Published online Apr 14, 2008. doi: 10.3748/wjg.14.2262
Revised: February 21, 2008
Published online: April 14, 2008
AIM: To evaluate the diagnostic value of endoscopy in patients with gastrointestinal graft-versus-host disease (GI GVHD).
METHODS: We identified 8 patients with GI GVHD following allogeneic hematopoietic stem cell trans-plantation (HSCT). GVHD was defined histologically as the presence of gland apoptosis, not explained by other inflammatory or infectious etiologies.
RESULTS: The symptoms of GI GVHD included anorexia, nausea, vomiting, watery diarrhea, abdominal pain, GI bleeding, etc. Upper endoscopic appearance varied from subtle mucosal edema, hyperemia, erythema to obvious erosion. Colonoscopic examination showed diffuse edema, hyperemia, patchy erosion, scattered ulcer, sloughing and active bleeding. Histological changes in GI GVHD included apoptosis of crypt epithelial cells, dropout of crypts, and lymphocytic infiltration in epithelium and lamina propria. The involvement of stomach and rectocolon varied from diffuse to focal.
CONCLUSION: Endoscopy may play a significant role in early diagnosis of GI GVHD patients following allogeneic HSCT, and histologic examination of gastrointestinal biopsies is needed to confirm the final diagnosis.
- Citation: Xu CF, Zhu LX, Xu XM, Chen WC, Wu DP. Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J Gastroenterol 2008; 14(14): 2262-2267
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2262.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2262
Allogenic hematopoietic stem cell transplantation (HSCT) is increasingly performed for a variety of disorders, including acute and chronic leukemia, hematologic malignancies, and marrow failure states[1]. Graft-versus-host disease (GVHD) is the leading cause of morbidity and mortality after allogeneic HSCT[23]. Gastrointestinal (GI) complaints are relatively common within the first 100 d following allogeneic HSCT[45]. Although nausea, vomiting, anorexia and high-volume diarrhea are the common manifestations of GI GVHD, they may also be attributable to chemoradiation toxicity, medication side effects, or a variety of bacterial, fungal, viral infections. Thus, it is very difficult to establish the diagnosis of GI GVHD based on the clinical grounds alone[6].
Endoscopy with biopsy has been shown to be accurate in the identification of GVHD. Although previous reports have documented a high yield for rectal biopsy[78], upper GI biopsies are superior to rectal or rectosigmoid biopsies in the diagnosis of GVHD[910]. Thus further evaluation may be needed to establish the best diagnostic approach to GI GVHD.
Our aim in the present study was to demonstrate the endoscopic and histological features of GI GVHD. Eight patients with proven GI GVHD were included in the study, and we intended to evaluate the significance of endoscopy and biopsy in the diagnosis of GI-GVHD.
From January 2002 to December 2006, eight patients with suspected GI GVHD 20 d following allogenetic HSCT at the First Affiliated Hospital of Soochow University were enrolled in this study. All patients were interviewed and the following data were recorded: age, gender, underlying disease and transplantation type, stool per day, stool volume, nausea, vomiting, diarrhea, anorexia, gastrointestinal bleeding and skin rash. Laboratory studies including liver chemistry tests were also recorded. Routine stool examination and bacterial culture were performed for all patients. Cytomegalovirus (CMV) antigenemia was monitored twice weekly after conditioning regimens.
One patient with acute myeloid leukemia (AML) underwent 2-locus mismatched unrelated donor transplant. Four patients with chronic myeloid leukemia (CML) were recipients of a matched related donor (MRD) transplant. Three patients with acute lymphoblastic leukemia (ALL) received haploid related donor transplant. Peripheral blood hematopoietic stem cells were collected from donors in all but one case. One case with AML had hematopoietic stem cells harvested from bone marrow through Taiwan Marrow Donor Registry.
Following conditioning regimens were used: BuCy (busulfan 4 mg/kg per day for 4 d and cyclophosphamide 60 mg/kg per day for 2 d) for standard transplantation in patients 1, 3 and 4; Bu-Fludara-ATG (antihuman thymocyte globulin) (busulfan 4 mg/kg per day for 2 d, fludarabine 30 mg/m2 per day for 6 d, and antithymocyte globulin 2.5 mg/kg per day for 4 d) for non-myeloablative transplantation in patients 2, 5; Me-CCNU (semustine)-TBI (total body radiation)-Cy (Me-CCNU 250 mg/m2, -d8; TBI 8Gy, -d7; Ara-C (arabinosylcytosin) 4 g/m2, -d6, -d5; Cy 1.8 g/m2, -d4, -d3) for all ALL patients.
All patients received cyclosporine A (CSA) with short-course MTX (methotrexate) for the prophylaxis of GVHD. For patient 1 who underwent 2-locus mismatched unrelated transplantation, ATG and MMF (mycophenolic mofetil) were added. Treatment for patients with ALL was intensified and prolonged by using the combination of cyclosporine A, MMF, ATG and anti-CD25 antibody for GVHD prophylaxis.
GI GVHD could be diagnosed according to its clinical manifestations, endoscopic appearance and histopathological evaluation. Medication-induced side effects, chemoradiation toxicity or GI infections must be excluded. Specific histological criteria could establish the diagnosis of GI GVHD. Focal dropout and apoptosis of GI crypt epithelial cells are usually regarded as golden standard to diagnose GVHD. Acute GVHD (aGVHD) is defined as occurring within 20 to 100 d after transplantation and chronic GVHD (cGVHD) occurring 100 d after transplantation[5]. A clinical grading system based upon the degree of involvement for each of the organ systems was originally developed by investigators in Seattle[2]: (1) gradeI: 500-1000 cc stool/d, accompanied with anorexia and vomiting; (2) grade II: 1000-1500 cc stool/d, histologically proven GVHD by endoscopic biopsies; (3) grade III: 1500-2000 cc stool/d; (4) grade IV: over 2000 cc stool/d, accompanied with ileus and severe abdominal pain.
If patients had persistent unexplained GI symptoms (diarrhea, nausea, vomiting, anorexia, abdominal pain or gastrointestinal bleeding) after transplantation, then upper endoscopy and/or colonoscopy were performed. Upper endoscopy with gastric biopsies of both antrum and body were performed in one patient, colonoscopy was performed with multiple biopsies of the ileum, right colon and rectosigmoid colon in 6 patients. A combination of upper endoscopy with colonoscopy and multiple biopsies was performed in another patient. For each patient, biopsies were systematically performed in the GI tract, two of which were transmitted to the microbiology department and studied further for bacterial, viral, or fungal pathogens. Another two biopsy specimens were immediately snap-frozen in liquid nitrogen and used for CMV immunohistochemical study. The remaining two biopsy specimens were fixed in formaldehyde, and further processed for paraffin embedding. Paraffin blocks were sectioned at 4 &mgr;m and stained with hematoxylin and eosin for routine histopathological examination.
Of the eight patients, two developed grade III acute GI GVHD, and four grade IV acute GI GVHD, the remaining suffered from limited chronic GI GVHD. Detailed data are listed in Table 1. The clinical manifestations of upper GI GVHD included nausea, vomiting, anorexia, and abdominal pain. Lower GI symptoms manifested as voluminous secretory diarrhea accompanied with abdominal bloating or pain. Three patients had intestinal bleeding, and only one patient had gastric bleeding (Table 2).
| Case No./Sex/Age | Diagnosis | Conditioning regimens | Donor HLA match | GVHD prophylaxis | Stage grading |
| 1/F/29 | AML-M2 | BU/CY | HLA 2-locus mismatched unrelated donor | CSA, ATG, MMFMTX | aGVHD grade IV |
| 2/M/47 | CML-CR | Fludarabine, Bu, ATG | HLA-identical sibling donor | CSA, MTX | cGVHD Limited |
| 3/M/39 | CML -CR | BU/CY | HLA-identical sibling donor | CSA, MTX | cGVHD/Limited |
| 4/M/23 | CML-CR | BU/CY | HLA-identical sibling donor | CSA, MTX | aGVHD grade IV |
| 5/M/63 | CML-CR | Fludarabine, Bu, ATG | HLA-identical sibling donor | CSA, MTX | aGVHD grade IV |
| 6/F/35 | ALL-CR | Me-CCNU, TBI, Ara-C, CY | HLA 2-locus mismatched related donor | CSA, MTX, MMF, ATG | aGVHD grade IV |
| 7/M/42 | ALL-CR | BU/CY, Me-CCNU, Ara-C | HLA 2-locus mismatched related donor | CSA, MTX, MMF, ATG | aGVHD grade III |
| 8/F/23 | ALL-CR | Me-CCNU, TBI, Ara-C, CY | HLA 2-locus mismatched related donor | CSA, MTX, MMF, ATG Anti-CD25 | aGVHD grade III |
| Case No./Sex/Age | Nausea | Vomiting | Anorexia | Abdominal pain | Diarrhea | Gastrointestinal bleeding |
| 1/F/29 | + | + | + | + | + | + |
| 2/M/47 | + | + | + | - | - | - |
| 3/M/39 | - | - | - | - | + | + |
| 4/M/23 | + | + | - | + | + | + |
| 5/M/63 | - | - | - | + | + | - |
| 6/F/35 | + | - | - | + | + | + |
| 7/M/42 | + | - | + | + | + | - |
| 8/F/23 | + | + | - | - | + | - |
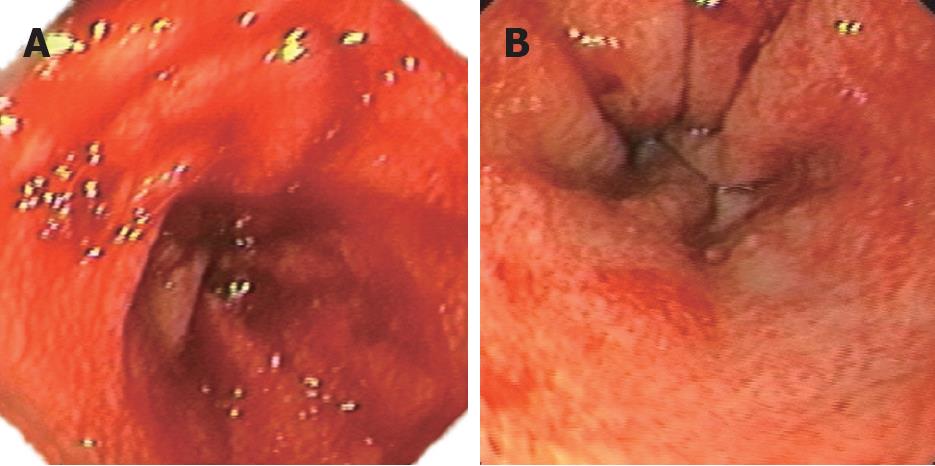
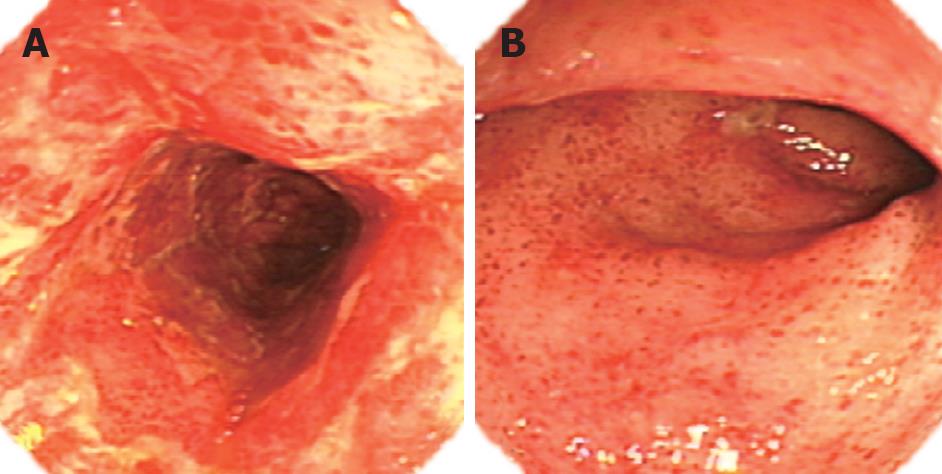
The endoscopic findings varied greatly. The first endoscopy for patient 1 with grade IV acute GVHD showed diffuse erythema with mucosal ozzing in the antrum and body of the stomach (Figure 1A). Because nausea, vomiting, melena and hematemesis persisted despite empiric treatment, emergency upper endoscopy and biopsy were repeated 1 wk later. The endoscopic appearance revealed a pale mucosal surface with reticulated submucosal small vessels accompanied with erosion and erythema in the antrum (Figure 1B). For the same patient, colonoscopy was performed after gastric bleeding was controlled, and disclosed extensive mucosal hyperemia and edema in the colon. In patient 2 with chronic GI GVHD, the upper endoscopic examination showed subtle mucosal edema with erythema in the antrum, but the appearance of the esophagus and duodenum was grossly normal. In patient 3 with chronic GVHD five months after transplantation, colonoscopic examination disclosed hemorrhagic spots, patchy erosions, and active bleeding. Patients 4, 5 and 6 showed similar diffuse damages, namely widespread erythema, multiple erosions and small ulcer. Two of the three patients had active bleeding in the colon (Figure 2A). Hemorrhagic spots and multiple shallow ulcers could be detected on the surface of rectocolon (Figure 2B). Patients 7 and 8 demonstrated widespread edema, erythema with multiple erosions without active bleeding in the total rectocolon.
In patient 1, histologic examination of gastric biopsy specimens showed focal dropout of crypt epithelial cells, variable lymphocytic infiltration of the epithelium and lamina propria, and colonic biopsies showed nonspecific inflammation. Gastric biopsies disclosed a crypt with multiple apoptotic cells in patient 2. Extensive mucosal erosions, shallow ulcer, sloughing and apoptosis of epithelial cells were found in patient 3. Extensive colonic mucosal erosion and necrosis were observed in patients 4 and 5, and biopsies of the colon in these patients showed clear histological evidence of acute GVHD. Biopsy specimens from patients 6, 7 and 8 illustrated numerous apoptotic bodies in crypts, and small lymphocytic infiltration of the adjacent lamina propria. CMV infection was not confirmed on biopsy specimens from seven patients by immunohistochemical study except for one patient with HLA 2-locus mismatched, in which colonic mucosa was weakly positive, but late antigen was negative 205 d after transplantation. Because this patient had concomitantly severe GI GVHD and skin involvement 24 d after allogeneic bone marrow transplantation (BMT), GI GVHD coexisted with CMV infection.
GVHD is the leading cause of morbidity and mortality after allogenic HSCT, occurring in up to 75% of patients[11]. According to the degree of involvement in each of the organ systems, acute GVHD can be clinically classified as grades I-IV. High risk factors include HLA disparity, unrelated-donor transplantation, donor-recipient gender difference, old age, and infection[12]. In the present study, one young female patient who underwent two-locus HLA-mismatched unrelated BMT suffered from grade IV acute GI GVHD 24 d after transplantation.
The principal organs with involvement of acute GI GVHD include stomach, small intestine, and rectocolon[13], but esophageal acute GVHD is uncommon[1415]. Roy et al[10] found that GVHD limited to the upper GI tract accounts for 18% of patients, GVHD involving the lower and upper GI tract accounts for 10%, and 26% of patients. The most prominent symptoms of GVHD involving the upper GI tract are anorexia, dyspepsia, nausea, vomiting, and, occasionally, abdominal pain[16]. Lower GI GVHD manifests as voluminous watery diarrhea (typically secretory in nature) accompanied with abdominal bloating, ileus, and occasionally overt intestinal bleeding[1718]. In contrast to acute GI GVHD, chronic GI GVHD differs markedly in distribution and histopathology. Esophageal involvement of chronic GI GVHD is not uncommon, but the stomach and intestine are rarely involved[19].
In the present study, colonoscopy disclosed scattered hemorrhagic spots and mucosal erosion in one patient with chronic GVHD.
Obviously, clinical manifestations of GI GVHD are nonspecific. There is a wide overlap of symptoms with many GI diseases. Toxicity from the regimen of cytoreductive therapy given before transplantation can cause symptoms of anorexia, nausea, vomiting, all of which are also characteristic of GVHD[220]. For most conditioning regimens, this variable is less important 20 d after transplantation, when toxicity to intestinal mucosa has largely resolved. A variety of bacterial, fungal and viral infections may affect the diagnosis of GI GVHD. Clinical manifestations of intestinal bacterial infection are mainly bloody stool and pathogenic bacteria can be confirmed from excreta. Endoscopy can also disclose mucosal erosion and pus moss. Fungal infections of the GI tract have become unusual since the routine use of prophylactic fluconazole, and fungas can be identified by examining stool specimens. In addition, since clinical symptoms of enteric CMV infection can resemble GVHD, all patients must undergo viral surveillance. Histologic identification of CMV infection is less sensitive than viral culture. Therefore, viral immunohistology and culture should be done if the patient is at a high risk for CMV infection. For more sensitive detection of CMV reactivation, polymerase chain reaction is also recommended[2122].
As stated previously, patients with and without GI GVHD cannot be distinguished based entirely upon clinical findings. Accurate and timely diagnosis is essential, as early recognition and intervention may significantly improve the outcome[2324]. Endoscopy combined with tissue biopsy is usually required to establish the diagnosis of acute GI GVHD. In a retrospective study, Terdiman and colleague[25] confirmed that acute upper GI GVHD is sensitive to many drugs if early diagnosis could be properly made. While treatment fails, upper GI GVHD may progress to lower GI. Therefore, upper GI GVHD is an early event. Our study revealed that upper endoscopic appearance of GVHD ranged from normal mucosa to erythema, erosion, ulceration and active bleeding. Normal endoscopic examinations have been reported in up to 21% of patients with histologically confirmed acute GVHD[20]. Sloughing of the mucosa is uncommon but high specific[26]. It is noted that discordance may be seen in different regions of the gut. In the present study, mucosal lesions in the antrum and body were more severe than those in the funds and duodenum, whereas the esophagus was less involved.
Enteric acute GVHD exhibits diffuse hyperemia, edema, erosion, and slough of mucosa, which can resemble severe ulcerative colitis[27]. In the present study, the grossly visible mucosal damage was uneven in distribution, sometimes appearing severely abnormal in one area while being unremarkable at other locations.
Since endoscopic appearance of GVHD is also nonspecific, endoscopic diagnosis cannot replace histopathological examinations. At present, endoscopy with biopsies remains the gold standard for the diagnosis of acute GI GVHD[2]. Histological criteria for GVHD are the presence of epithelial single-cell apoptosis and crypt cell dropout[28]. However, the reported mucosal site with the highest diagnostic yield (upper and/or lower) varies in studies. In a prospective study of HSCT patients with diarrhea and upper GI symptoms, Cox and his companies[20] discovered that the positive rate of gastric mucosal biopsies was 85% in 29 GVHD patients who were confirmed by histopathology and 58% in biopsies from duodenum and rectum-sigmoid colon. In another prospective study of 24 patients undergoing both upper and lower endoscopy[23], biopsies were obtained from the stomach, duodenum, ileum, right and rectosigmoid colon, while the biopsy site with the highest yield was the distal colon (82%), and a combination of upper endoscopy with sigmoidoscopy and colonscopy with ileal biopsies was equivalent (94%), suggesting that multiple biopsies should be obtained from stomach, duodenum, and rectum-sigmoid colon, in order to improve the accuracy and sensitivity of diagnosis. Many factors (chemoradiation toxicity, medication side effects, particularly CMV infection), can interfere with the histologic interpretation. Proton pump inhibitor (PPI) therapy is associated with increased apoptosis in antral biopsies. Biopsy from gastric fundus rather than from antrum may be preferable for the diagnosis of upper GI GVHD[3]. It is, therefore, important to rule out these factors in making a histologic diagnosis of GVHD after transplantation.
There is a discrepancy between endoscopic and histologic assessments of the severity of the disease[29]. Mucosal edema and erythema that are endoscopically impressive will be subtle when corresponding biopsies are assessed microscopically. In contrast, normal mucosa may display focal crypt epithelial apoptosis characteristic of GVHD. Thus, the correlation between endoscopic and histologic findings requires further investigation.
A clinical grading system based on the degree of lower GI symptoms (diarrhea volume, etc) does not consider the upper GI symptoms and endoscopic findings. Thus, an alternative, revised grading system needs to be proposed that takes into account the upper GI symptoms and endoscopic findings.
Roy et al[7] showed upper GI involvement is more common than lower GI in patients with GVHD confirmed by skin biopsy. Weisdorf et al[30] also confirmed that 59.7% of patients with GI GVHD have skin GVHD. Therefore, endoscopy with tissue biopsies may acquire positive results in patients with negative skin biopsies. It is noted that GI GVHD is not correlated with hepatic venous occlusion diseases (VOD).
It was reported that endoscopic examination is usually safe for patients with GVHD or occasional intestinal perforation, and oozing at the biopsy site due to thrombocytopenia[27]. Thus, a platelet count of more than 50 × 109/L is needed before endoscopic examination.
Because of the lack of sufficient samples, diagnostic endoscopic findings need further evaluation. In addition, endoscopists should cooperate with specialists in bone marrow transplantation to standardize the biopsy location and the number of specimens, method and time to undertake gastroscopy and/or colonoscopy[23].
In summary, endoscopic findings are highly variable in diagnosis of GI GVHD. There is a discrepancy between endoscopic and histologic assessments of the severity of GI GVHD. Gastrointestinal biopsies are needed to confirm the diagnosis of GI GVHD.
Allogenic hematopoietic stem cell transplantation (HSCT) is increasingly performed for a variety of disorders, such as acute and chronic leukemia, but many patients undergoing HSCT develop acute graft-versus-host disease (GVHD). GVHD involving the gastrointestinal (GI) tract is common, but it is difficult to establish the diagnosis of GI GVHD because of the nonspecific GI symptoms. Recognition of GI GVHD is critical for directing its specific therapy.
The diagnosis of GI GVHD often depends on an endoscopic evaluation. The endoscopic appearance of GI GVHD can range from normal to mild edema or erythema to dramatic mucosal slough, but the mucosal damage caused by chemoradiation toxicity, side effects of medications, and enteric infections with viruses, bacteria, and fungi may occur. Although endoscopy with biopsy is commonly used in the evaluation of suspected GI GVHD, the best diagnostic approach remains undefined.
There is no standardized protocol for upper or lower endoscopy, biopsy number and location. This study demonstrated that endoscopic examinations and histologic evaluation of biopsies could be used to diagnose GI GVHD. There is a discrepancy between endoscopic and histologic assessments of the severity of GI GVHD.
The present study further demonstrated the endoscopic role in diagnosing GI GVHD in patients following allogeneic HSCT, and histologic examination of GI biopsies is needed to confirm the final diagnosis.
GVHD: a condition that occurs following bone marrow transplantation or peripheral blood stem cell transplantation, in which lymphocytes from the graft attack specific tissues in the host. The skin, gut, and liver are the most severely affected. Drugs that suppress the immune reaction, such as steroids and cyclosporin A, reduce the severity of rejection.
The present study reported eight patients with proven GI GVHD and demonstrated the role of endoscopic examinations and histologic evaluation of biopsies in diagnosing GI GVHD, which is very important in clinical practice.
| 1. | Oomori S, Takagi S, Kikuchi T, Utsunomiya K, Yokoyama H, Negoro K, Tohmiya Y, Aihara H, Yamada M, Takahashi S. Significance of colonoscopy in patients with intestinal graft-versus-host disease after hematopoietic stem cell transplantation. Endoscopy. 2005;37:346-350. [Cited in This Article: ] |
| 2. | Bombi JA, Nadal A, Carreras E, Ramirez J, Munoz J, Rozman C, Cardesa A. Assessment of histopathologic changes in the colonic biopsy in acute graft-versus-host disease. Am J Clin Pathol. 1995;103:690-695. [Cited in This Article: ] |
| 3. | Welch DC, Wirth PS, Goldenring JR, Ness E, Jagasia M, Washington K. Gastric graft-versus-host disease revisited: does proton pump inhibitor therapy affect endoscopic gastric biopsy interpretation? Am J Surg Pathol. 2006;30:444-449. [Cited in This Article: ] |
| 4. | Fallows G, Rubinger M, Bernstein CN. Does gastroenterology consultation change management of patients receiving hematopoietic stem cell transplantation? Bone Marrow Transplant. 2001;28:289-294. [Cited in This Article: ] |
| 5. | Schulenburg A, Turetschek K, Wrba F, Vogelsang H, Greinix HT, Keil F, Mitterbauer M, Kalhs P. Early and late gastrointestinal complications after myeloablative and nonmyeloablative allogeneic stem cell transplantation. Ann Hematol. 2004;83:101-106. [Cited in This Article: ] |
| 6. | Schulenburg A, Kalhs P, Rabitsch W. Recommendations for diagnosis of acute gastrointestinal graft-versus-host disease in the small intestine. Transplantation. 2005;79:1767. [Cited in This Article: ] |
| 7. | Nydegger A, Catto-Smith AG, Tiedemann K, Hardikar W. Diagnosis of gastrointestinal graft-versus-host disease--is rectal biopsy enough? Pediatr Blood Cancer. 2007;48:561-566. [Cited in This Article: ] |
| 8. | Ross WA, Couriel D. Colonic graft-versus-host disease. Curr Opin Gastroenterol. 2005;21:64-69. [Cited in This Article: ] |
| 9. | McDonald GB, Shulman HM, Sullivan KM, Spencer GD. Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology. 1986;90:460-477. [Cited in This Article: ] |
| 10. | Roy J, Snover D, Weisdorf S, Mulvahill A, Filipovich A, Weisdorf D. Simultaneous upper and lower endoscopic biopsy in the diagnosis of intestinal graft-versus-host disease. Transplantation. 1991;51:642-646. [Cited in This Article: ] |
| 11. | Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667-674. [Cited in This Article: ] |
| 12. | Izumi N, Furukawa T, Sato N, Okazuka K, Tsukada N, Abe T, Yano T, Kurasaki T, Masuko M, Toba K. Risk factors for acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: retrospective analysis of 73 patients who received cyclosporin A. Bone Marrow Transplant. 2007;40:875-880. [Cited in This Article: ] |
| 13. | Iqbal N, Salzman D, Lazenby AJ, Wilcox CM. Diagnosis of gastrointestinal graft-versus-host disease. Am J Gastroenterol. 2000;95:3034-3038. [Cited in This Article: ] |
| 14. | Sodhi SS, Srinivasan R, Thomas RM. Esophageal graft versus host disease. Gastrointest Endosc. 2000;52:235. [Cited in This Article: ] |
| 15. | Otero Lopez-Cubero S, Sale GE, McDonald GB. Acute graft-versus-host disease of the esophagus. Endoscopy. 1997;29:S35-S36. [Cited in This Article: ] |
| 16. | Wakui M, Okamoto S, Ishida A, Kobayashi H, Watanabe R, Yajima T, Iwao Y, Hisamatsu T, Hibi T, Ikeda Y. Prospective evaluation for upper gastrointestinal tract acute graft-versus-host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;23:573-578. [Cited in This Article: ] |
| 17. | Jiang Q, Huang XJ, Chen H, Xu LP, Liu DH, Chen YH, Zhang YC, Liu KY, Guo NL, Lu DP. Severe gastrointestinal bleeding after allogeneic hematopoietic stem cell transplantation--15 case analysis. Zhonghua Xueyexue Zazhi. 2005;26:277-280. [Cited in This Article: ] |
| 18. | Nevo S, Enger C, Swan V, Wojno KJ, Fuller AK, Altomonte V, Braine HG, Noga SJ, Vogelsang GB. Acute bleeding after allogeneic bone marrow transplantation: association with graft versus host disease and effect on survival. Transplantation. 1999;67:681-689. [Cited in This Article: ] |
| 19. | Akpek G, Chinratanalab W, Lee LA, Torbenson M, Hallick JP, Anders V, Vogelsang GB. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46-51. [Cited in This Article: ] |
| 20. | Cox GJ, Matsui SM, Lo RS, Hinds M, Bowden RA, Hackman RC, Meyer WG, Mori M, Tarr PI, Oshiro LS. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107:1398-1407. [Cited in This Article: ] |
| 21. | Tsai KS, Hsieh HJ, Chow KC, Lin TY, Chiang SF, Huang HH. Detection of cytomegalovirus infection in a patient with febrile ulceronecrotic Mucha-Habermann's disease. Int J Dermatol. 2001;40:694-698. [Cited in This Article: ] |
| 22. | Gerna G, Lilleri D. Monitoring transplant patients for human cytomegalovirus: Diagnostic update. Herpes. 2006;13:4-11. [Cited in This Article: ] |
| 23. | Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 2006;38:371-376. [Cited in This Article: ] |
| 24. | Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G, Kalloo AN, Lee LA. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy. 2002;34:808-813. [Cited in This Article: ] |
| 25. | Terdiman JP, Linker CA, Ries CA, Damon LE, Rugo HS, Ostroff JW. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy. 1996;28:680-685. [Cited in This Article: ] |
| 26. | Watanabe N, Okazaki K, Yazumi S, Nishi T, Matsuura M, Chiba T. Acute graft-versus-host disease in the small intestine. Gastrointest Endosc. 2002;55:716. [Cited in This Article: ] |
| 27. | Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612-621. [Cited in This Article: ] |
| 28. | Melson J, Jakate S, Fung H, Arai S, Keshavarzian A. Crypt loss is a marker of clinical severity of acute gastrointestinal graft-versus-host disease. Am J Hematol. 2007;82:881-886. [Cited in This Article: ] |
| 29. | Yeh SP, Liao YM, Hsu CH, Chen CL, Shen YC, Hsueh CT, Huang HH, Chiu CF. Gastric bleeding due to graft-vs-host disease: discrepancy between endoscopic and histologic assessment. Am J Clin Pathol. 2004;122:919-925. [Cited in This Article: ] |