Published online Apr 7, 2008. doi: 10.3748/wjg.14.2085
Revised: February 27, 2008
Published online: April 7, 2008
AIM: To examine the effects of 10% diluted honey, which has been shown to be scolicidal, on the liver and biliary system and determine whether it could be used as a scolicidal agent in the presence of biliary-cystic communication.
METHODS: Thirty Wistar-Albino rats were divided into two groups. Honey with 10% dilution in the study group and 0.9% saline (NaCl) in the control group were injected into the common bile ducts of rats through a 3-mm duodenotomy. The animals were sacrificed 6 mo after the procedure. Histopathological, biochemical, and radiological examinations were performed for evaluation of side effects.
RESULTS: At the end of the sixth month, liver function tests were found to be normal in both groups. The tissue samples of liver and ductus choledochus of the honey group showed no histomorphologic difference from the control group. No stricture on the biliary tree was detected on the retrograde cholangiograms.
CONCLUSION: According to these results, we concluded that 10% diluted honey could be used as scolicidal agent safely in the presence of biliary-cystic communication.
- Citation: Kilicoglu B, Kismet K, Kilicoglu SS, Erel S, Gencay O, Sorkun K, Erdemli E, Akhan O, Akkus MA, Sayek I. Effects of honey as a scolicidal agent on the hepatobiliary system. World J Gastroenterol 2008; 14(13): 2085-2088
- URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2085.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2085
Hydatid disease is still an important endemic problem in Turkey and in many other parts of the world. Dissemination of protoscolex-rich fluid during surgery is a major cause of recurrence. Instillation of scolicidal agent into a hepatic hydatid cyst is the most commonly employed measure to prevent this serious complication[1]. Biliary system strictures or “caustic sclerosing cholangitis” can develop from the caustic effect of the scolicidal solution diffused from the cyst into the biliary system, during the surgical intervention of hydatid cysts of the liver[2]. Surgical treatment has been used around the world for years as the primary choice of treatment. Although percutaneous treatment of hydatid cysts was considered to be contraindicated due to potential risks of anaphylactic shock and dissemination of the hydatid fluid into the abdomen, this method has been used successfully for treatment of hydatid cysts since 1980s[3]. Up to date, many scolicidal agents have been used for inactivation of the cyst content, but there is no ideal agent that is both effective and safe[4].
The surgical treatment of hydatid disease of the liver includes evacuation of the cyst with scolocidal irrigation and either excision or drainage of the cyst[5]. The objectives of surgical treatment are inactivating scolices, preventing spillage of cyst contents, eliminating all viable elements of the cyst, and managing the residual cavity of the cyst. Inactivation of scolices with various scolicidal agents has been tried with varying success[6].
Honey is the foodstuff made by honeybees from the nectar of flowers or secretions from other parts of the plants, which they gather, transform together with their own specific materials, and store in honeycomb. Honey is considered as healthy and wholesome food with curative properties. It has antimicrobial effects against many bacteria and this property may be due to its osmolarity, acidity, flavonoids, aromatic acid substances, and hydrogen peroxide[7].
In a previous study, 10% diluted honey has been shown to be highly effective on protoscolices[8].
The aim of the present study was to investigate whether diluted honey would cause caustic sclerosing cholangitis when injected directly into the common bile duct of rats.
Thirty Wistar-Albino female rats, weighing 225 ± 25 g, were included in this study. Animals were deprived of food 12 h before anesthesia, but had free access to water 2 h before anesthesia. No enteral or parenteral antibiotics were administered at any time. Rats were housed under constant temperature (21°C ± 2°C) individually in wire cages with 12 h light-dark cycle. Rats that died during the study were excluded. The procedures in this experimental study were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and approved by the Animal Ethics Committee of Ankara Research and Training Hospital.
The rats were randomly divided into two equal groups of 15 rats each. Rats were anesthetized by an intramuscular injection of ketamine HCl (Ketalar, Parke-Davis, Eczacibasi, Istanbul, Turkey; 40 mg/kg body weight) and xylazine (Rompun, Bayer, Leverkusen, Germany; 5 mg/kg body weight). All animals were allowed to breath spontaneously during the experiments. After the abdomen was shaved and cleaned with povidone iodine, a midline laparotomy was carried out, and the intestines were covered with sterile gauze pads soaked with isotonic saline at 36°C-38°C. A 3-mm duodenotomy was performed. Test solutions, 0.15 mL, either sterile isotonic saline solution (control group) or 10% dilutions of honey (study group) (Anzer honey, Rize, Turkey), were injected without pressure into the common bile duct with 27 gauge syringe. Immediately after the injection, the common bile duct was clamped with an atraumatic vascular clamp (bulldog). The catheter was then withdrawn. The clamp was removed 5 min later, and the duodenotomy was closed with a 6-0 polypropylene (Prolene) suture. There was no operative mortality. The study animals were kept for 6 mo, during which time they were fed with rat chow ad libitum and tap water and kept at room temperature (18°C-20°C) in separate cages.
Blood samples were obtained 1 wk after the surgical procedure and at the end of the experimental study (6 mo after the procedure) for liver function tests including bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma glutamyl transferase (GGT).
Six months after the procedure, retrograde cholangio-graphy was performed under ketamine hydrochloride anesthesia. Midline laparotomy was carried out, and a 3-mm duodenotomy was performed. Radiopaque solution, 0.15 mL, was injected without pressure into the common bile duct using a 27 gauge syringe. Immediately after the injection, the common bile duct was clamped with an atraumatic vascular clamp and antero-posterior cholangiograms were obtained. Following cholangiography, blood samples for determination of liver function tests were obtained. Liver, common bile duct and duodenum were excised en-bloc for histopathological examination.
The biochemical analyses were made by an autoanalyzer (Olympus AU 640, Japan) using commercial kits.
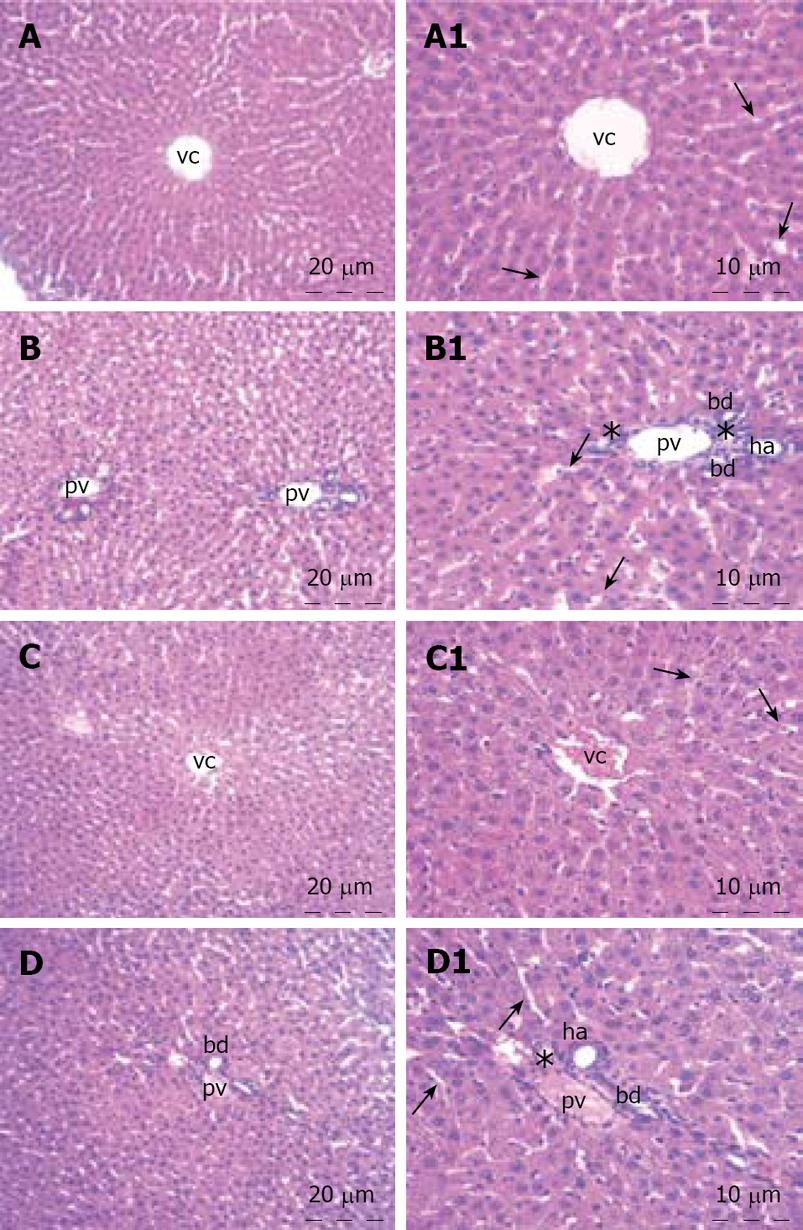
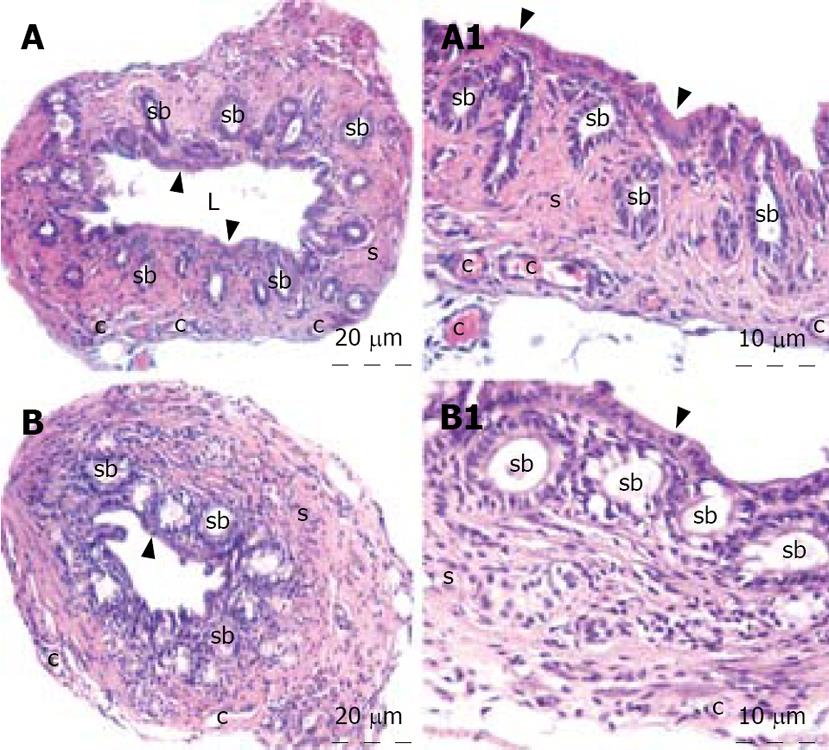
The liver specimens of the right and left lobes and common bile duct were taken and immediately fixed in 10% neutral buffered formalin solution for one week. Tissues were washed in flowing water and dehydrated with rising concentrations of ethanol (50%, 75%, 96% and 100%). After dehydration, specimens were put into xylene to obtain transparency and were then infiltrated with and embedded in paraffin. Histological sections of the specimens in thickness of 6 &mgr;m from all the groups were stained with hematoxylin and eosin. The whole tissue blocks were sectioned and histopathological examinations were performed on systematically randomly sampled preparations by a blinded researcher. The specimens were photographed by Nikon eclipse E 600. Liver specimens were evaluated to assess the morphology of the hepatocytes, portal areas, sinusoidal lesions, cellular infiltration in the lobule or portal spaces and parenchymal lesions. Histopathological examination of the common bile duct was performed to assess the histomorphology of the epithelium, connective tissue, inflammation, fibroblastic proliferation and necrosis.
Differences between the groups were analyzed with Mann-Whitney U test. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 13.0 for Windows (SPSS Inc., Chicago, USA). P values less than 0.05 were considered to be significant.
Five rats from each group died within 5 d after the procedure. Two of ten died in the early postoperative period possibly due to anesthesia, and the others died because of trauma to the common bile duct and leakage into the peritoneum. The remaining 20 rats were alive until the end of the study without any problem.
Liver function tests were slightly elevated 1 wk after the procedure in both groups, and this might be due to the cannulation and injection of common bile duct and there was no difference between the groups. At the end of the first and sixth month, liver function tests were found to be normal in both groups.
No stricture in the biliary tree was found on the retrograde cholangiograms.
The histopathological evaluation of the liver sections did not reveal any difference between the control and honey groups. Polygonal shaped hepatocytes radiating outward from a central vein in the center and arranging into hepatic cords separated by adjacent sinusoids were demonstrated. Both the hepatic portal vein and hepatic artery branch and also the bile ductules in the corners of hepatic lobules were in normal architecture histologically. There was neither inflammatory cellular infiltration nor bile pigment accumulation in either group (Figure 1). When we examined the tissue samples of the common bile duct, no adverse effect of the honey was demonstrated. The epithelium of the common bile duct was penetrated into the stroma to form pits known as the sacculi of Beale. The dense connective tissue and smooth muscle were in regular architecture in both groups. All three layers, mucosa, muscularis and serosa of the common bile duct were viewed normal. There was no inflammation and/or fibroblastic proliferation in the stroma of the control and honey groups. The lining cells of the sacculi of Beale were mucous gland cells that were clearly viewed in the honey group. The tissue samples of liver and common bile duct of the honey group showed no histomorphologic difference from the control group (Figure 2).
The ideal treatment for hepatic hydatid disease should completely eliminate the parasite and prevent recurrence of the disease with minimum morbidity and mortality. There are three available therapeutic modalities for hepatic hydatid disease: systemic chemotherapy, surgery, and percutaneous treatment[9]. Meticulous packing of the operative field is necessary irrespective of the surgical technique employed with sponges soaked in scolicidal agents to inactivate the scolices which may leak from the cyst during surgical manipulation. In conventional or minimally invasive hydatid disease surgery, inactivation of the cyst content is essential, justifying the routine use of scolicidal solutions. In the presence of cystobiliary communications, the passage of these solutions may cause hepatic stasis, edema and necrosis in the hepatic tissue as well as histopathological changes in the biliary tree[2]. Various experimental studies investigated the effects of 95% alcohol, 10% povidone iodine, 0.9%, 5.0%, 10.0%, 20.0% NaCl, 3% H2O2, 5% formalin, 0.5% AgNO3, cetrimide on the liver and the biliary tree. Severe hepatobiliary complications have been reported for formalin, alcohol and 10%-20% NaCl[1011].
Sclerosing cholangitis may be due to immunological, infectious, vascular, or chemical factors. In patients with hydatid disease of the liver, various factors, including injection of scolicidal agent into the cyst cavity, a communication between the cyst and biliary tree, and a particular sensitivity to the scolicidal agent seems to be necessary to promote caustic sclerosing cholangitis[5].
Histopathological changes in sclerosing cholangitis is spotty necrosis in the liver parenchyma, widening of sinusoids, regenerative changes in hepatocytes, Kupffer cell hyperplasia, pigment accumulation, periductal fibrosis, inflammation, fibroblastic proliferation, and necrosis in the extrahepatic biliary ducts[512]. In our study, no histopathological difference was detected in honey group when compared with the control group. None of the above mentioned pathological changes was present in the liver and common bile duct specimens of honey group.
The retrograde cholangiograms were all within normal limits without any evidence for biliary stricture. Liver function tests at the end of the first and sixth month were also within the normal ranges.
In a previous study, we found that honey was a potent scolicidal agent in vitro[8]. Honey concentrations of 10% or greater killed all protoscolices. The scolicidal effects of honey began at the end of the third minute. Intraperitoneal application of honey resulted in adverse effects with this concentration. According to these results, we concluded that honey might be used as a potent scolicidal agent after the evaluation of side effects on hepatobiliary system and the in vivo activity. The ideal scolicidal agent should have rapid and complete scolicidal effects with minimal local and systemic side effects[13]. No systemic side effects, such as anaphylactic reaction or hyperglycemia, and no local side effects in peritoneal surface developed with intraperitoneal administration[8]. Since an important and life-threatening side effect of scolicidal agents is sclerosing cholangitis, we planned to evaluate the effects of honey on hepatobiliary system with the present study in which we did not find any side effects on hepatobiliary system evaluated by using biochemical, histological, and radiologic parameters.
In conclusion, although sclerosing cholangitis is a major complication that restrict the use of many scolicidal agents in the presence of a biliary-cystic communication in hydatid liver disease, our experience from the present study shows that honey can be used safely in this situation.
To examine the effects of 10% diluted honey, which has been shown to be scolicidal, on the liver and biliary system whether it could be used as a scolicidal agent in the presence of biliary-cystic communication.
The present study investigated whether diluted honey would cause caustic sclerosing cholangitis when injected directly into the common bile duct of rats.
Sclerosing cholangitis is a major complication that restricts the use of many scolicidal agents in the presence of a biliary-cystic communication in hydatid liver disease, our experience from the present study shows that honey can be used safely in this situation.
Evaluates the effects of a 10% honey solution on the liver as a possible scolicidal agent in the treatment of hydatid cysts.
The rationale behind this study is that the authors have previously shown that a 10% honey solution has scolicidal properties in vitro and that current scolicidal agents often cause sclerosing cholangitis. It is an interesting paper.
| 1. | Tozar E, Topcu O, Karayalcin K, Akbay SI, Hengirmen S. The effects of cetrimide-chlorhexidine combination on the hepato-pancreatico-biliary system. World J Surg. 2005;29:754-758. [Cited in This Article: ] |
| 2. | Belghiti J, Benhamou JP, Houry S, Grenier P, Huguier M, Fekete F. Caustic sclerosing cholangitis. A complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162-1165. [Cited in This Article: ] |
| 3. | Akhan O, Ozmen MN. Percutaneous treatment of liver hydatid cysts. Eur J Radiol. 1999;32:76-85. [Cited in This Article: ] |
| 4. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [Cited in This Article: ] |
| 5. | Sahin M, Eryilmaz R, Bulbuloglu E. The effect of scolicidal agents on liver and biliary tree (experimental study). J Invest Surg. 2004;17:323-326. [Cited in This Article: ] |
| 6. | Sayek I, Onat D. Diagnosis and treatment of uncomplicated hydatid cyst of the liver. World J Surg. 2001;25:21-27. [Cited in This Article: ] |
| 7. | Orsolic N, Basic I. Antimetastatic effect of honey. Mellifera. 2004;4:38-43. [Cited in This Article: ] |
| 8. | Kilicoglu B, Kismet K, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Akkus MA. The scolicidal effects of honey. Adv Ther. 2006;23:1077-1083. [Cited in This Article: ] |
| 9. | Sayek I, Tirnaksiz MB, Dogan R. Cystic hydatid disease: current trends in diagnosis and management. Surg Today. 2004;34:987-996. [Cited in This Article: ] |
| 10. | Yetim I, Erzurumlu K, Hokelek M, Baris S, Dervisoglu A, Polat C, Belet U, Buyukkarabacak Y, Guvenli A. Results of alcohol and albendazole injections in hepatic hydatidosis: experimental study. J Gastroenterol Hepatol. 2005;20:1442-1447. [Cited in This Article: ] |
| 11. | Topcu O, Aydin C, Arici S, Duman M, Sen M, Koyuncu A. The effects of various scolicidal agents on the hepatopancreatic biliary system. Chir Gastroenterol. 2006;22:185-190. [Cited in This Article: ] |
| 12. | Houry S, Languille O, Huguier M, Benhamou JP, Belghiti J, Msika S. Sclerosing cholangitis induced by formaldehyde solution injected into the biliary tree of rats. Arch Surg. 1990;125:1059-1061. [Cited in This Article: ] |
| 13. | Altindis M, Arikan Y, Cetinkaya Z, Polat C, Yilmaz S, Akbulut G, Dilek ON, Gokce O. Octenidine hydrochloride in hydatid disease. J Invest Surg. 2004;17:41-44. [Cited in This Article: ] |