or bismuth-based regimen, and 2328 received a comparison regimen (Figure 1). Thirty-three of the trials were found in fully published form, and two were only published as abstracts[29,44]. Seven of the RCTs used more than one bismuth-containing regimen[7,20,31,34,35,41,47].
Detailed trial characteristics are provided in Table 1. Nineteen of the trials were conducted in Europe[15-18,20,23-25,27-30,37,40,42-45,47], eight in the Far East[21,22,32,35,36,38,46,48], four in the USA[31,33,34,41], one in the Middle East[26], one in South America[19], one in Australia[7], and one was a multi-national study[39]. Eleven of the studies were multi-centre RCTs[15,21,25,29,31,32,34,39,41,44,45]. Duration of bismuth therapy ranged from 7 to 56 d, with a total daily dose of between 400 mg and 2100 mg. Nineteen studies used ranitidine bismuth citrate[15,17,20,22,24,28-32,34,36,39-41,43,45,46,48], ten studies colloidal bismuth subcitrate[7,16,18,21,26,27,37,38,42,44], two studies tripotassium dictrato bismuthate[23,35], two studies bismuth subsalicylate[25,33], one study bismuth subnitrate[19], and one study both bismuth subnitrate and colloidal bismuth subcitrate[47]. Comparison regimens were proton pump inhibitor or H2-receptor antagonist (H2-RA)-based eradication therapy in 23 studies[15,17-24,26-30,32,35,36,39,40,43,45,46,48], antibiotics alone in four studies[16,38,44,47], antibiotics or placebo in three studies[31,34,41], H2-RA alone in two studies[25,37], placebo alone in two studies[33,42], and H2-RA in combination with either one antibiotic or placebo in one study[7]. The mean age of individuals in included studies ranged from 36.7 years to 50.5 years, and the proportion of male patients varied between 32 percent and 78 percent. The number of participants in each RCT ranged from 20 to 530 individuals.
Thirteen of the trials were double-blind randomised studies[7,15,20,22,31,33,34,37-39,41,42,47], the remainder being either single-blind or open. Five of the single-blind trials specifically stated that assessors were blinded to treatment allocation[21,24,45,46,48]. Ten of the studies reported the method of generation of the randomization schedule[15,18,20,26,30,32,37,39,47,48], but only four the method of concealment of allocation[27,32,47,48]. Four of the studies recorded adverse events using a questionnaire[20,24,28,40], but only two of these stated that the questionnaire was validated[24,28]. Seven studies collected information concerning adverse events using a diary or diary cards[21,27,32,34-36,48], two via face-to-face interview[43,45], and one via telephone interview[46]. The remainder of trials did not state how they collected adverse events data.
There were no serious adverse events such as death or neurotoxicity in either arm of any of the included RCTs. Twenty-five trials reported the total number of individuals experiencing any adverse event with bismuth or bismuth-containing regimens versus comparison regimen[15-20,23-27,30,32,33,35-40,42,44,45,47,48]. Three of these studies utilized more than one regimen[20,35,47], allowing 28 comparisons to be made. The relative risk of an adverse event with bismuth or bismuth-containing regimens versus comparison regimen was 1.01 (95% CI: 0.87 to 1.16) (Figure 2 and Table 2). There was statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 30.3%). The Egger test did not suggest any trend for funnel plot asymmetry (P = 0.16). Sensitivity analysis according to trial setting, country of origin, dose of bismuth salt used, type of bismuth salt used, mean age of patients included in the study, and proportion of males included in the study failed to reveal any obvious explanation for the observed heterogeneity.
Abdominal pain: Thirteen trials reported the total number of individuals experiencing abdominal pain with bismuth or bismuth-containing regimens versus comparison regimen[17,18,20,21,24,26,28,30,34,39,40,46,47]. Three of these studies utilized more than one regimen[20,34,47], allowing 16 comparisons to be made. The relative risk of abdominal pain with bismuth or bismuth-containing regimens versus comparison regimen was 1.06 (95% CI: 0.64 to 1.74) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 22.0%), and the Egger test did not suggest any trend for funnel plot asymmetry (P = 0.15).
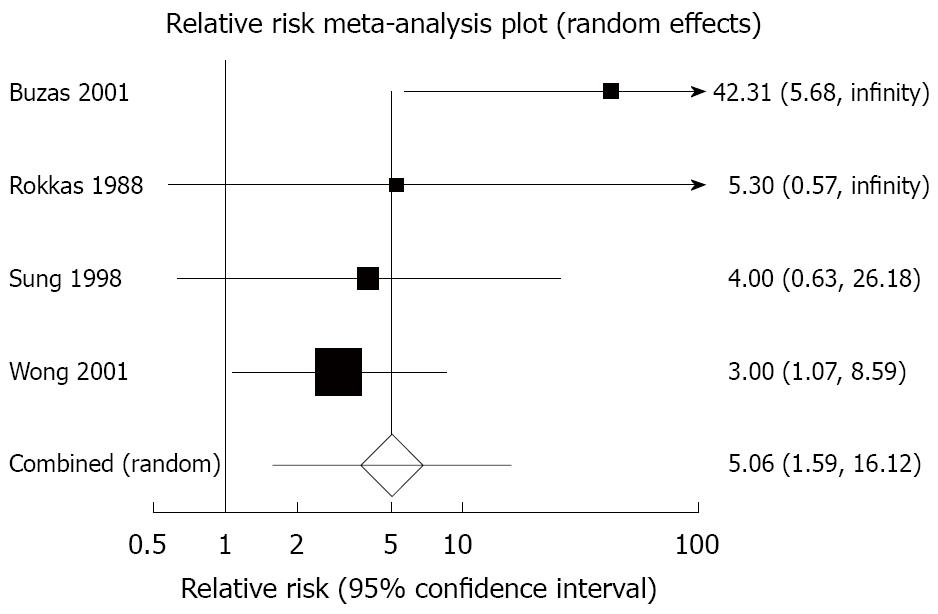
Dark stools: Four trials reported the total number of individuals experiencing dark stools with bismuth or bismuth-containing regimens versus comparison regimen[17,42,46,48]. The relative risk of dark stools with bismuth or bismuth-containing regimens versus comparison regimen was 5.06 (95% CI: 1.59 to 16.12) (Figure 3 and Table 2). There was marginal statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 25.2%), but no obvious causes were found, and the Egger test did not suggest any trend for funnel plot asymmetry (P = 0.28). The number of patients needed to harm with bismuth or bismuth-containing regimen versus comparison regimen to cause one case of dark stools was 7.5 (95% CI: 4 to 71).
Diarrhoea: Twenty-two trials reported the total number of individuals experiencing diarrhoea with bismuth or bismuth-containing regimens versus comparison regimen[7,17,18,20,24-28,30-32,34,36,39-42,45-48]. Six of these studies utilized more than one regimen[7,20,31,34,41,47], allowing 28 comparisons to be made. The relative risk of diarrhoea with bismuth or bismuth-containing regimens versus comparison regimen was 1.01 (95% CI: 0.72 to 1.42) (Table 2). There was marginal statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 26.2%), but no obvious causes were found, and the Egger test did not suggest any trend for funnel plot asymmetry (P = 0.75).
Dizziness: Eight trials reported the total number of individuals experiencing dizziness with bismuth or bismuth-containing regimens versus comparison regimen[21,26,31,32,35,41,46,48]. Three of these studies utilized more than one regimen[31,35,41], allowing 11 comparisons to be made. The relative risk of dizziness with bismuth or bismuth-containing regimens versus comparison regimen was 1.18 (95% CI: 0.81 to 1.72) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%), and the Egger test did not suggest any trend for funnel plot asymmetry (P = 0.20).
Headache: Fourteen trials reported the total number of individuals experiencing headache with bismuth or bismuth-containing regimens versus comparison regimen[17,18,20,24-26,30,31,34,39-41,46,47]. Five of these studies utilized more than one regimen[20,31,34,41,47], allowing 19 comparisons to be made. The relative risk of headache with bismuth or bismuth-containing regimens versus comparison regimen was 1.31 (95% CI: 0.81 to 2.11) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%), and the Egger test did not suggest any trend for funnel plot asymmetry (P = 0.83).
Metallic taste: Fourteen trials reported the total number of individuals experiencing metallic taste with bismuth or bismuth-containing regimens versus comparison regimen[17,20,24,27,30,34,35,39-42,45,46,48]. Four of these studies utilized more than one regimen[20,34,35,41], allowing 18 comparisons to be made. The relative risk of metallic taste with bismuth or bismuth-containing regimens versus comparison regimen was 1.02 (95% CI: 0.81 to 1.28) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%), though the Egger test suggested there was funnel plot asymmetry (P = 0.01).
Nausea and/or vomiting: Twenty trials reported the total number of individuals experiencing nausea and/or vomiting with bismuth or bismuth-containing regimens versus comparison regimen[17,18,20,21,24-28,30-32,34,35,39-42,46,47]. Six of these studies utilized more than one regimen[20,31,34,35,41,47], allowing 26 comparisons to be made. The relative risk of nausea and/or vomiting with bismuth or bismuth-containing regimens versus comparison regimen was 1.16 (95% CI: 0.89 to 1.52) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%), and the Egger test did not suggest that there was any evidence of funnel plot asymmetry (P = 0.85).
Twenty-eight trials reported the total number of individuals who terminated therapy due to experiencing adverse events with bismuth or bismuth-containing regimens versus comparison regimen[16-18,20,22-32,34-37,39-43,45-48]. Six of these studies utilized more than one regimen[20,31,34,35,41,47], allowing 34 comparisons to be made. The relative risk of withdrawal of therapy due to adverse events with bismuth or bismuth-containing regimens versus comparison regimen was 0.86 (95% CI: 0.54 to 1.37) (Table 2). There was no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%), though the Egger test suggested that there was evidence of funnel plot asymmetry (P = 0.05).
The duration of bismuth therapy was one month or more in eleven of the included studies[7,18,25,31,34,37-39,41,42,47]. There were sufficient trials to pool data to examine the effect of duration of therapy on total number of adverse events, some of the specific individual adverse events (including diarrhoea, headache, and nausea and/or vomiting), and withdrawal of therapy due to adverse events.
Total number of adverse events: Seven trials provided data on total number of adverse events in 945 individuals (467 of whom were assigned to bismuth)[18,25,37-39,42,47], and one study utilized more than one regimen allowing eight comparisons to be made[47]. The relative risk of an adverse event with bismuth or bismuth-containing regimens used for one month or more versus comparison regimen was 1.20 (95% CI:1.00 to 1.44), with no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%).
Diarrhoea: Nine studies provided data on the incidence of diarrhoea with one month or more of bismuth in 1601 patients (859 of whom were assigned to bismuth)[7,18,25,31,34,39,41,42,47], with five of the studies utilizing more than one regimen allowing fourteen comparisons to be made[7,31,34,41,47]. The relative risk of diarrhoea with bismuth or bismuth-containing regimens used for one month or more versus comparison regimen was 1.72 (95% CI: 1.14 to 2.60), with no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%).
Headache: Seven studies provided data on the incidence of headache with one month or more of bismuth in 1435 patients (778 of whom were allocated to bismuth)[18,25,31,34,39,41,47], with four of the studies utilizing more than one regimen allowing eleven comparisons to be made[31,34,41,47]. The relative risk of headache with bismuth or bismuth-containing regimens used for one month or more versus comparison regimen was 1.39 (95% CI: 0.76 to 2.53), with no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%).
Nausea and/or vomiting: Eight studies provided data on the incidence of nausea and/or vomiting with one month or more of bismuth in 1501 patients (810 of whom were allocated to bismuth)[18,25,31,34,39,41,42,47], with four of the studies utilizing more than one regimen allowing twelve comparisons to be made[31,34,41,47]. The relative risk of nausea and/or vomiting with bismuth or bismuth-containing regimens used for one month or more versus comparison regimen was 1.47 (95% CI: 0.87 to 2.48), with no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%).
Withdrawal of therapy due to adverse events: Nine studies provided data on the incidence of withdrawal of therapy due to adverse events with one month or more of bismuth in 1554 patients (837 of whom were allocated to bismuth)[18,25,31,34,37,39,41,42,47], with four of the studies utilizing more than one regimen allowing thirteen comparisons to be made[31,34,41,47]. The relative risk of withdrawal of therapy due to adverse events with bismuth or bismuth-containing regimens used for one month or more versus comparison regimen was 0.86 (95% CI: 0.47 to 1.57), with no statistically significant heterogeneity detected between trial results (heterogeneity test: I2= 0%).
This is, to our knowledge, the first systematic review and meta-analysis to examine the safety profile of bismuth compounds used either alone or in combination with antibiotics for the treatment of H pylori infection, or H pylori-related diseases. This information is very important because there have been previous concerns surrounding the issue of potential for toxicity with use of the drug in some countries, particularly in France, where severe neurological adverse events related to the prolonged use of bismuth, given in large quantities, led to the complete withdrawal of all bismuth compounds. This is in contrast to much of the rest of the world, particularly North America, where these drugs are still available without prescription over the counter.
Serfontein et al[11], when reviewing blood bismuth levels in patients following administration of therapeutic bismuth formulations, concluded that levels less than 50 μg/mL were highly unlikely to be associated with any meaningful toxicity in man. The authors also reported site-specific toxicity issues depending on whether the complexes of bismuth were water or lipid soluble, the former being associated with renal toxicity, the latter with neurotoxicity. In both cases the doses used and the duration of treatment leading to such adverse effects were much greater than the ones used in the context of H pylori eradication therapy. When bismuth-based compounds are used in the treatment of H pylori they are usually only given for 1 to 2 wk at low doses, so it would be expected that in this situation the incidence of severe adverse events such as death or neurotoxicity would be lower. These data, with no reported deaths or neurotoxicity in any of the included RCTs, would support this hypothesis. Less serious adverse events are still important though, particularly from the patient’s perspective. These may affect compliance with therapy, which is important as successful eradication of H pylori is likely to lead to successful ulcer healing and prevention of ulcer relapse[49], and may also improve symptoms in a small but significant proportion of those with functional dyspepsia[50,51]. In a previous analysis of factors that determine the likely success of H pylori eradication with bismuth-based triple therapies, patient compliance was shown to be the most important predictor of response[52].
No statistically significant difference was detected in the total number of side-effects between those receiving bismuth-based therapy and comparison regimen in this meta-analysis. In addition, there was no statistically significant difference detected in individual adverse events such as abdominal pain, diarrhoea, dizziness, headache, metallic taste, and nausea and/or vomiting with bismuth compounds versus comparison regimen. Finally, there was no statistically significant increase detected in the number of individuals requiring cessation of therapy as a direct result of adverse events with bismuth-based therapy versus comparison regimen. The number of individuals reporting dark stools with bismuth was significantly higher, though there were fewer studies reporting this adverse event, which probably explains the wider confidence interval. This is unlikely to have any serious consequences related to patient safety, but it is still important to warn patients that this is an expected side-effect of therapy. This observation also has implications for the successful blinding of patients allocated to bismuth therapy in double-blind RCTs.
Total number of side-effects did appear to increase slightly when only those trials that used one month or more of bismuth therapy were included in the meta-analysis, though this did not achieve statistical significance. Diarrhoea was significantly more common with bismuth compounds when only studies using more than one month of therapy were included, but no statistically significant difference was detected in the incidence of other adverse events reported, where there were sufficient trials to examine this issue. Again, there was no increase in cessation of therapy in individuals assigned to bismuth-based therapy, even if treatment was for one month or more. As mentioned earlier, most current bismuth-based H pylori eradication regimens are given for 1 or 2 wk only, so these observations related to longer duration of bismuth therapy are unlikely to have any significant implications in the majority of patients.
The strengths of this systematic review and meta-analysis are that it has been conducted using rigorous methodology and contains a large number of RCTs, which have provided data from in excess of 2000 patients for most of the analyses. In addition, the fact that the data of interest to this meta-analysis were not the primary endpoint of any of the included trials means that the results of the current study are likely to be free from publication bias, as evidenced by the funnel plots for many of the outcomes we assessed. Disadvantages, as with any systematic review, arise from the methodology of the trials included. Many studies were not double-blind and few reported that assessors were blinded to the treatment allocation of the patients either, and this may have led to under-reporting of adverse events in those assigned to “active treatment” with bismuth therapy. Most studies also failed to report either the method of generation of the randomization schedule or the method of concealment of allocation. Finally, only four of the studies used a questionnaire to collect adverse event data, and only two stated that this questionnaire was validated. This may mean that adverse event data were inaccurate in many of the trials and we cannot exclude the possibility that this may have biased the results of the current meta-analysis towards the null hypothesis.
In summary, this systematic review and meta-analysis provides strong evidence that bismuth compounds used either alone, or in combination with antibiotics and acid-suppression therapy, for the treatment of H pylori are safe and well-tolerated. The only observation of note was that dark stools were significantly more common in those assigned to bismuth-based therapies.
Bismuth compounds are often used as part of eradication therapy for Helicobacter pylori (H pylori). There are concerns about toxicity of these compounds in some countries, particularly as a result of their potential neurological sequelae.
Data concerning toxic effects of bismuth compounds are mainly derived from studies that have used these compounds at a high dose for a prolonged period of time. We conducted a meta-analysis of adverse events resulting from a 1 to 2-wk course of bismuth based H pylori eradication therapy.
The current study demonstrated that bismuth compounds, when used short-term for 1 to 2 wk in H pylori eradication therapy, are safe. The only adverse event that occurred more frequently in patients receiving bismuth was dark stools.
Potential adverse events from bismuth compounds in a 1 to 2-wk course of H pylori eradication therapy are now quantified. Gastroenterologists can be assured that these compounds are safe to use.
The number needed to harm is the number of patients that would need to be treated with bismuth compounds for one patient to experience an adverse event.
There are now problems in obtaining satisfactory eradication rates of H pylori with PPI-based triple therapies, so the use of bismuth containing regimens has been recommended as a potential first line therapy in the Maastricht guidelines. Furthermore, there are now new bismuth combinations commercially available. For these reasons it is important to be sure of the safety of bismuth compounds.
Peer reviewer: Francis Mégraud, Professor, Department of Bacteriology, Universite Victor Segalen Bordeaux 2, 146 rue Leo Saignat, Bordeaux 33076, France
S- Editor Li LF L- Editor Stewart GJ E- Editor Ma WH
| 1. | Tillman LA, Drake FM, Dixon JS, Wood JR. Review article: safety of bismuth in the treatment of gastrointestinal diseases. Aliment Pharmacol Ther. 1996;10:459-467. [Cited in This Article: ] |
| 2. | Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321:1273-1275. [Cited in This Article: ] |
| 3. | Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol. 2007;21:315-324. [Cited in This Article: ] |
| 4. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [Cited in This Article: ] |
| 5. | McNulty CA, Gearty JC, Crump B, Davis M, Donovan IA, Melikian V, Lister DM, Wise R. Campylobacter pyloridis and associated gastritis: investigator blind, placebo controlled trial of bismuth salicylate and erythromycin ethylsuccinate. Br Med J (Clin Res Ed). 1986;293:645-649. [Cited in This Article: ] |
| 6. | Coghlan JG, Gilligan D, Humphries H, McKenna D, Dooley C, Sweeney E, Keane C, O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987;2:1109-1111. [Cited in This Article: ] |
| 7. | Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow ED, Blackbourn SJ, Phillips M, Waters TE, Sanderson CR. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;2:1437-1442. [Cited in This Article: ] |
| 8. | Graham DY, Lew GM, Evans DG, Evans DJ Jr, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann Intern Med. 1991;115:266-269. [Cited in This Article: ] |
| 9. | Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ Jr, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705-708. [Cited in This Article: ] |
| 10. | Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233-1235. [Cited in This Article: ] |
| 11. | Serfontein WJ, Mekel R. Bismuth toxicity in man II. Review of bismuth blood and urine levels in patients after administration of therapeutic bismuth formulations in relation to the problem of bismuth toxicity in man. Res Commun Chem Pathol Pharmacol. 1979;26:391-411. [Cited in This Article: ] |
| 12. | Spenard J, Aumais C, Massicotte J, Tremblay C, Lefebvre M. Influence of omeprazole on bioavailability of bismuth following administration of a triple capsule of bismuth biskalcitrate, metronidazole, and tetracycline. J Clin Pharmacol. 2004;44:640-645. [Cited in This Article: ] |
| 13. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [Cited in This Article: ] |
| 14. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [Cited in This Article: ] |
| 15. | Bujanda L, Herrerias JM, Ripolles V, Pena D, Chaves da Cruz ATC, Fueyo A. Efficacy and tolerability of three regimens for Helicobacter pylori eradication: A multicentre, double-blind, randomised clinical trial. Clin Drug Investig. 2001;21:1-7. [Cited in This Article: ] |
| 16. | Burette A, Glupczynski Y, De Prez C. Evaluation of various multi-drug eradication regimens for Helicobacter pylori. Eur J Gastroenterol Hepatol. 1992;4:817-823. [Cited in This Article: ] |
| 17. | Buzas GM, Illyes G, Szekely E, Szeles I. Six regimens for the eradication of Helicobacter pylori (Hp) in duodenal ulcer patients: three consecutive trials (1995-1999). J Physiol Paris. 2001;95:437-441. [Cited in This Article: ] |
| 18. | Carpintero P, Blanco M, Pajares JM. Ranitidine versus colloidal bismuth subcitrate in combination with amoxicillin and metronidazole for eradicating Helicobacter pylori in patients with duodenal ulcer. Clin Infect Dis. 1997;25:1032-1037. [Cited in This Article: ] |
| 19. | Carvalho AF, Fiorelli LA, Jorge VN, Da Silva CM, De Nucci G, Ferraz JG, Pedrazzoli J. Addition of bismuth subnitrate to omeprazole plus amoxycillin improves eradication of Helicobacter pylori. Aliment Pharmacol Ther. 1998;12:557-561. [Cited in This Article: ] |
| 20. | Catalano F, Branciforte G, Catanzaro R, Cipolla R, Bentivegna C, Brogna A. Helicobacter pylori-positive duodenal ulcer: three-day antibiotic eradication regimen. Aliment Pharmacol Ther. 2000;14:1329-1334. [Cited in This Article: ] |
| 21. | Xiao SD, Liu WZ, Hu PJ, Ouyang Q, Wang JL, Zhou LY, Cheng NN. A multicentre study on eradication of Helicobacter pylori using four 1-week triple therapies in China. Aliment Pharmacol Ther. 2001;15:81-86. [Cited in This Article: ] |
| 22. | Chuang CH, Sheu BS, Yang HB, Wu JJ, Lin XZ. Ranitidine bismuth citrate or omeprazole-based triple therapy for Helicobacter pylori eradication in Helicobacter pylori-infected non-ulcer dyspepsia. Dig Liver Dis. 2001;33:125-130. [Cited in This Article: ] |
| 23. | Dal Bo’ N, Di Mario F, Battaglia G, Buda A, Leandro G, Vianello F, Kusstatscher S, Salandin S, Pilotto A, Cassaro M. Low dose of clarithromycin in triple therapy for the eradication of Helicobacter pylori: one or two weeks? J Gastroenterol Hepatol. 1998;13:288-293. [Cited in This Article: ] |
| 24. | Danese S, Armuzzi A, Romano A, Cremonini F, Candelli M, Franceschi F, Ojetti V, Venuti A, Pola P, Gasbarrini G. Efficacy and tolerability of antibiotics in patients undergoing H. pylori eradication. Hepatogastroenterology. 2001;48:465-467. [Cited in This Article: ] |
| 25. | Eberhardt R, Kasper G. Effect of oral bismuth subsalicylate on Campylobacter pylori and on healing and relapse rate of peptic ulcer. Rev Infect Dis. 1990;12 Suppl 1:S115-S119. [Cited in This Article: ] |
| 26. | Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2004;19:89-93. [Cited in This Article: ] |
| 27. | Forne M, Viver JM, Espinos JC, Coll I, Tresserra F, Garau J. Impact of colloidal bismuth subnitrate in the eradication rates of Helicobacter pylori infection-associated duodenal ulcer using a short treatment regimen with omeprazole and clarithromycin: a randomized study. Am J Gastroenterol. 1995;90:718-721. [Cited in This Article: ] |
| 28. | Gasbarrini A, Ojetti V, Pitocco D, Armuzzi A, Silveri NG, Pola P, Ghirlanda G, Gasbarrini G. Efficacy of different Helicobacter pylori eradication regimens in patients affected by insulin-dependent diabetes mellitus. Scand J Gastroenterol. 2000;35:260-263. [Cited in This Article: ] |
| 29. | Georgopolous S, Karatapanis S, Ladas S, Papamrkos D, Vretou N, Artikis V, Mentis A, Raptis S. Lansoprazole vs ranitidine bismuth citrate based short-term triple therapies for Helicobacter pylori (H. pylori) eradication: A randomised study with 6-month follow-up. Gut. 1999;44:A120-A121. [Cited in This Article: ] |
| 30. | Gisbert JP, Carpio D, Marcos S, Gisbert JL, Garcia Gravalos R, Pajares JM. One-week therapy with pantoprazole versus ranitidine bismuth citrate plus two antibiotics for Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2000;12:489-495. [Cited in This Article: ] |
| 31. | Graham DY, Breiter JR, Ciociola AA, Sykes DL, McSorley DJ. An alternative non-macrolide, non-imidazole treatment regimen for curing Helicobacter pylori and duodenal ulcers: ranitidine bismuth citrate plus amoxicillin. The RBC H. pylori Study Group. Helicobacter. 1998;3:125-131. [Cited in This Article: ] |
| 32. | Hung WK, Wong WM, Wong GS, Yip AW, Szeto ML, Lai KC, Hu WH, Chan CK, Xia HH, Yuen MF. One-week ranitidine bismuth citrate, amoxicillin and metronidazole triple therapy for the treatment of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther. 2002;16:2067-2072. [Cited in This Article: ] |
| 33. | Lanza FL, Skoglund ML, Rack MF, Yardley JH. The effect of bismuth subsalicylate on the histologic gastritis seen with Campylobacter pylori: a placebo-controlled, randomized study. Am J Gastroenterol. 1989;84:1060-1064. [Cited in This Article: ] |
| 34. | Lanza FL, Sontag SJ, Ciociola AA, Sykes DL, Heath A, McSorley DJ. Ranitidine bismuth citrate plus clarithromycin: a dual therapy regimen for patients with duodenal ulcer. Helicobacter. 1998;3:212-221. [Cited in This Article: ] |
| 35. | Liu WZ, Xiao SD, Shi Y, Wu SM, Zhang DZ, Xu WW, Tytgat GN. Furazolidone-containing short-term triple therapies are effective in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1999;13:317-322. [Cited in This Article: ] |
| 36. | Mao HV, Lak BV, Long T, Chung NQ, Thang DM, Hop TV, Chien NN, Hoan PQ, Henley KS, Perez-Perez GI. Omeprazole or ranitidine bismuth citrate triple therapy to treat Helicobacter pylori infection: a randomized, controlled trial in Vietnamese patients with duodenal ulcer. Aliment Pharmacol Ther. 2000;14:97-101. [Cited in This Article: ] |
| 37. | Masci E, Colombo E, Testoni PA, Fanti L, Guslandi M, Tittobello A. Colloid bismuth versus famotidine in the treatment and prevention of duodenal ulcer relapse: results of a double-blind, double dummy randomized study. Fundam Clin Pharmacol. 1995;9:280-283. [Cited in This Article: ] |
| 38. | Nafeeza MI, Shahimi MM, Kudva MV, Ahmad H, Isa MR, Sood IM, Mazlam MZ, Jamal F, Suboh Y. Evaluation of therapies in the treatment of Helicobacter pylori associated non-ulcer dyspepsia. Singapore Med J. 1992;33:570-574. [Cited in This Article: ] |
| 39. | Pare P, Farley A, Romaozinho JM, Bardhan KD, French PC, Roberts PM. Comparison of ranitidine bismuth citrate plus clarithromycin with omeprazole plus clarithromycin for the eradication of Helicobacter pylori. Aliment Pharmacol Ther. 1999;13:1071-1078. [Cited in This Article: ] |
| 40. | Perri F, Festa V, Merla A, Quitadamo M, Clemente R, Andriulli A. Amoxicillin/tetracycline combinations are inadequate as alternative therapies for Helicobacter pylori infection. Helicobacter. 2002;7:99-104. [Cited in This Article: ] |
| 41. | Peterson WL, Ciociola AA, Sykes DL, McSorley DJ, Webb DD. Ranitidine bismuth citrate plus clarithromycin is effective for healing duodenal ulcers, eradicating H. pylori and reducing ulcer recurrence. RBC H. pylori Study Group. Aliment Pharmacol Ther. 1996;10:251-261. [Cited in This Article: ] |
| 42. | Rokkas T, Pursey C, Uzoechina E, Dorrington L, Simmons NA, Filipe MI, Sladen GE. Non-ulcer dyspepsia and short term De-Nol therapy: a placebo controlled trial with particular reference to the role of Campylobacter pylori. Gut. 1988;29:1386-1391. [Cited in This Article: ] |
| 43. | Spadaccini A, De Fanis C, Sciampa G, Russo L, Silla M, Pantaleone U, Di Virgilio M, Pizzicanella G. Triple regimens using lansoprazole or ranitidine bismuth citrate for Helicobacter pylori eradication. Aliment Pharmacol Ther. 1998;12:997-1001. [Cited in This Article: ] |
| 44. | Spiliadis C, Georgopolous S, Stambolos P, Mentis A, Gianikaki L, Manika Z, Skandalis N. Evaluation of the efficacy of clarithromycin in the eradication of Helicobacter pylori. Gut. 1998;43:A85. [Cited in This Article: ] |
| 45. | Spinzi GC, Boni F, Bortoli A, Colombo E, Ballardini G, Venturelli R, Minoli G. Seven-day triple therapy with ranitidine bismuth citrate or omeprazole and two antibiotics for eradication of Helicobacter pylori in duodenal ulcer: a multicentre, randomized, single-blind study. Aliment Pharmacol Ther. 2000;14:325-330. [Cited in This Article: ] |
| 46. | Sung JJ, Leung WK, Ling TK, Yung MY, Chan FK, Lee YT, Cheng AF, Chung SC. One-week use of ranitidine bismuth citrate, amoxycillin and clarithromycin for the treatment of Helicobacter pylori-related duodenal ulcer. Aliment Pharmacol Ther. 1998;12:725-730. [Cited in This Article: ] |
| 47. | Whitehead MW, Phillips RH, Sieniawska CE, Delves HT, Seed PT, Thompson RP, Powell JJ. Double-blind comparison of absorbable colloidal bismuth subcitrate and nonabsorbable bismuth subnitrate in the eradication of Helicobacter pylori and the relief of nonulcer dyspepsia. Helicobacter. 2000;5:169-175. [Cited in This Article: ] |
| 48. | Wong BC, Wong WM, Wang WH, Fung FM, Lai KC, Chu KM, Yuen ST, Leung SY, Hu WH, Yuen MF. One-week ranitidine bismuth citrate-based triple therapy for the eradication of Helicobacter pylori in Hong Kong with high prevalence of metronidazole resistance. Aliment Pharmacol Ther. 2001;15:403-409. [Cited in This Article: ] |
| 49. | Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833-1855. [Cited in This Article: ] |
| 50. | Moayyedi P, Soo S, Deeks J, Forman D, Mason J, Innes M, Delaney B. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. Dyspepsia Review Group. BMJ. 2000;321:659-664. [Cited in This Article: ] |
| 51. | Moayyedi P, Deeks J, Talley NJ, Delaney B, Forman D. An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepancy between systematic reviews. Am J Gastroenterol. 2003;98:2621-2626. [Cited in This Article: ] |
| 52. | Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ Jr, Klein PD, Alpert LC, Genta RM. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102:493-496. [Cited in This Article: ] |