Published online Oct 14, 2008. doi: 10.3748/wjg.14.5927
Revised: August 27, 2008
Accepted: September 3, 2008
Published online: October 14, 2008
Dedifferentiated liposarcoma is a variant of liposarcoma with a more aggressive course. Mutations of the p53 gene have been found in different types of soft tissue sarcoma. It is generally accepted that p53 mutations in human malignant tumors are often related to a poor prognosis. In our case, analysis of p53 gene mutation in tumor samples was performed. p53 gene mutation was observed in dedifferentiated tumor tissue samples but not in well-differentiated tumor tissue samples. It has been reported that p53 gene mutation occurs most commonly in the retroperitoneum and rarely in other anatomic locations. Herein we report a case of dedifferentiated liposarcoma located at intraperitoneum.
- Citation: Karaman A, Kabalar ME, Özcan &, Koca T, Binici DN. Intraperitoneal dedifferentiated liposarcoma: A case report. World J Gastroenterol 2008; 14(38): 5927-5929
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5927.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5927
Liposarcoma is one of the most frequent malignant soft tissue tumors and currently classified into five main subgroups: well-differentiated, myxoid, round cell, pleomorphic, and dedifferentiated[1]. It is generally accepted that p53 mutations in human malignant tumors are often related to a poor prognosis[2,3]. Taubert et al[4] reported that patients with nonframeshift mutations have a rather poor prognosis. Moreover, p53 overexpression alone is associated with a poor clinical outcome[5,6]. Mutations of the p53 gene have been found in different types of soft tissue sarcoma[7]. Well-differentiated liposarcoma is the most common variant. Dedifferentiated liposarcoma, a variant of liposarcoma with a worse prognosis, has a less frequency and occurs most commonly in retroperitoneum[8,9].
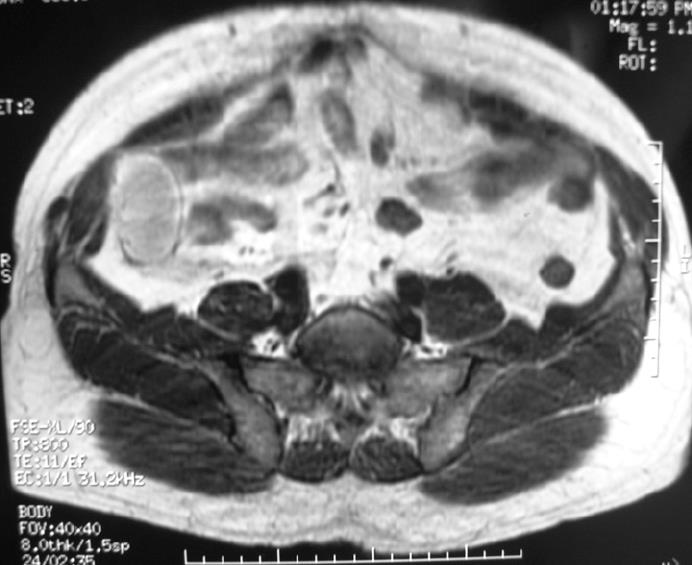
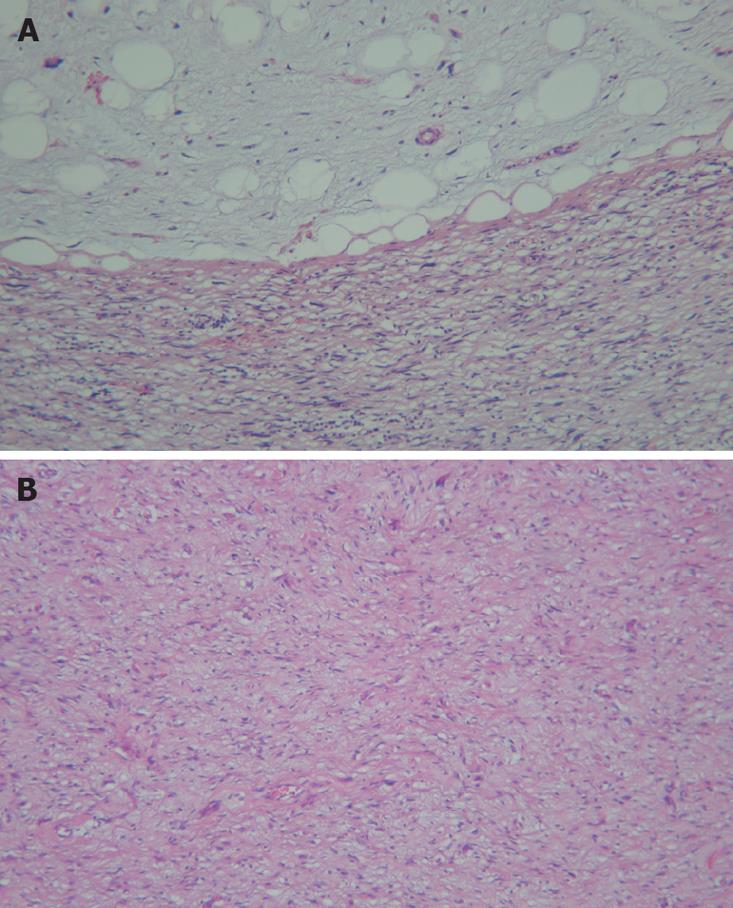
A 62-year-old man presented with a 3-mo history of constant right flank discomfort, fatigue, recent weight loss, loss of appetite and abdominal fullness. Physical examination revealed an abdominal mass in the right lower quadrant. T1-weighted magnetic resonance image (MRI) of the abdomen demonstrated a high-intensity mass. The tumor, located at intraperitoneum, was in the inferio-lateral to the cecum (Figure 1). Laboratory tests including tumor markers showed no abnormality. Surgery was performed to expose the tumor through a right lower abdominal incision. Gross examination revealed a 10 cm yellow, fleshy mass. Pathological examination revealed a dedifferentiated liposarcoma (Figure 2). Immunolabeling for S-100 was positive, while CD117 (C-KIT) and CD34 were negative in the well-differentiated tumor tissue samples, thus ruling out a gastrointestinal stromal tumor. Based on the histology and immunoprofile, a diagnosis of dedifferentiated liposarcoma was established. Furthermore, we investigated the occurrence of p53 gene mutations in the tumor samples.
Tissue samples were incubated for 15 min at 65°C, then overnight at 37°C in a lytic solution containing 1 mg/mL proteinase K, 10 mmol/L Tris/HCl, 10 mmol/L ethylene diamine tetraacetic acid, 150 mmol/L NaCl, 0.4% sodium dodecyl sulfate. DNA was extracted twice with an equal volume of 1:1 SS-phenol/chloroform, and precipitated for 2 h at -20°C after addition of 0.1 volume of 3 mol/L sodium acetate, 20 μg of glycogen as a carrier, and 2.5 volume of 100% ethanol. After centrifugation, the precipitate was washed with 2 mL of 80% ethanol, dried with a Speed Vac concentrator, and reconstituted with 50 μL of TE buffer.
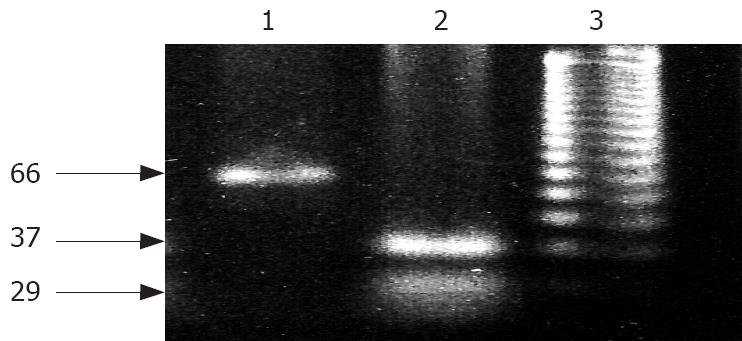
PCR was performed using a thermal cycle (PE 9700) with 50 ng of genomic DNA extracted from biopsy samples, 20 pmol of each primer, four deoxynucleotide triphosphates (dNTPs), reaction buffer and 1 μL (5 units) of fermentase Taq polymerase in a reaction volume of 100 μL. The PCR conditions consisted of an initial cycle at 95°C for 10 min, at 57°C for 1 min, and at 72°C for 10 s followed by 34 cycles at 95°C for 1 min, at 57°C for 30 s, and at 72°C for 10 s. The sequences of primer used are sense: 5'-CAGATGAAGCTCCCAGAA-3' (upstream) and anti-sense: 5'-GTGTAGGAGCTGCTGGTG-3' (downstream). An amplicon of 66 bp was produced, which was cleaved into 29 bp and 37 bp fragments with the enzyme BstU1, if its recognition site (CGCG) was present. The primer amplified a region containing codon 72 in exon 4 of p53[10,11]. Mutation of the p53 gene was only observed in dedifferentiated tumor tissue samples but not in well-differentiated tumor tissue samples (Figure 3).
Mutation or allelic deletion of p53 gene appears to play an important role in the development of human carcinoma[12]. It has been established that accumulation of wild type p53 protein results in two pathways: cell cycle arrest and programmed cell death, both of which are involved in tumor suppressor functions[13]. Therefore, mutation of p53 leads to disruption of these pathways, a selective growth advantage for tumor cells, and loss of function may increase proliferation activity and development of tumor[14].
Liposarcoma arising in extremities or retroperi-toneum affects middle-aged and old patients, and tend to follow a relatively indolent clinical course with local recurrences after resection and occasional distant metastasis, mainly to the lungs[1]. Dedifferentiated liposarcoma is defined histologically by a transition from well-differentiated liposarcoma to a non-lipogenic sarcoma with variable histological grade[1]. The dedifferentiated tumor can resemble any sarcoma, but often mimics a malignant fibrous histiocytoma (MFH)[9].
Dedifferentiated liposarcoma, despite its high-grade histology, has a less aggressive clinical course than other types of high grade sarcoma, although the underlying mechanism is unclear[1]. Compared to well-differentiated liposarcoma, dedifferentiated liposarcoma has similar genetic changes, ring or giant marker chromosomes, but a worse prognosis[1]. Approximately 40% of dedifferentiated liposarcomas will recur locally, 17% will metastasize, and 28% of the patients will ultimately die as a result of tumor[1].
It was reported that dedifferentiated liposarcoma occurs most commonly in retroperitoneum but rarely in other anatomic locations[9]. Five cases of dedifferentiated liposarcoma in small bowel mesentery have been described[15]. In addition, a case of dedifferentiated liposarcoma has been documented in the sigmoid mesocolon[16]. The present tumor, located at intraperitoneum.
Immunohistochemically, dedifferentiated liposarco-ma is usually negative for CD117 and CD34 in dedifferentiated tissue and positive for S100 protein in well-differentiated tissue. Dedifferentiated liposarcoma needs to be distinguished from other high-grade sarcomas such as MFH because these high-grade sarcomas have a much worse prognosis[17].
We investigated the exon 4 of p53 gene in adipose tissue tumor by PCR observed mutation of p53 gene in dedifferentiated liposarcoma samples. Similarly, Taubert et al[4] detected mutations of p53 gene in 5/32 of liposarcomas. It was reported that wild type p53 protein can induce cell apoptosis, whereas intracellular accumulation of mutant p53 protein can inhibit cell apoptosis, promote cell transformation and proliferation, resulting in carcinogenesis[5,14].
The patient underwent adjuvant radiation therapy and was asymptomatic during the 15-mo follow-up period. However, because the recurrence rate of dedifferentiated liposarcoma is very high, it is necessary to follow up carefully for a long term.
Peer reviewer: Alastair John Watson, Professor, The Henry Wellcome Laboratory, Nuffield Building, University of Liverpool, Crown St., Liverpool, L69 3GE, United Kingdom
S- Editor Xiao LL L- Editor Wang XL E- Editor Yin DH
| 1. | Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of soft tissue and bone. Lyon: IARC Press 2002; 40-44, 227-232. [Cited in This Article: ] |
| 2. | Ichikawa A, Kinoshita T, Watanabe T, Kato H, Nagai H, Tsushita K, Saito H, Hotta T. Mutations of the p53 gene as a prognostic factor in aggressive B-cell lymphoma. N Engl J Med. 1997;337:529-534. [Cited in This Article: ] |
| 3. | de Anta JM, Jassem E, Rosell R, Martínez-López E, Jassem J, Monzó M, Sánchez-Hernández JJ, Moreno I, Sánchez-Céspedes M. TP53 mutational pattern in Spanish and Polish non-small cell lung cancer patients: null mutations are associated with poor prognosis. Oncogene. 1997;15:2951-2958. [Cited in This Article: ] |
| 4. | Taubert H, Meye A, Wurl P. Prognosis is correlated with p53 mutation type for soft tissue sarcoma patients. Cancer Res. 1996;56:4134–4136. [Cited in This Article: ] |
| 5. | Drobnjak M, Latres E, Pollack D, Karpeh M, Dudas M, Woodruff JM, Brennan MF, Cordon-Cardo C. Prognostic implications of p53 nuclear overexpression and high proliferation index of Ki-67 in adult soft-tissue sarcomas. J Natl Cancer Inst. 1994;86:549-554. [Cited in This Article: ] |
| 6. | Kawai A, Noguchi M, Beppu Y, Yokoyama R, Mukai K, Hirohashi S, Inoue H, Fukuma H. Nuclear immunoreaction of p53 protein in soft tissue sarcomas. A possible prognostic factor. Cancer. 1994;73:2499-2505. [Cited in This Article: ] |
| 7. | Diller L, Sexsmith E, Gottlieb A, Li FP, Malkin D. Germline p53 mutations are frequently detected in young children with rhabdomyosarcoma. J Clin Invest. 1995;95:1606-1611. [Cited in This Article: ] |
| 8. | Kindblom LG, Meis-Kindblom JM, Enzinger FM. Variants of liposarcoma. Am J Surg Pathol. 1995;19:605-606; author reply 606-608. [Cited in This Article: ] |
| 9. | Goldblum JR, Weiss SW: Enznger and Weiss’s Soft Tissue Tumors. 4th ed. St Louis: Mosby 2001; 641-693. [Cited in This Article: ] |
| 10. | Meltzer SJ, Yin J, Huang Y, McDaniel TK, Newkirk C, Iseri O, Vogelstein B, Resau JH. Reduction to homozygosity involving p53 in esophageal cancers demonstrated by the polymerase chain reaction. Proc Natl Acad Sci USA. 1991;88:4976-4980. [Cited in This Article: ] |
| 11. | Fujita M, Inoue M, Tanizawa O, Iwamoto S, Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992;52:5323-5328. [Cited in This Article: ] |
| 12. | Imazeki F, Omata M, Nose H, Ohto M, Isono K. p53 gene mutations in gastric and esophageal cancers. Gastroenterology. 1992;103:892-896. [Cited in This Article: ] |
| 13. | Janus F, Albrechtsen N, Dornreiter I, Wiesmüller L, Grosse F, Deppert W. The dual role model for p53 in maintaining genomic integrity. Cell Mol Life Sci. 1999;55:12-27. [Cited in This Article: ] |
| 14. | Zhou X, Wang XW, Xu L, Hagiwara K, Nagashima M, Wolkowicz R, Zurer I, Rotter V, Harris CC. COOH-terminal domain of p53 modulates p53-mediated transcriptional transactivation, cell growth, and apoptosis. Cancer Res. 1999;59:843-848. [Cited in This Article: ] |
| 15. | Hasegawa T, Seki K, Hasegawa F, Matsuno Y, Shimodo T, Hirose T, Sano T, Hirohashi S. Dedifferentiated liposarcoma of retroperitoneum and mesentery: varied growth patterns and histological grades--a clinicopathologic study of 32 cases. Hum Pathol. 2000;31:717-727. [Cited in This Article: ] |
| 16. | Winn B, Gao J, Akbari H, Bhattacharya B. Dedifferentiated liposarcoma arising from the sigmoid mesocolon: a case report. World J Gastroenterol. 2007;13:4147-4148. [Cited in This Article: ] |
| 17. | Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271-281. [Cited in This Article: ] |