INTRODUCTION
The presence of blood into the biliary tree, as a result of hemorrhage through the biliary tract, has first been reported by Francis Glisson[1–3]. The term hemobilia, however, which is used to describe this phenomenon, was coined by Sandblom[1–3]. Hemobilia usually presents with one or more constituents of the following triad of symptoms and signs: upper quadrant pain, upper gastrointestinal bleeding and jaundice[3]. Hemobilia can occur due to trauma (both accidental and iatrogenic), gallstone disease, acalculous cholecystitis, cholangitis, ascariasis or hydatid, hepatic abscess, malignancies of the liver and pancreas and biliary tract, polyarteritis nodosa, vascular malformations of hepatic artery aneurysm or coagulopathy[3]. As far as coagulopathies are concerned, Bernard-Soulier syndrome[4], idiopathic thrombocytopenic purpura[5], treatment with anticoagulants[6] and hemophilia[78] have been implicated in the induction of hemobilia. To our knowledge, seven cases of hemobilia in patients with hemophilia have been described[7–13], five of them following liver biopsy[9–13] and only two cases of spontaneous hemobilia[78], while several cases of malignancy-associated hemobilia[14–16] have been reported. A case, however, which combines all the three conditions, has never been published. We describe a case of hemobilia as the initial manifestation of cholangiocarcinoma in a patient with hemophilia B.
CASE REPORT
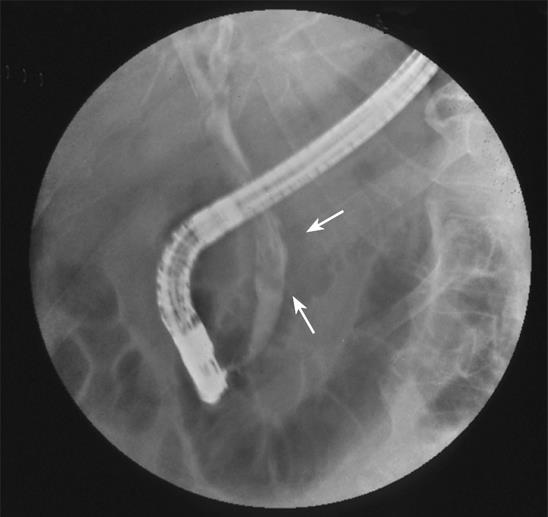
A 70-year-old male was admitted to our department during his second episode of hematemesis, melena, fever, and right upper quadrant pain. He was a known hemophilia B patient with liver cirrhosis, who was seropositive for hepatitis C virus and referred to our department three months ago due to a similar episode. He had a history of cholecystitis and underwent a papillotomy for cholelithiasis and common bile duct stones two years ago. The work-up performed during his first admission revealed higher than normal serum levels of cancer antigen (CA) 19-9 (112.5 U/mL), along with ejection of a blood clot through the sphincterotomized papilla of Vater-hemobilia by side-viewing endoscopy and the presence of string-like filling defects in the biliary tree via endoscopic retrograde cholangiopancreatography (ERCP, Figure 1). Magnetic resonance angiography (MRA) showed no evidence of hepatic artery pseudoaneurysm or any other vascular malformation. No potential cause for hemobilia other than the underlying coagulopathy was identified since ultrasonography, magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP) and ERCP were not suggestive of another etiology. The patient was treated at that time with factor IX concentrate, fresh frozen plasma (FFP) and blood transfusions until the prolonged activated partial thromboplastin time (APTT) and the decreased hematocrit reached their normal levels. Moreover, cholestasis resulting from intrabiliary clots was successfully managed by removing these clots with a baloon catheter during ERCP, which led to lysis of the remaining smaller clots and a significant decrease in levels of bilirubin, gamma-glutamyltransferase (gamma-GT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). As soon as the above-mentioned markers returned to normal and the patient’s clinical condition improved, he was discharged and remained free of any bleeding or cholestasis-related manifestations for almost 3 mo. Then, he was hospitalized for a week at a peripheral hospital with low-grade fever and right upper quadrant pain. During hospitalization, he had episodes of hematemesis and melena, but the site of bleeding was not identified. The patient was then referred to our hospital.
Figure 1 Endoscopic retrograde cholangiopancreatography showing string-like filling defects (arrows) suggestive of intraductal blood clots.
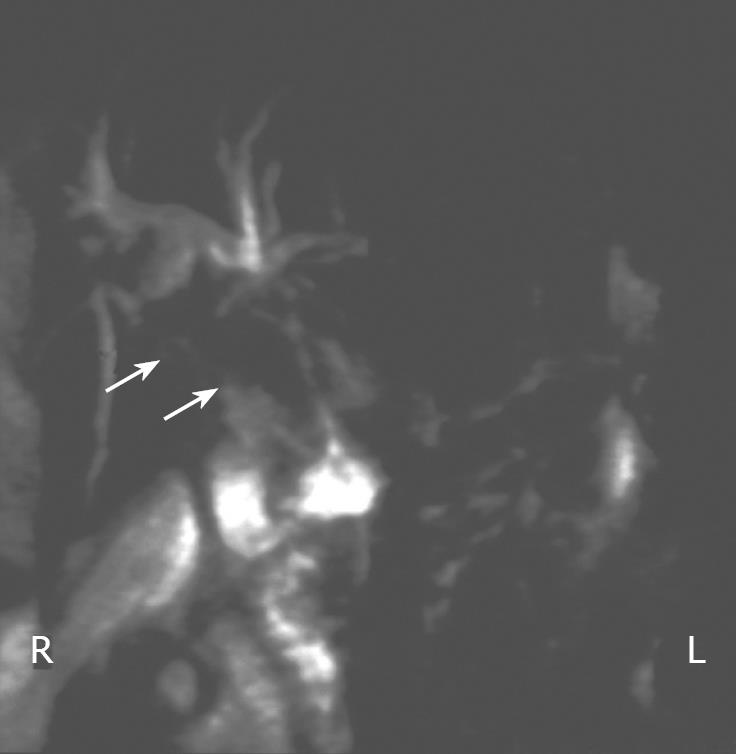
Physical examination at admission revealed that his temperature was 37.4°C, blood pressure was 160/70 mmHg and heart rate was 55 beats per minute. Right upper quadrant tenderness, jaundice and melena were also present. Laboratory findings included 7.2 g/dL hemoglobin, 2.65 × 106/&mgr;L erythrocytes, 227 × 103/&mgr;L platelets, 6.1 ×103/&mgr;L leucocytes with 55.6% polymorphs, 6.9 mg/dL total serum bilirubin, 4.03 mg/dL direct bilirubin, 199 IU/L AST, 53 IU/L ALT, 633 IU/L gamma-GT, 1466 IU/L alkaline phosphatase (ALP), 36.2 s APTT. Moreover, the determination of serum tumor markers revealed significantly elevated levels of CA 19-9 (3456 U/mL). Abdominal ultrasonography showed echogenic non-shadowing debris in the common bile duct and dilation of the intrahepatic bile ducts in the right liver lobe. Side-viewing endoscopy revealed blood in the lumen of the second part of the duodenum and a prominent blood clot at the papilla of Vater. Finally, MRCP revealed dilation of the intrahepatic bile ducts and identified the cause of hemobilia: a cholangiocarcinoma involving a 3 cm-long proximal segment of the common bile duct (Figure 2). These findings were confirmed by ERCP. The intrabiliary blood clots were removed using a baloon catheter and a 10 cm-long (10 Fr) stent was placed to stop the bleeding and decrease the level of bilirubin, gamma-GT and ALP. Factor IX concentrates, erythrocyte concentrates and FFP were also transfused until APTT, hemoglobin and erythrocyte count levels returned to normal. In addition, he was also treated with antibiotics. The patient was doing well, no recurrence of bleeding or cholangitis was recorded and he was discharged. Two weeks later, almost 6 wk before a scheduled stent was replaced, he presented with high-grade fever, jaundice and upper quadrant pain. The patient was septic at admittance. Despite full antibiotic coverage, he died of septic shock soon.
Figure 2 MRCP image showing stricture (arrows) of the proximal segment of the common bile duct.
DISCUSSION
Hemophilia is a rare cause of non-iatrogenic hemobilia. To our knowledge, only two cases of spontaneous hemobilia in hemophiliacs have been described so far[78]. Malignancies, on the other hand, are a more common cause of hemobilia, compared to hemophilia. Malignant tumors, however, do not account for more than 10% of total hemobilia incidents[3]. Hemophilia and malignancy, two potential causes for hemobilia, were both present in our patient. Hemorrhagic diathesis induced by hemophilia B, increased the susceptibility to bleeding originating from a tumor-infiltrated ductal system. What seems to be of some importance is the fact that hemobilia was proved to be an early manifestation of the underlying cholangiocarcinoma, and that ultrasonography, ERCP and MR techniques (MRI, MRCP, MRA) failed to identify it. A mild elevation of CA 19-9 was also recorded during the patient’s first admission. It was reported that use of this tumor marker in the diagnosis of cholangiocarcinoma shows a sensitivity of 53%, when a CA19-9 value > 100 U/mL is used[17], and is considered to be misleading when such patients have gallstone disease and/or cholangitis[18]. In our case, the elevation of CA19-9 was initially attributed to chronic ascending cholangitis following papillotomy which in turn could affect the arterial wall, thus leading to the formation of hepatic artery pseudoaneurysm, a known cause of hemobilia[19].
Although cholangiocarcinoma was not detected during the initial work-up, hemobilia was successfully recorded during both work-ups. The combination of upper quadrant pain, upper gastrointestinal bleeding (hematemesis, melena) and jaundice as well as the evidence provided by the abdominal ultrasonography were all indicative of hemobilia. It was not, however, until side-viewing endoscopy and ERCP were performed, hemobilia was diagnosed.
In view of the classical triad of hemobilia, all the three constituents are present in up to 22% of patients[3]. Side-viewing endoscopy performed independently or as a part of ERCP can reveal active bleeding[620] or blood clots at the papilla of Vater (as in this case). Moreover, cholangiography may show string-like, as reported here, or spherical filling defects[3]. Angiography also plays a significant role in the confirmation and management of hemobilia and in the identification of vascular malformations as a potential cause of bleeding[3]. Ultrasonography, on the other hand, offers circumstantial evidence of bile duct dilation along with the presence of echogenic, non-shadowing, non-mobile formations of blood clots that evolve less reflective masses[3]. Computed tomography[21] and radioisotope studies[3] can also be used.
As far as the management of hemobilia is concerned, different approaches have been applied, depending on the etiology and the underlying diseases. It was reported that decompression of the biliary tree encourages resolution of jaundice and contributes to the arrest of hemobilia[38]. As shown in a study performed by Sandblom, bile enzymes can perform lysis of fibrin, but a free flow of bile is needed so that this lytic activity can occur[22]. Embolization techniques, such as transarterial embolization[23] or even surgery[3], have also been applied in the management of hemobilia. In our case, decompression of the biliary tree (by means of a balloon catheter and stenting during ERCP) along with correction of the patient’s bleeding diathesis was adequate for both resolution of jaundice and control of bleeding.
In summary, underlying malignancy should be suspected in cases of non-traumatic, non-iatrogenic hemobilia irrespective of any coincident coagulopathies, such as hemophilia. Hemobilia should not be easily attributed to hemophilia, even when the first work-up fails to identify a potential cause other than coagulopathy. ERCP may serve as a diagnostic tool for both hemobilia and underlying malignancy as well as a therapeutic technique for the management of obstructive jaundice and, in part, of bleeding. The correction of bleeding diathesis by treatment with factor IX, FFP and erythrocyte concentrates is mandatory for the arrest of hemobilia.
Peer reviewer: Giammarco Fava, MD, via Gervasoni 12, Ancona 60129, Italy