Published online Jun 14, 2008. doi: 10.3748/wjg.14.3563
Revised: March 20, 2008
Accepted: March 27, 2008
Published online: June 14, 2008
AIM: To analyze the imaging findings of hepatic malignancy with right atrial (RA) embolus.
METHODS: Forty-six patients with an embolus in the RA were diagnosed, including 44 patients with hepatocellular carcinoma (HCC), 1 patient with cholangiocellular carcinoma and 1 patient with hepatic carcinoma metastasis. The diagnosis was confirmed by clinical examination, serum α-fetoprotein and imaging. Seventeen patients underwent transcatheter arterial chemoembolization (TACE).
RESULTS: On enhancement computer tomography (CT) or magnetic resonance (MR) imaging, a nodular filling defect in the RA could be easily found, with a slight enhancement in the arterial phase. The coronal images of CT or MR showed the extent of lesion. Lipiodol entered the embolus after TACE, hence reducing the speed of embolus growth. There was a survival benefit for patients receiving anticancer treatment.
CONCLUSION: Patients with HCC, showing a filling defect of the inferior vena cava (IVC), hepatic vein (HV) and RA on images, can be diagnosed with RA embolus. Encroachment of the RA is very rare in patients with hepatic malignancies. Furthermore, a prolongation of survival time is found in those patients who underwent TACE.
- Citation: Cheng HY, Wang XY, Zhao GL, Chen D. Imaging findings and transcatheter arterial chemoembolization of hepatic malignancy with right atrial embolus in 46 patients. World J Gastroenterol 2008; 14(22): 3563-3568
- URL: https://www.wjgnet.com/1007-9327/full/v14/i22/3563.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3563
Malignant tumors of various organs and tissues, originating from all over the body, may disseminate to the right atrium (RA) and form a nodular embolus. Current literature reports that malignant tumors, such as hepatocellular carcinoma (HCC), pancreatic carcinoma, adrenal carcinoma, testicular cancer, lymphoma, leiomyosarcoma, hysterocarcinoma, nasopharyngeal cancer, esophageal cancer, Ewing’s sarcoma and Wilm’s tumor can encroach on the RA[1–3].
HCC is the most prevalent of the liver tumors, and the portal vein (PV), hepatic vein (HV), and the inferior vena cava (IVC) are often affected. Although it is rare, tumors may also intrude on the RA. This study describes the imaging findings in 46 patients with malignant hepatic tumors that formed a tumor embolus in the RA.
From August, 2001 to May, 2007, 46 patients with hepatic malignancy and RA embolus were enrolled (41 men and 5 women, aged 23-75 years; average 52.5 years). The patients were diagnosed by computed tomography (CT) and magnetic resonance (MR), laboratory values (i.e., α-fetoprotein) and clinical examination. The malignant hepatic tumors were designated as HCC (n = 44), intrahepatic cholangiocellular carcinoma (n = 1) and lung and liver metastasis from post-operation retroperitoneal sarcoma (n = 1). All these patients had encroachment of the IVC and the RA.
Two of the 46 patients underwent surgery while 17 patients had transcatheter arterial chemoembolization (TACE). TACE was performed 4 times in 4 patients, twice in 10 patients and once in 3 patients. The remaining 27 patients had serious liver function damage. These patients were not treated with anti-tumor drugs, but were given liver protective medication until discharged.
CT was performed using multi-slice spiral CT (Lightspeed QX/i, GE) scanning with high-quality scanning mode, thickness 5 mm, 120 kV, 270-300 mA. The contrast medium was 100 mL Ioversol Injection 350 (Mallinckrodt, Canada), and the velocity was 3 mL/s.
The MR image (Signa Excite Twin Speed 1.5T, GE) was preformed with a T2-weighted image using RFSE+FS, TR: 5000-10 000 ms, TE: 80-130 ms, ETL: 16-23; the T1-weighted image using FSPGR, TR: 175 ms, TE: 4.2 ms; and the 2D Fiesta coronal image using TR: 4.2 ms, TE: 1.5 ms. The contrast medium was 30 mL gadopentetic acid dimeglumine salt injection (Schering, Germany), and the velocity was 4 mL/s. Dynamic CT and MR images were produced with an arterial phase of 25 s, a portal phase of 60 s, and a delayed phase of 3 min.
Plate digital subtraction angiography (DSA, INNOVA 4100, GE) with 40 mL of contrast medium, and a velocity of 6 mL/s) were used in TACE treatment. Angiography was initially performed on the abdominal cavity and superior mesenteric artery to determine the variant hepatic arterial supply, the blood supply type, and size, number and position of tumors in all patients. Then, the celiac axis was selected. A 5 Fr Yashiro angiographic catheter (Terumo) was advanced over a 0.035 inch guide wire (Terumo) into the desired hepatic artery branch, depending on the tumor location. Because the optimal agents or doses for TACE have not been determined,[4] the dose of anticancer drug and lipiodol (Lipiodol Ultra Fluide, Guerbet, France) was determined based on the size, number, and blood supply of the tumor, and also on the basis of practical infusion procedures during the operation[5]. Anticancer drugs used were epirubicin hydrochloride (20-40 mg), hydroxycamptothecin (OPT, 10 mg) and ultra-liquefied lipiodol (10-30 mL). Before infusion, epirubicin hydrochloride and lipiodol were fully mixed together to form an emulsion. At the end, some gel foam sponge powder was infused to decrease blood flow. Lidocaine (5 mL) was also given intra-arterially after chemoembolization for pain control.
The relationship between RA embolus and hepatic cancer was observed to determine the development pathway.
Patients were monitored from the moment of discovery of the RA embolus to an occurrence of hard end-point (i.e. patients’ death) or to the end of the study period (May 30, 2007). The growth velocity of the embolus was monitored in 10 cases. In these cases the RA embolus was discovered during follow-up and the patients had no RA embolus at first examination. The relationship between the type of intrahepatic tumor and RA embolus was observed.
The types of intra-hepatic malignancy found were: huge-mass type in 37 cases (80.4%), multinodular type in 8 cases (17.4%) and single nodular type in 1 case. The location of the tumor was the right hepatic lobe in 39 cases, the left hepatic lobe in 5 cases, and between the right and left lobe in 2 cases. There were 4 cases of lung metastasis and 5 cases of abdominal lymph node metastasis.
On imaging, the RA embolus was commonly a regular, slight irregular round, orbicular-ovate, or lobulated type. The maximum size was 6.1 cm × 12 cm, almost filling the entire RA. The minimum size was 1.5 cm × 2.1 cm.
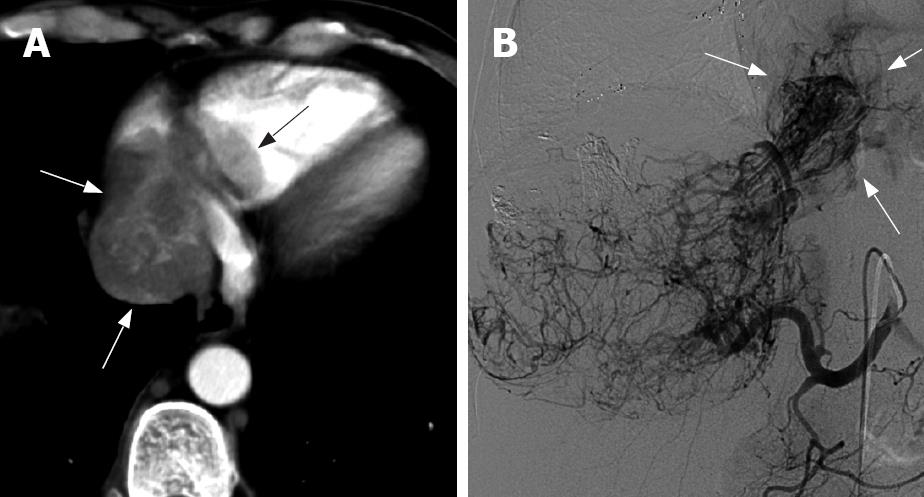
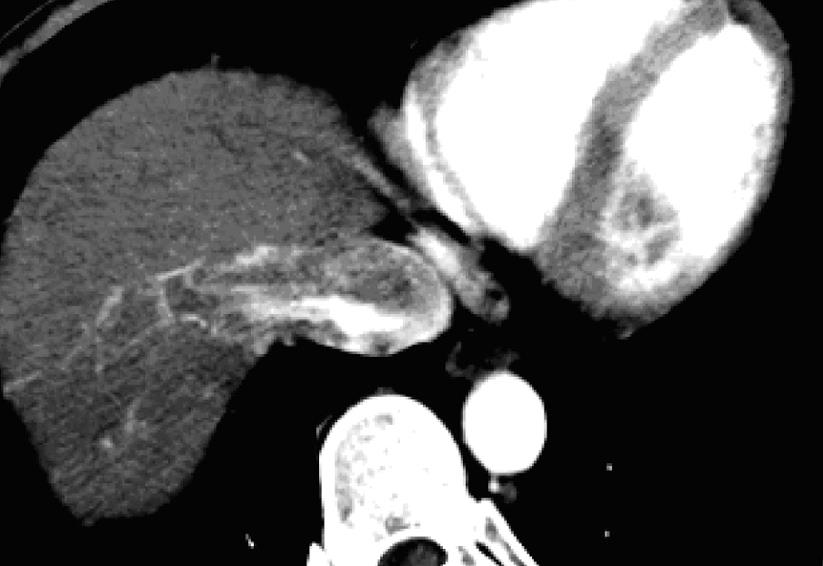
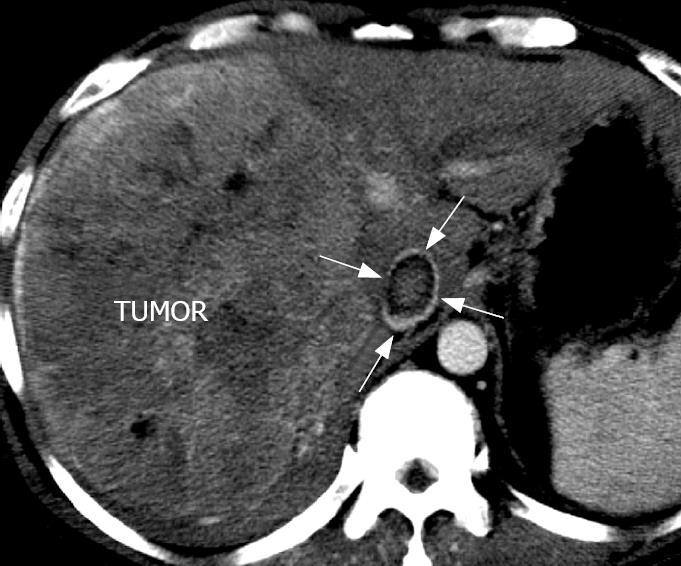
A plain CT scan showed low-density or iso-density, which corresponded to a normal cordis density. The CT value was 27-52 HU, without an obvious density difference and sometimes hardly differentiated. There was a low density line around the embolus edge. On the arterial phase scan, the contrast medium filled the cordis intracavity and the embolus showed a well-defined filling defect. On this arterial phase scan, the embolus had a slight inhomogenous “stick”-like enhancement (Figure 1A), while the tumor encroaching the IVC could be seen as a “stick”-like enhancement arterial vessel inside the IVC (Figure 2). The CT value of the embolus increased by about 30 HU following enhancement. On the portal phase scan, the contrast medium in the cordis intracavity was partially discharged, and the density of the embolus was slightly increased or decreased. The edge of the embolus was comparatively blurred. On the delay phase scan, the density of the embolus decreased, but the edge was clear; hence there was a small increase in embolus size when compared to the arterial phase scan.
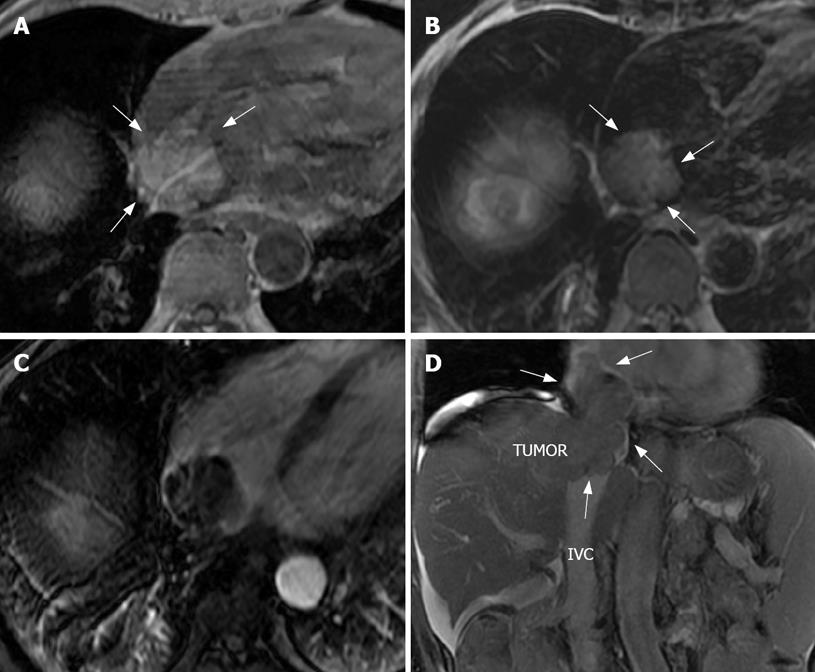
The embolus showed a nodular high or iso-signal in the T1-weighted image (Figure 3A). A higher signal was found in the T2-weighted image (Figure 3B) because in this image, larger vessels, the atrium and the ventricles, have a flowing void effect. There was a low signal in the lumina and cavity of the greater vessels and cordis, while the thrombus signal was an indicator of intrahepatic HCC with a higher signal. In the arterial phase image, the thrombus was shown as a low signal filling defect with a slightly “stick”-like enhancement (Figure 3C). The portal and delay phase images showed low nodular signals. In the coronal image, the relationship between the intrahepatic tumor, IVC, and RA embolus could be seen very clearly (Figure 3D and Figure 4B).
Angiography images showed that the tumor vessel entered the RA and was a grating-like type (Figure 1B). After TACE with lipiodol perfusion, the lipiodol was retained in the HCC and the embolus and there were higher density group masses in the intra-liver area and the RA (Figure 4C). This suggested that TACE could be used successfully to treat an embolus of the HCC.
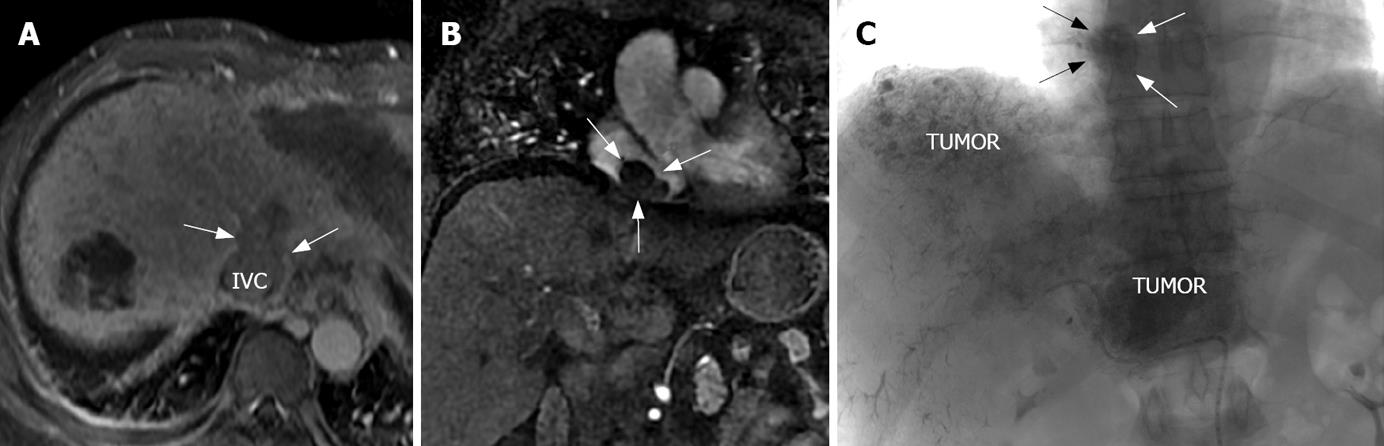
Invasion of the PV, HV, and IVC was seen in 38 cases, 11 cases, and all cases, respectively. The range of invasion of IVC varied in size in different patients and the longest invasion size was 8.3 cm. The coronal images from CT and MR showed the relationship between the intra-liver tumor, IVC and the RA embolus best (Figures 3D, 4A and B). Vein invasion was discovered by the presence of a filling defect in the vena cava. In a portal phase cross section image there was no contrast medium in the center of the IVC cava with embolus and there was clear enhancement around the embolus, which showed up as a “ring” sign (Figure 5).
There were two routes of HCC invasion into the RA. One directly invaded into the IVC, then upward into the RA and downward into IVC. The second one was invasion into the HV, and then into the IVC and RA. The first route was more common.
Thirty-six of the 46 patients had an RA embolus when imaging examination was performed, and the embolus was discovered during follow-up in 10 cases. The growth velocity of the embolus in these 10 cases was slow growth of 3 cm in 6 mo, and quick growth of 4 cm in 1 mo. The embolus may remain in steady state for a long time after TACE treatment.
Twenty-seven patients without anticancer treatment died after 6 mo. The shortest time to death was 1 mo, and longest time to death was 8 mo (average 3.5 mo). In 17 patients who received TACE treatment the longest survival time was 19 mo, and the shortest survival time was 4 mo (average 8.4 mo). The survival time of 2 patients who underwent surgery were 5 and 7 mo, respectively.
About 70% patients with HCC have HV and PV invasion, but encroachment into the RA is very rare. According to the literature[36–10], less than 50 cases have been reported. A combination of literature and our hospital clinical data indicates HCC encroachment into the RA may not be an overt symptom. However, when the embolus obstructs the tricuspid orifice and coronary sinus opening, it may result in a severe hemodynamic disorder. This can include venous engorgement of both upper extremities and the chest, hydroncus, pleural effusion, and flustered or even dyspneic respiration. An electrocardiogram will show right bundle branch block. When the IVC is obstructed, engorgement of the veins of the lower extremities will appear, leading quickly to Budd-Chiari syndrome[1011], and in this situation the prognosis of patients is very bad. Even when the HCC and embolus are surgically resected the patient may quickly die due to the severity of the pathogenetic condition or cardiac failure[12–15].
The formation of the RA embolus had a growth process. Firstly, the tumor perforated, involving the wall of the HV and/or IVC, then the tumor extended into the lumen of the vein and grew there. The tumor within the lumen can grow upward and/or downward, and if it grows upward it may get into the RA and continue slow growth there. On the basis of overview data from 10 patients, the quickest velocity of embolus growth was 4 cm in 1 mo, and the slowest velocity of growth was 3 cm in 6 mo (average growth of 3.7 cm in 3.2 mo). Slow growth of the embolus was due to receiving TACE. TACE treatment was effective and controlled hepatic malignancy and tumor embolus.
Using the plain CT scan, the density difference between the embolus and cardiac tissue was not obvious, but sometimes there was a low density single band appearance between them. When this appearance is found, we should distinguish between normal interventricular septum, interatrial septum and tumor in the cordis as the location of each was not the same.
In the arterial phase scan, the malignant embolus was mildly enhanced. The heart chambers were filled with a high concentration of contrast medium whereas the embolus showed a filling defect so that there was a very significant contrast and a clear density difference. The arterial phase scan produced an image showing the embolus. In the portal phase scan, the embolus continued to display slight enhancement. In the delay phase, most of the contrast medium in the heart chambers was discharged, but the density of the embolus was still lower than that of cardiac tissue. In this phase, the size of the embolus was increased compared with the arterial phase. This was because the high density contrast medium submerged part of the edge of the embolus in the arterial phase.
CT and MR images can display and distinguish between a tumor, thrombi, and a malignant embolus[16]. The malignant embolus was round, orbicular-ovate or cauliflower in shape. The embolus was part of the arterial blood supply. On angiography, a lot of blood vessels and signs of “stria” are seen with a hypervascular tumor, but there is no enhancement or staining for hypovascular tumors. The thrombus was not enhanced when it has lower density and there was no intrahepatic malignant tumor. Cardiac tumors can be divided into 2 types: primary tumor and metastasis. The most common is the primary myxoma; 93.5% of them are located in the left atrium and 6% are located in the RA. A myxoma can activate and the stem can cohere on the atrial septum. But when the malignant embolus was solid and was not transfigured, the stem cohered on the IVC.
MR images showed the embolus better than CT as MR showed it from different direction of view.
The PV, IVC and HV may be encroached by HCC, and show a filling defect of the venous lumen. Most patients with HCC had PV encroachment. IVC encroachment was relatively rare. The tumor encroached from the IVC to the RA and formed an embolus. When the tumor grew in the IVC the image showed a prismatic filling defect. The vessel wall was not destroyed unless the tumor encroached directly upon the wall. There was a gap between the embolus and vessel wall and the contrast medium flowed through the gap. This gap was shown as a high density ring-shape with low density in the center on the arterial phase scan, like a “ring”. Tumors in the right hepatic lobe usual encroach upon the hepatic vein and directly affect the IVC, and tumors in the left lobe firstly encroach upon the left hepatic vein and middle hepatic vein, get into IVC, then re-enter the RA.
Patients who could not take TACE generally had PV embolus, pulmonary metastasis, retroperitoneal lymph node metastasis, medium dose or high dose ascites, and a grade C Child-Pugh hepatic function classification. Patients could take TACE treatment if they had class A or B hepatic function and if they could tolerate TACE treatment. The tumor could be controlled for a certain time and to a certain degree after TACE treatment, and the survival time of patients could be prolonged[717–19].
CT and MR images can exhibit and distinguish between tumor, thrombi and malignant embolus[16]. The malignant embolus was round, orbicular-ovate or cauliflower shape. The embolus was arterial blood supply, on the angiography, it may show a lot of blood vessel and “stria” sign for hypervascular tumor, but for hypovascuar tumor it showed no enhancement and stain. The thrombus was no enhancement with more low density and no intrahepatic malignant tumor.
Malignant tumors of various organs may disseminate to the right atrium (RA) and form a nodular embolus. Hepatocellular carcinoma (HCC) is the most prevalent of the liver tumors, and the portal vein (PV), hepatic vein (HV) and the inferior vena cava (IVC) are often affected. Although rare, the RA may show signs of tumor intrusion.
Nodular filling defects in the RA could be easily found on enhancement computer tomography (CT) or magnetic resonance (MR) imaging. The coronal images of CT or MR show the extent of the lesion. Lipiodol can enter the embolus after transcatheter arterial chemoembolization (TACE) and reduce the speed of embolus growth.
Lipiodol can enter the embolus after TACE and reduce the speed of embolus growth. There was a survival benefit associated with anticancer treatment.
CT and MR images can exhibit and distinguish between the tumor, thrombi and a malignant embolus. MR images showed the embolus better than CT because MR can show it from a different direction of view.
TACE: One of most commonly used technologies for liver cancer therapy. The chemotherapeutic agents and embolic agents (and/or particles) were delivered via selective catheter placement to the tumor(s) feeding vessels in an attempt to achieve cytoreduction by enabling more focused drug delivery or deposition of a higher concentration of drug within the liver cancer.
This study reported imaging characteristics of an RA tumor embolus in patients with advanced HCC. It arouses interest for readers and provides an important clue for diagnosing and treating patients with tumor emboli.
| 1. | Cheng HY, Xu AM, Chen D, Xu W, Jia YC. Multi-slice helical CT findings of tumor thrombus in the right atrium from hepatocellular carcinoma. Zhonghua Fangshexue Zazhi. 2003;37:989-991. [Cited in This Article: ] |
| 2. | Nokamura K, Kohmoto T, Kawada M, Shimizu S, Kuroko Y, Kawabata T, Sano S. [Left atrial thrombus in an 80-year-old woman scheduled to have an operation for uterus cancer; report of a case]. Kyobu Geka. 2005;58:838-840. [Cited in This Article: ] |
| 3. | Miyazawa M, Torii T, Asano H, Yamada M, Toshimitsu Y, Shinozuka N, Koyama I. Does a surgery for hepatocellular carcinoma with tumor thrombus highly occupying in the right atrium have significance? A case report and review of the literature. Hepatogastroenterology. 2005;52:212-216. [Cited in This Article: ] |
| 4. | Reidy DL, Schwartz JD. Therapy for unresectable hepatocellular carcinoma: review of the randomized clinical trials-I: hepatic arterial embolization and embolization-based therapies in unresectable hepatocellular carcinoma. Anticancer Drugs. 2004;15:427-437. [Cited in This Article: ] |
| 5. | Cheng HY, Xu AM, Chen D, Jia YC. [Adjustment of lipiodol dose according to tumor blood supply during the transcatheter arterial chemoembolization for large hepatic carcinoma by mulitdetector helical CT]. Zhonghua Zhongliu Zazhi. 2003;25:186-189. [Cited in This Article: ] |
| 6. | Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527-1536. [Cited in This Article: ] |
| 7. | Wallace MJ. Transatrial stent placement for treatment of inferior vena cava obstruction secondary to extension of intracardiac tumor thrombus from hepatocellular carcinoma. J Vasc Interv Radiol. 2003;14:1339-1343. [Cited in This Article: ] |
| 8. | Wilson K, Guardino J, Shapira O. Pulmonary tumor embolism as a presenting feature of cavoatrial hepatocellular carcinoma. Chest. 2001;119:657-658. [Cited in This Article: ] |
| 9. | Yogita S, Tashiro S, Harada M, Kitagawa T, Kato I. Hepatocellular carcinoma with extension into the right atrium: report of a successful liver resection by hepatic vascular exclusion using cardiopulmonary bypass. J Med Invest. 2000;47:155-160. [Cited in This Article: ] |
| 10. | Wu CC, Hseih S, Ho WM, Tang JS, Liu TJ, P'eng FK. Surgical treatment for recurrent hepatocellular carcinoma with tumor thrombi in right atrium: using cardiopulmonary bypass and deep hypothermic circulatory arrest. J Surg Oncol. 2000;74:227-231. [Cited in This Article: ] |
| 11. | Saisse J, Hardwigsen J, Castellani P, Caus T, Le Treut YP. Budd-Chiari syndrome secondary to intracardiac extension of hepatocellular carcinoma. Two cases treated by radical resection. Hepatogastroenterology. 2001;48:836-839. [Cited in This Article: ] |
| 12. | Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002;17:401-405. [Cited in This Article: ] |
| 13. | Nonami T, Nakao A, Harada A, Kaneko T, Kurokawa T, Takagi H. Hepatic resection for hepatocellular carcinoma with a tumor thrombus extending to inferior vena cava. Hepatogastroenterology. 1997;44:798-802. [Cited in This Article: ] |
| 14. | Zaczek M, Franczyk M, Mikulski A. [Right atrial and right ventricular thrombus in a patient with hepatic carcinoma - a case report]. Kardiol Pol. 2003;59:321-324. [Cited in This Article: ] |
| 15. | Valera JM, Merino R, Palavecino P, Sepulveda L, Smok G, Fernandez M, Brahm J. [Hepatocellular carcinoma with cardiovascular invasion. Report of five cases]. Rev Med Chil. 2004;132:1517-1522. [Cited in This Article: ] |
| 16. | Krombach GA, Spuentrup E, Buecker A, Mahnken AH, Katoh M, Temur Y, Higgins CB, Gunther RW. [Heart tumors: magnetic resonance imaging and multislice spiral CT]. Rofo. 2005;177:1205-1218. [Cited in This Article: ] |
| 17. | Izaki K, Matsumoto S, Konishi J, Higashino T, Tsurusaki M, Fukuda T, Akasaka Y, Mori T, Sugimoto K, Fujii M. [Temporary placement of inferior vena cava filter prior to transcatheter arterial embolization (TAE) for hepatocellular carcinoma with IVC tumor thrombus--prevention of pulmonary tumor emboli after TAE]. Gan To Kagaku Ryoho. 2001;28:1708-1711. [Cited in This Article: ] |
| 18. | Chang JY, Ka WS, Chao TY, Liu TW, Chuang TR, Chen LT. Hepatocellular carcinoma with intra-atrial tumor thrombi. A report of three cases responsive to thalidomide treatment and literature review. Oncology. 2004;67:320-326. [Cited in This Article: ] |
| 19. | Yogita S, Tashiro S, Harada M, Kitagawa T, Kato I. Hepatocellular carcinoma with extension into the right atrium: report of a successful liver resection by hepatic vascular exclusion using cardiopulmonary bypass. J Med Invest. 2000;47:155-160. [Cited in This Article: ] |